文章信息
- 代永欣, 王林, 万贤崇
- Dai Yongxin, Wang Lin, Wan Xianchong
- 遮荫和环剥对刺槐、侧柏苗木碳素分配和水力学特性的影响
- Effects of Shading and Girdling on Carbon Allocation and Hydraulic Architecture of Robinia pseudoacacia and Platycladus orientalis Seedlings
- 林业科学, 2017, 53(7): 37-44.
- Scientia Silvae Sinicae, 2017, 53(7): 37-44.
- DOI: 10.11707/j.1001-7488.20170704
-
文章历史
- 收稿日期:2016-12-05
- 修回日期:2017-03-29
-
作者相关文章
2. 山西农业大学林学院 太谷 030800
2. College of Forestry Science, Shanxi Agricultural University Taigu 030800
水力学特性的维持和碳平衡都是植物生存必不可少的基础。在逆境条件下2种代谢的平衡可能会被打破,如在干旱胁迫下木质部负压增大,导致气穴栓塞的大量发生,限制植物的水分输导,而发生水力学失败(Tyree, 2003; 万贤崇等, 2007)。为应对干旱有些植物会通过关闭气孔来避免由于过分失水而导致的水力学失败,但气孔关闭又会造成碳摄取限制,导致碳饥饿。水力学失败和碳饥饿被认为是干旱导致植物死亡的2种主要机制(McDowell et al., 2008)。近年来的研究表明2种机制的交互作用或共同作用在干旱导致树木死亡中起重要作用(Anderegg et al., 2013; Sevanto et al., 2014; Salmon et al., 2015)。对于两者之间的相互作用,目前比较明确的是水力学失败对碳饥饿的影响。水力学失败可以导致气孔关闭,进而诱发碳饥饿(McDowell et al., 2008; Menezes-Silva et al., 2015; Pangle et al., 2015)。水力学失败也会限制碳在韧皮部的运输(Sevanto et al., 2014; Woodruff, 2014),导致植物出现局部碳饥饿(McDowell et al., 2010; Hartmann et al., 2013)。因而水力学失败会促进碳饥饿的发生,加重碳饥饿的影响。
关于碳饥饿对水力学特性的影响研究较少。非结构性碳(NSC),通常指可溶性糖和淀粉,是植物生命活动中碳和能量的储备和缓冲,其对维持树木生长、抵御病虫害和维持水力学特性有重要作用(Dietze et al., 2014)。NSC不足会减缓树木的生长速率,降低植物抵抗病虫害的能力,影响植物渗透调节和木质部导管的栓塞修复过程(McDowell, 2011)。碳限制对植物生长的抑制作用已形成共识(McDowell, 2011; Sala et al., 2012),但对于碳限制如何影响水力学特性还有一些环节不清楚,如碳限制条件下NSC的分配过程(Dietze et al., 2014),同时也不了解NSC在呼吸、渗透调节、栓塞修复等生理功能上是否存在优先次序(Hartmann, 2011),以致难以全面理解碳限制对水力学特性的影响。在全球气候变化导致干旱发生频率增加、食叶害虫危害加大的背景下(Allen et al., 2010),明确水力学失败和碳饥饿之间的相互作用方式可以更好地理解植物如何应对气候变化。
