文章信息
- 全文选, 丁贵杰
- Quan Wenxuan, Ding Guijie
- 干旱胁迫下马尾松幼苗针叶挥发性物质与内源激素的变化
- Dynamic of Volatiles and Endogenous Hormones in Pinus massoniana Needles under Drought Stress
- 林业科学, 2017, 53(4): 49-55.
- Scientia Silvae Sinicae, 2017, 53(4): 49-55.
- DOI: 10.11707/j.1001-7488.20170406
-
文章历史
- 收稿日期:2016-10-26
- 修回日期:2017-02-15
-
作者相关文章
2. 贵州省森林资源与环境研究中心 贵阳 550025;
3. 贵州师范大学山地环境重点实验室 贵阳 550001
2. Research Center for Forest Resources & Environment, Guizhou Province Guiyang 550025;
3. Key Laboratory of Mountainous Environmental Protection, Guizhou Normal University Guiyang 550001
马尾松(Pinus massoniana)以速生、丰产、适应性强、综合利用程度高、用途广等优良特性成为我国最重要的乡土用材树种,广泛分布于秦岭、淮河以南,云贵高原以东的17个省区市(丁贵杰等,2006)。干旱灾害严重影响马尾松的生长和繁殖,但马尾松强大的根系吸水能力增强了对逆境的适应性,从而使其成为了植树造林的主要先锋树种之一。以往对马尾松胁迫生态学研究主要集中在生物和非生物胁迫方面(Wang et al., 2013; Hu et al., 2014; Fan et al., 2016),而提高树种的抗旱性是森林培育的主要目标之一,因此强化研究马尾松对干旱环境的适应机制至关重要。
近年来,植物信号研究成为国内外研究热点,受到广泛关注。当植物遭受环境胁迫时,针叶可以释放挥发性物质,这些物质具有直接和间接的信号调控作用(Laothawornkitkul et al., 2008)。植物释放挥发性物质是其重要的防御性反应,挥发性物质包括萜烯类和脂肪酸衍生物等,是植物抵抗逆境条件的重要信号(Dixon,2001; Loreto et al., 2010)。植物内源激素参与其适应胁迫的全过程,包括激素的合成、运输和信号控制(Santner et al., 2009; Ross et al., 2011),不同内源激素在胁迫条件下作用不同(Peleg et al., 2011)。
植物叶片的抗逆性有助于提高整株植物的抵抗力(Sack et al., 2006),因此深入分析不同干旱条件下马尾松针叶的次生代谢变化十分必要。本研究的科学意义:针叶挥发性物质和内源激素作为马尾松感受环境胁迫的一种信号,是否可以作为定量指标来评价其对干旱胁迫的响应状况?通过研究马尾松幼苗对不同干旱胁迫的调节能力,从次生代谢物的变化方面深入探讨马尾松对干旱胁迫的适应与抗逆机制,是对该造林树种抗逆性的进一步研究和认识,并为速生丰产林的培育提供科学依据。
1 材料与方法 1.1 试验材料与设计2016年3月,在贵州省麻江县苗圃选取长势一致的马尾松1年生实生苗作为试验材料。干旱胁迫试验在贵州师范大学温室进行,盆栽缓苗后于6月15日在温室内对幼苗进行干旱处理。采用盆栽称质量控水法,设计4个水分梯度,即最大田间持水量的75% ~ 80% (对照,CK)、55% ~ 60% (轻度干旱,LD)、40% ~ 45% (中度干旱,MD)和30% ~ 35% (重度干旱,SD)。每处理20盆,每盆1株。统一浇水管理,每天18:00通过称质量法维持土壤相对含水量在试验设计的范围内,60天后进行指标测定。