遮荫和环剥是经常用于模拟植物碳限制的研究手段(Domec et al., 2008)。遮荫可以限制植物的光合作用以影响碳摄取(O’Brien et al., 2014),环剥通过限制光合产物向根部的运输,可用于模拟根部碳限制(Sellin et al., 2013)。本研究以刺槐(Robinia pseudoacacia)和侧柏(Platycladus orientalis)为试验材料,通过90%遮荫造成植物整体处于低NSC水平和环剥造成植物根部的低NSC水平,研究碳限制对水力学特性的影响。刺槐和侧柏是中国华北和西北地区重要的人工林树种(Zhang et al., 2015),这些地区的植物经常受到季节性干旱的胁迫。刺槐和侧柏有着不同的抗旱策略(靳欣等, 2011),近年来就干旱对这2个树种水分平衡的影响进行了较广泛的研究(靳欣等, 2011; 王林等, 2013a;2013b;2016),但目前尚不了解这2个树种在碳限制条件下水力学特性的响应。本研究通过比较这2个树种对2种不同处理的响应异同,了解不同树种对光强变化的响应及不同处理对NSC的影响,进而了解碳限制对水力学特性的影响。
1 材料与方法 1.1 试验材料与试验设计本研究在山西省太谷县山西农业大学林学院实验基地(112°18′12″E, 37°15′36″N,海拔780 m)进行,于2014年3月初将2年生刺槐苗和3年生侧柏苗定植到花盆(直径35 cm,高35 cm)中,每盆栽植1株。盆土采用2/3田间表层土+1/3沙,保持土壤含水量在田间持水量的80%左右,缓苗3个月。2014年6月中旬开始试验,每个树种选择生长基本一致苗木36株,随机分为3组,每组12株,分别进行遮荫、环剥和对照处理,每个处理中6株用于测定水力学特性和光合指标,6株用于测定生物量和非结构性碳。遮荫处理用遮光90%的遮阳网将盆栽苗的顶部和四周完全遮蔽,并用照度计确认遮阳网的遮荫效果,处理2个月后逐步揭开遮阳网让植株适应自然光照环境,10天以后选择连续晴天测定各指标。环剥处理的时间与遮荫处理相同,在距离树干基部8 cm处剥掉2 cm宽的皮层,剥离皮层后用小刀小心地清除韧皮部残留,此后用保水硅胶包裹,防止木质部失水干燥。为防止伤口处的韧皮部重新生长连接,每15天检查1次伤口情况。
1.2 试验方法 1.2.1 生物量和非结构性碳浓度的测定苗木收获后,分离植株的细根(直径<2 mm)、粗根(直径>2 mm)、茎和叶片,将茎和粗根的韧皮部和木质部分开,105 ℃杀青15 min后于70 ℃烘干48 h,之后称量,计算不同部位的生物量。
烘干样品用于测定根和茎非结构性度(可溶性糖+淀粉)碳浓,将韧皮部去除老皮,烘干样品用粉碎机粉碎后过100目筛,取过筛粉末用于测定可溶性糖和淀粉含量,测定采用硫酸蒽酮法,具体方法参照Mitchell等(2013)。
1.2.2 根系导水率测定根系导水率采用植物高压导水率测量仪(HPFM-Gen3, Dynamax, USA)测定,在距离主干基部5 cm处截去主干,并用解剖刀将切口修平,根部连接植物高压导水率测量仪,测定根系导水率。
1.2.3 水势、导水率损失值和气孔导度的测定气孔导度选择晴天上午9:00—11:00用稳态气孔计(SC-1Decagon, Pullman, USA)测定,气孔导度测定之后再测定水势和导水率损失值(Percentage loss of hydraulic conductance, PLC)。采用压力室(PMS1505D-EXP,USA)分别测定枝条凌晨和正午水势,凌晨水势测定时间为日出前5:00—5:30,正午水势于中午12:00—14:00测定。同时测定枝条凌晨PLC,采样时间同凌晨水势。栓塞去除刺槐采用高压法,0.175 MPa高压水冲洗5 min,侧柏采用真空法,将茎段浸没在0.025 mol·L-1的KCl溶液中,放入真空干燥器中,用真空泵抽出真空干燥器中的空气,保持4 h以脱除茎段中的栓塞,试验参考王林等(2015)的方法。
1.2.4 数据处理采用SPSS 13.0软件进行数据统计分析,对照和处理的均值比较采用单因素方差分析,不同处理间用最小显著差数法(LSD)进行多重比较。利用SigmaPlot 10.0软件制图,所有指标测定重复5次以上。
2 结果与分析 2.1 遮荫和环剥对刺槐、侧柏生物量分配的影响由表 1可知,遮荫和环剥处理刺槐的苗高和基径均显著低于对照(P < 0.05),遮荫和环剥处理侧柏的苗高与对照之间无显著差异,但基径显著低于对照(P < 0.05)。
|
|
由图 1可知,遮荫和环剥处理均显著降低2个树种各部位的生物量(P < 0.05)。遮荫和环剥处理刺槐的细根生物量分别仅为对照的6.4%和4.3%,而遮荫和环剥处理侧柏细根生物量分别为对照的39.7%和22.7%。遮荫和环剥处理刺槐不同部位生物量之间均无显著差异,而遮荫处理侧柏的细根、粗根和叶片生物量均显著高于环剥处理。
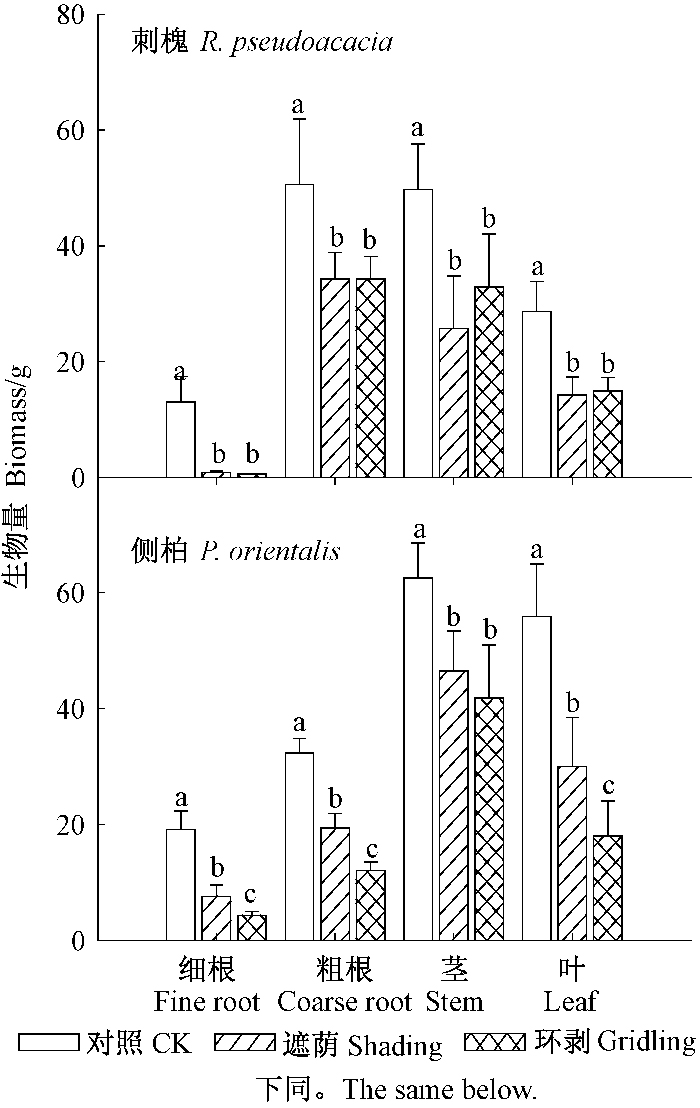
|
图 1 遮荫和环剥处理对刺槐和侧柏生物量分配的影响 Fig.1 Biomass allocation of R. pseudoacacia and P. orientalis under different treatments |
由图 2可知,遮荫显著降低刺槐和侧柏不同部位的NSC浓度(P < 0.05)。环剥显著降低2个树种根部的NSC浓度(P < 0.05),其中刺槐茎韧皮部NSC浓度略有降低,木质部该值显著升高,侧柏茎韧皮部和木质部NSC均显著升高。在遮荫和环剥处理之间刺槐根韧皮部和木质部NSC浓度差异不显著,而侧柏环剥处理的根韧皮部和木质部NSC均显著低于遮荫处理(P < 0.05)。
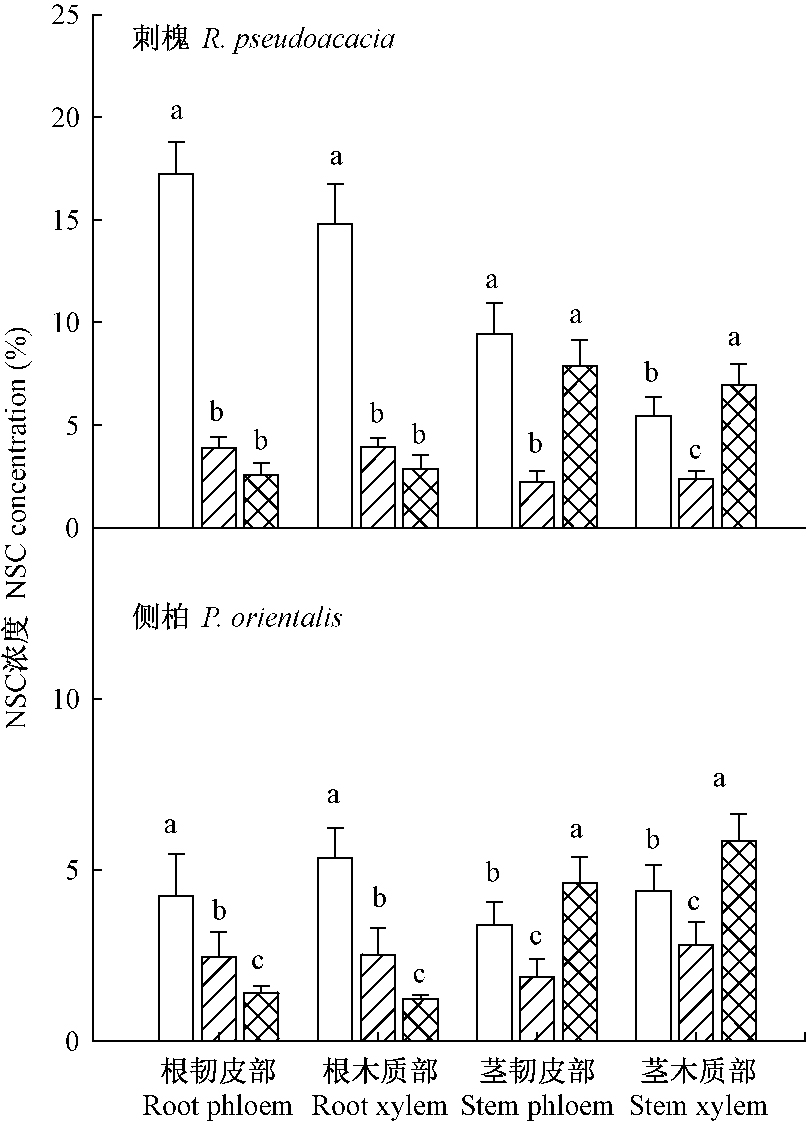
|
图 2 遮荫和环剥处理对刺槐和侧柏NSC浓度的影响 Fig.2 The effect of shading and girdling on NSC concentration for R. pseudoacacia and P. orientalis |
由图 3可知,2个树种遮荫和环剥处理根系导水率均显著低于对照(P < 0.05)。在遮荫和环剥处理之间刺槐根系导水率无显著差异,遮荫和环剥处理根系导水率分别为对照的3.7%和2.9%;遮荫处理侧柏根系导水率显著高于环剥处理,遮荫和环剥处理根系导水率分别为对照的21.0%和7.6%。
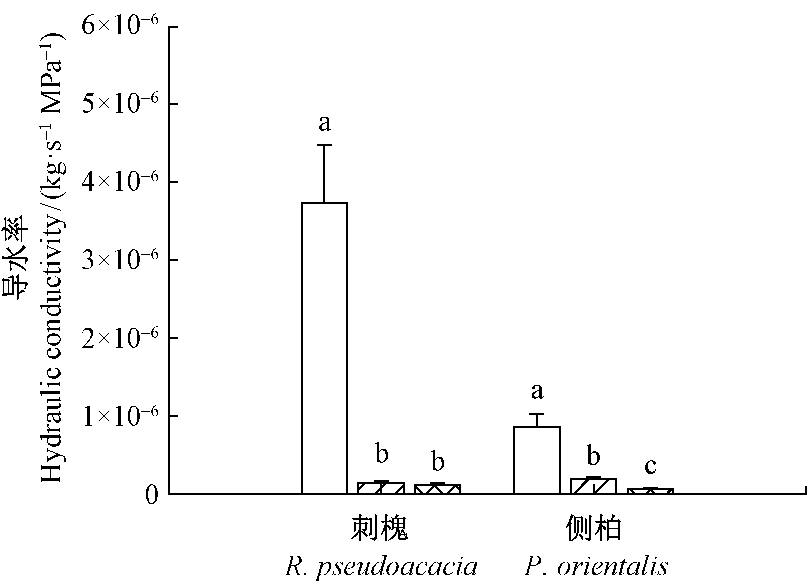
|
图 3 遮荫和环剥处理对刺槐和侧柏根系导水率的影响 Fig.3 The effect of shading and girdling on root hydraulic conductivity for R. pseudoacacia and P. orientalis |
由图 4可知,遮荫和环剥均显著降低刺槐和侧柏的凌晨水势和正午水(P < 0.05),刺槐凌晨水势、正午水势在2个处理之间均没有显著差异,遮荫处理侧柏的凌晨水势和正午水势均显著高于环剥处理。
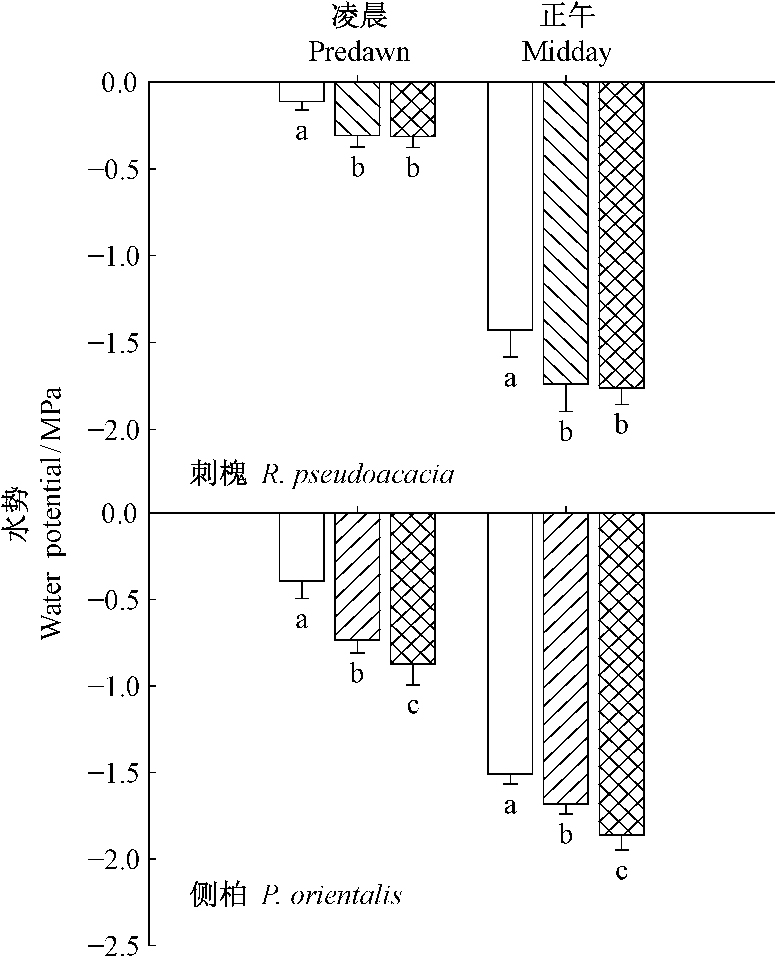
|
图 4 遮荫和环剥处理对刺槐和侧柏凌晨和正午水势的影响 Fig.4 The effect of shading and girdling on predawn and midday water potential for R. pseudoacacia and P. orientalis |
对PLC的测定结果(图 5)表明,遮荫和环剥均显著加大2个树种根和枝条PLC。在2个处理之间刺槐根、枝条PLC均没有显著差异,而侧柏环剥处理根和枝条PLC均显著高于遮荫处理(P < 0.05)。
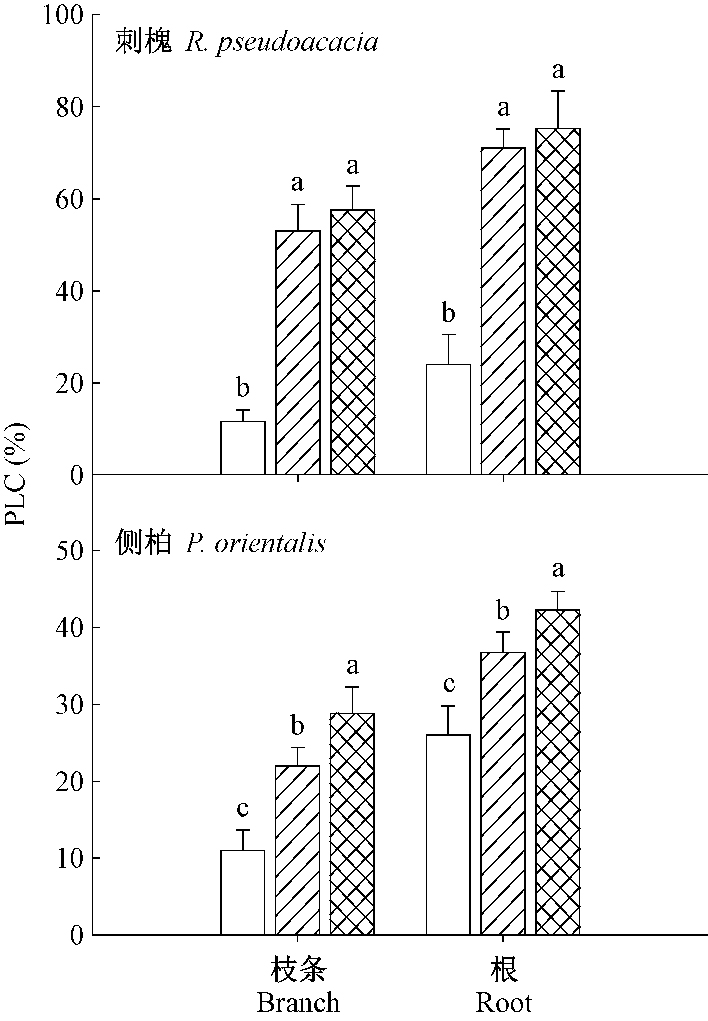
|
图 5 遮荫和环剥处理对刺槐和侧柏凌晨栓塞的影响 Fig.5 The effect of shading and girdling on predawn PLC for R. pseudoacacia and P. orientalis |
对气孔导度测定结果(图 6)表明,遮荫和环剥处理均显著降低刺槐和侧柏的气孔导度(P < 0.05),遮荫和环剥处理的刺槐气孔导度分别为对照的33.7%和26.1%,侧柏的气孔导度分别为对照的46.9%和23.4%。环剥处理刺槐的气孔导度比遮荫处理略低,但二者之间差异不显著。侧柏环剥处理的气孔导度显著低于遮荫处理。
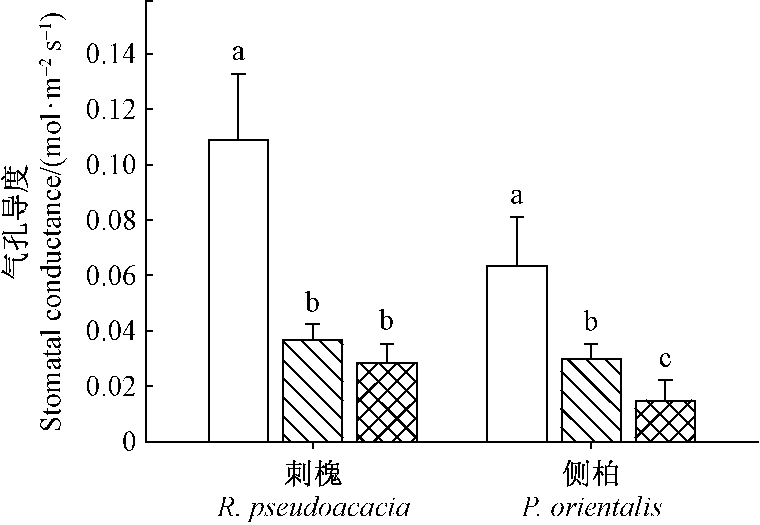
|
图 6 遮荫和环剥处理对刺槐和侧柏气孔导度的影响 Fig.6 The effect of shading and girdling on stomatal conductance for R. pseudoacacia and P. orientalis |
本研究中,遮荫显著降低2种植物不同部位的NSC浓度,环剥一定程度上增加2个树种茎NSC浓度,但显著降低根系NSC浓度。然而,2种形式的碳限制都显著降低2个树种不同器官的生物量,根部碳限制与植物整体碳限制一样对生长起到显著抑制作用,这与前人的研究结果一致(Wiley et al., 2012; Anderegg et al., 2012; Landhäusser et al., 2012)。碳限制树木生长的机制一直以来存在2种不同观点,即“碳限制”和“汇限制”,通常认为碳的可利用性降低导致的生长受限为“碳限制”,而当树木利用碳的能力受限(如环境或营养因素)而导致的生长受限为“汇限制”(Wiley et al., 2012, 2013; Palacio et al., 2014)。在胁迫条件下树木往往以牺牲生长为代价而把NSC主动储存起来,这样有利于树木应对可能发生的碳饥饿以及之后的生长恢复,从而导致生长受到抑制(碳限制)(Wiley et al., 2012; Sala et al., 2012)。本研究中遮荫使得碳摄取受到严重限制,树木几乎没有多余的NSC用于储存,只能依赖于储藏NSC来维持基本的生命活动,从而导致NSC浓度和生长同时显著下降,这与Sevanto等(2014)对可食松(Pinus edulis)遮荫的研究结果一致。
环剥阻碍韧皮部运输,使得光合产物不能输送到根部,植物只能动用根部储存的碳来维持根系的生命活动,从而导致根部生长和NSC的积累严重下降。细根属于周转较快的器官,所以受到的生长抑制也最严重。由于环剥处理的植物地上部分能维持一定的光合作用,以及韧皮部运输受到限制,导致茎NSC的积累,且茎的生长也受到明显抑制。植物生长需要各器官整体配合,环剥限制根的生长和代谢活动,进而限制其对水分和矿质元素的吸收,反过来抑制地上部分的生长(Kosola et al., 2001; Sigurdsson et al., 2013);另外韧皮部运输障碍限制NSC的转运和利用会产生反馈抑制,这也可能是限制地上部分生长的原因之一(McDowell et al., 2010; Sevanto et al., 2014)。
3.2 碳限制对水力学特性的影响遮荫或环剥均造成刺槐和侧柏根系的碳限制;同时遮荫或环剥处理根系导水率显著降低、根系木质部栓塞程度(以PLC为指标)增大,表明根的水分吸收能力受到限制。遮荫或环割处理导致细根生物量显著下降,即降低根系吸收面积。另一方面,根系吸水过程需要NSC提供渗透调节作用,碳限制会影响根系主动吸水能力(郭建荣, 2017),吸收面积和主动吸水能力的下降都导致根系水分吸收功能下降。根系吸水能力下降会打破整个植物的水分平衡,2种处理的刺槐和侧柏枝条水势明显下降、枝条木质部栓塞显著增加,表明2种植物的地上部分水分状况变差,水分输导能力受限。2种处理的2种植物根PLC均高于枝条,可能是由于“脆弱性分区”(Tyree et al., 1993),根的栓塞脆弱性高于枝条,使得在同样木质部张力下根更容易发生气穴栓塞。
NSC可以为栓塞修复过程提供渗透势梯度(Secchi et al., 2011),NSC下降会影响栓塞修复能力,如环剥会降低木质部的栓塞修复能力(Salleo et al., 1996, 2004; Christman et al., 2012)。碳限制也可能会导致木质部抗栓塞能力下降(Anderegg et al., 2012)。植物木质部栓塞产生和修复是一个动态过程,栓塞修复能力和抗栓塞能力的下降必然会导致木质部水分输导受限,进而导致气孔导度下降(Domec et al., 2006)。本研究中环剥处理导致茎NSC浓度的上升并没有改善其地上部分的水势和栓塞程度。植物根系水分吸收和叶片蒸腾失水是维持植物水分平衡的主要机制。根系吸水能力的限制使得吸水无法满足地上部分的蒸腾失水,必然导致水分失衡。其结果引起木质部(包括枝条木质部)张力增加,随之造成气穴栓塞。另外,环剥导致韧皮部运输障碍,使得NSC不能被运输到需要的部位(McDowell et al., 2010; McDowell, 2011; Sevanto et al., 2014)。环剥植物地上部分光合产物的积累也可能会抑制光合作用(Araya et al., 2006),这会进一步限制植物的碳摄取,导致碳代谢和水分代谢之间的恶性循环。气孔导度下降虽然会减少植物的蒸散,但并没有缓解水分吸收和输导能力下降导致的植物水分状况恶化。
3.3 遮荫和环剥处理对刺槐和侧柏水力学特性影响的异同本研究中遮荫和环剥处理都导致2种植物水分吸收和输导功能受限,主要原因是2种处理都导致根生物量和NSC浓度的显著降低,进而限制根的水分吸收和输导功能。根是植物水分吸收和输导的重要器官,而且根位于植物茎的生物学下端,根的水分输导能力下降直接造成地上部分水分供应能力的下降,也就造成地上部分水分状况的恶化。这表明根的水分吸收和输导能力在植物水力学特性维持中起重要作用,根部碳限制会严重影响植物的水力学特性。地上部分NSC浓度上升并不能缓解根碳限制导致的水分状况恶化。
比较2种植物对遮荫和环剥的差别响应,发现刺槐的根系导水率、根和茎PLC、水势和气孔导度在2种处理之间无显著差异,而遮荫对侧柏的影响显著小于环剥,环剥处理的侧柏根系导水率显著低于遮荫处理,凌晨和正午水势显著降低,根和茎PLC增大。同时也发现环剥对侧柏NSC的影响较遮荫严重,而2种处理对刺槐根部NSC的影响差别不大,这可能与2种植物的特性有关,刺槐属于典型的阳性树种,耐阴性较差(王林等, 2013b),而侧柏具有一定的耐阴性,耐弱光的能力好于刺槐,因此遮荫对侧柏的影响小于刺槐。
4 结论2种形式的碳限制都导致植物根生物量严重下降,根系导水率显著降低,根和茎木质部PLC显著升高,水势降低,气孔导度减小;环剥造成的地上部分NSC浓度增加并没有缓解地上部分的水分状况。碳限制影响细根发生和根木质部栓塞修复,从而影响根的水分吸收和输导能力,降低木质部水势,加重根和茎木质部栓塞程度,进而影响叶片气孔导度和碳摄取,加剧碳限制的影响,因而影响植物在逆境下的存活,而根部NSC浓度降低对植物水力学特性的影响更大。
| [] |
郭建荣. 2017. 木本植物银腺杨根压及其昼夜周期与影响因素的研究. 北京: 中国林业科学研究院博士学位论文. ( Guo J R. 2017. Researching of root pressure and its diurnal rhythm of 84K pupolar and the influencing factors.Beijing: PhD thesis of Chinese Academy of Forestry.[in Chinese]) |
| [] |
靳欣, 徐洁, 白坤栋, 等. 2011. 从水力结构比较3种共存木本植物的抗旱策略. 北京林业大学学报, 33(6): 135–141.
( Jin X, Xu J, Bai K D, et al. 2011. Comparison of drought strategies of three co-existing woody plants by their hydraulic structures. Journal of Beijing Forestry University, 33(6): 135–141. [in Chinese] ) |
| [] |
万贤崇, 孟平. 2007. 植物体内水分长距离运输的生理生态学机制. 植物生态学报, 31(5): 804–813.
( Wan X C, Meng P. 2007. Physiological and ecological mechanisms of long-distance water transport in plants: a review of recent issues. Chinese Journal of Plant Ecology, 31(5): 804–813. DOI:10.17521/cjpe.2007.0102 [in Chinese] ) |
| [] |
王林, 冯锦霞, 王双霞, 等. 2013a. 干旱和坡向互作对栓皮栎和侧柏生长的影响. 生态学报, 33(8): 2425–2433.
( Wang L, Feng J X, Wang S X, et al. 2013a. The interaction of drought and slope aspect on growth of Quercus variabilis and Platycladus orientalis. Acta Ecologica Sinica, 33(8): 2425–2433. [in Chinese] ) |
| [] |
王林, 冯锦霞, 万贤崇. 2013b. 土层厚度对刺槐旱季水分状况和生长的影响. 植物生态学报, 37(3): 248–255.
( Wand L, Feng J X, Wan X C. 2013b. Effects of soil thickness on dry-season water relations and growth in Robinia pseudoacacia. Chinese Journal of Plant Ecology, 37(3): 248–255. [in Chinese] ) |
| [] |
王林, 代永欣, 樊兴路, 等. 2015. 风对黄花蒿水力学性状和生长的影响. 生态学报, 35(13): 4454–4461.
( Wang L, Dai Y X, Fan X L, et al. 2015. Effects of wind on hydraulic properties and growth of Artemisia annua Linn. Acta Ecologica Sinica, 35(13): 4454–4461. [in Chinese] ) |
| [] |
王林, 代永欣, 郭晋平, 等. 2016. 刺槐苗木干旱胁迫过程中水力学失败和碳饥饿的交互作用. 林业科学, 52(6): 1–9.
( Wang L, Dai Y X, Guo J P, et al. 2016. Interaction of hydraulic failure and carbon starvation on Robinia pseudoacacia seedlings during drought. Scientia Silvae Sinicae, 52(6): 1–9. [in Chinese] ) |
| [] | Allen C D, Macalady A K, Chenchouni H, et al. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, 259(4): 660–684. DOI:10.1016/j.foreco.2009.09.001 |
| [] | Anderegg W R, Callaway E S. 2012. Infestation and hydraulic consequences of induced carbon starvation. Plant Physiology, 159(4): 1866–1874. DOI:10.1104/pp.112.198424 |
| [] | Anderegg W R, Anderegg L D. 2013. Hydraulic and carbohydrate changes in experimental drought-induced mortality of saplings in two conifer species. Tree Physiology, 33(3): 252–260. DOI:10.1093/treephys/tpt016 |
| [] | Araya T, Noguchi K, Terashima I. 2006. Effects of carbohydrate accumulation on photosynthesis differ between sink and source leaves of Phaseolus vulgaris L. Plant and Cell Physiology, 47(5): 644–652. DOI:10.1093/pcp/pcj033 |
| [] | Christman M A, Sperry J S, Smith D D. 2012. Rare pits, large vessels and extreme vulnerability to cavitation in aring-porous tree species. New Phytologist, 193(3): 713–720. DOI:10.1111/j.1469-8137.2011.03984.x |
| [] | Dietze M C, Sala A, Carbone M S, et al. 2014. Nonstructural carbon in woody plants. Annual Review of Plant Biology, 65(1): 667–687. DOI:10.1146/annurev-arplant-050213-040054 |
| [] | Domec J C, Scholz F G, Meinzer F C, et al. 2006. Diurnal and seasonal variation in root xylem embolism in neotropical savanna woody species: impact on stomatal control of plant water status. Plant Cell and Environment, 29(1): 26–35. DOI:10.1111/PCE.2006.29.issue-1 |
| [] | Domec J C, Pruyn M L. 2008. Bole girdling affects metabolic properties and root, trunk and branch hydraulics of young ponderosa pine trees. Tree Physiology, 28(10): 1493–1504. DOI:10.1093/treephys/28.10.1493 |
| [] | Hartmann H. 2011. Will a 385 million year-struggle for light become a struggle for water and for carbon?-How trees may cope with more frequent climate change-type drought events. Global Change Biology, 17(1): 642–655. DOI:10.1111/gcb.2010.17.issue-1 |
| [] | Hartmann H, Ziegler W, Trumbore S. 2013. Lethal drought leads to reduction in nonstructural carbohydrates in Norway spruce tree roots but not in the canopy. Functional Ecology, 27(2): 413–427. DOI:10.1111/fec.2013.27.issue-2 |
| [] | Kosola K R, Dickmann D I, Paul E A, et al. 2001. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia, 129(1): 65–74. DOI:10.1007/s004420100694 |
| [] | Landhäusser S M, Lieffers V J. 2012. Defoliation increases risk of carbon starvation in root systems of mature aspen. Trees, 26(2): 653–661. DOI:10.1007/s00468-011-0633-z |
| [] | McDowell N G, Pockman W T, Allen C D, et al. 2008. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought. New Phytologist,, 178(4): 719–739. DOI:10.1111/j.1469-8137.2008.02436.x |
| [] | McDowell N G, Sevanto S. 2010. The mechanisms of carbon starvation: How, when, or does it even occur at all. New Phytologist,, 186(2): 264–266. DOI:10.1111/j.1469-8137.2010.03232.x |
| [] | McDowell N G. 2011. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiology, 155(3): 1051–1059. DOI:10.1104/pp.110.170704 |
| [] | Menezes-Silva P E, Cavatte P C, Martins S C, et al. 2015. Wood density, but not leaf hydraulic architecture, is associated with drought tolerance in clones of Coffea canephora. Trees, 29(6): 1–11. |
| [] | Mitchell P J, O'Grady A P, Tissue D T, et al. 2013. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytologist, 197(3): 862–872. DOI:10.1111/nph.12064 |
| [] | O'Brien M J, Leuzinger S, Philipson C D, et al. 2014. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nature Climate Change, 4(8): 710–714. DOI:10.1038/nclimate2281 |
| [] | Palacio S, Hoch G, Sala A, et al. 2014. Does carbon storage limit tree growth. New Phytologist, 201(4): 1096–1100. DOI:10.1111/nph.12602 |
| [] | Pangle R E, Limousin J M, Plaut J A, et al. 2015. Prolonged experimental drought reduces plant hydraulic conductance and transpiration and increases mortality in a piñon-juniper woodland. Ecology and Evolution, 5(8): 1618–1638. DOI:10.1002/ece3.1422 |
| [] | Sala A, Woodruff D R, Meizer F C. 2012. Carbon dynamics in trees: feast or famine. Tree Physiology, 32(6): 764–775. DOI:10.1093/treephys/tpr143 |
| [] | Salleo S, Lo Gullo M A, Paoli D D, et al. 1996. Xylem recovery from cavitation-induced embolism in young plants of laurus nobilis: a possible mechanism. New Phytologist, 132(1): 47–56. DOI:10.1111/nph.1996.132.issue-1 |
| [] | Salleo S, Lo Gullo M A, Trifilò P, et al. 2004. New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of laurus nobilis L. Plant Cell and Environment, 27(8): 1065–1076. DOI:10.1111/pce.2004.27.issue-8 |
| [] | Salmon Y, Torres-Ruiz J M, Poyatos R, et al. 2015. Balancing the risks of hydraulic failure and carbon starvation: a twig scale analysis in declining Scots pine. Plant Cell and Environment, 38(12): 2575–2588. DOI:10.1111/pce.12572 |
| [] | Secchi F, Zwieniecki M. 2011. Sensing embolism in xylem vessels: the role of sucrose as a trigger for refilling. Plant, Cell and Environment, 34(3): 514–524. DOI:10.1111/pce.2011.34.issue-3 |
| [] | Sellin A, Niglas A, Õunapuu E. 2013. Impact of phloem girdling on leaf gas exchange and hydraulic conductance in hybrid aspen. Biologia Plantarum, 57(3): 531–539. DOI:10.1007/s10535-013-0316-2 |
| [] | Sevanto S, McDowell N G, Dickman L T, et al. 2014. How do trees die? A test of the hydraulic failure and carbon starvation hypotheses. Plant, Cell and Environment, 37(1): 153–161. DOI:10.1111/pce.2014.37.issue-1 |
| [] | Sigurdsson B D, Medhurst J L, Wallin G, et al. 2013. Growth of mature boreal Norway spruce was not affected by elevated. Tree Physiology, 33(11): 1192–1205. DOI:10.1093/treephys/tpt043 |
| [] | Tyree M T, Cochard H, Cruiziat P, et al. 1993. Drought-induced leaf shedding in walnut: evidence for vulnerability segmentation. Plant, Cell and Environment, 16(7): 879–882. DOI:10.1111/pce.1993.16.issue-7 |
| [] | Tyree M T. 2003. Plant hydraulics: the ascent of water. Nature, 423(6943): 923. DOI:10.1038/423923a |
| [] | Wiley E, Helliker B. 2012. Are-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist, 195(2): 285–289. DOI:10.1111/j.1469-8137.2012.04180.x |
| [] | Wiley E, Huepenbecker S, Casper B B, et al. 2013. The effects of defoliation on carbon allocation: can carbon limitation reduce growth in favour of storage. Tree Physiology, 33(11): 1216–1228. DOI:10.1093/treephys/tpt093 |
| [] | Woodruff D R. 2014. The impacts of water stress on phloem transport inDouglas-fir trees. Tree Physiology, 34(1): 5–14. DOI:10.1093/treephys/tpt106 |
| [] | Zhang T, Cao Y, Chen Y, et al. 2015. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees, 29(6): 1837–1849. DOI:10.1007/s00468-015-1265-5 |
 2017, Vol. 53
2017, Vol. 53