1.2 指标测定 1.2.1 挥发性物质测定条件GCMS-QP2010型气相色谱-质谱联用仪由日本岛津生产,SPME和100 μm PDMS萃取头由美国Supelco公司制造。每个水分梯度处理选取10株幼苗的茎段中部针叶鲜样剪碎混合后,取50 mg于10 mL顶空瓶中,放适量无水硫酸钠,插入老化后的萃取头,密闭,75 ℃恒温浴锅内萃取10 min后,进行GC-MS分析。
GC条件:色谱柱为VF-WAXms (30 m × 0.25 mm × 0.25 μm); 载气为氦气(99.999%); 柱前压49.5 Pa; 柱流量1 mL· min -1; 取样时间1 min。进样口温度230 ℃; 程序升温,40 ℃保持5 min,以5 ℃·min -1的速度升至250 ℃保持5 min。MS条件:电子轰击源为EI; 电子能量70 eV; 离子源温度200 ℃; 接口温度250 ℃; 溶剂切除时间3 min; 扫描质量范围40 ~ 450;扫描间隔0.5 s。
1.2.2 内源激素测定条件Agilent 1290液相色谱仪、Agilent 6460C三重四极杆质谱仪和配电喷雾离子源为美国安捷伦公司生产,4种内源激素ZT、IAA、ABA和GA标准品为色谱纯级,Sigma公司生产。色谱条件和质谱条件参照文献(Kojima et al., 2009; Diego et al., 2012),并在取样量和内源激素提取方法上加以改进。取针叶样品2 g,于液氮中研磨成粉末,分离上清液,过聚乙烯聚吡咯烷酮PVPP柱和二乙基氨基乙基交联葡聚糖凝胶(DEAE Sephadex A-25) 柱净化,过0.22 μm滤膜后检测。
1.3 数据处理根据总离子流峰面积归一化法计算各组分相对含量。挥发性物质鉴定基于NTST147及NTST127标准图谱库(Hewlett Packard,Palo Alto,California,USA)进行相似度检索(匹配度大于90%)和相关文献报道,确定挥发性物质的化学结构(Kim et al., 2013; Ioannou et al., 2014)。采用Origin 6.1(软件)绘图,SPSS18.0软件进行数理统计分析。
2 结果与分析 2.1 干旱胁迫对苗木生长形态的影响生物量、根冠比是评价植物耐干旱能力的重要指标,通常干旱胁迫显著抑制幼苗生物量增加。轻度、中度和重度胁迫时,马尾松苗木生物量均较对照显著降低; 不同干旱处理苗高增长量与对照均差异显著; 轻度、中度胁迫时苗木根冠比与对照差异显著,重度胁迫不显著(表 1)。
|
|
不同水分梯度幼苗针叶挥发性物质GC-MS总离子流色谱图见图 1。通过对总离子流色谱图中各组分图谱分析及资料核实,从对照、轻度、中度、重度胁迫均鉴定出13种挥发物,峰号依次为顺-3 -己烯醛(1)、α -蒎烯(2)、反-2 -己烯醛(3)、反-3 -己烯醇(4)、β -蒎烯(5)、月桂烯(6)、D -柠檬烯(7)、α -水芹烯(8)、4 -蒈烯(9)、醋酸冰片酯(10)、石竹烯(11)、α -石竹烯(12)、大根香叶烯D (13),其中单萜类6种、倍半萜类3种,其他类4种。

|
图 1 干旱胁迫下马尾松针叶挥发性物质的总离子流色谱 Fig.1 Total ion current of volatiles of masson pine needles under drought stresses |
由表 2可知,在鉴定出的挥发性物质中,相对含量较高的有α -蒎烯、α -水芹烯、石竹烯、大根香叶烯D、反-3 -己烯醇等,这些挥发物构成马尾松针叶的主要挥发性成分。对照针叶挥发性成分中,单萜类相对含量最高,占59.97%,其中以α -蒎烯(31.54%)和α -水芹烯(14.35%)为主; 倍半萜类占30.47%,主要是石竹烯(23.06%)。轻度胁迫时针叶挥发性成分中,单萜类相对含量最高,占48.64%,主要包括α -蒎烯(20.16%)、α -水芹烯(11.89%)等; 倍半萜类占32.26%,主要是石竹烯(21.78%)。中度胁迫时针叶挥发性成分中,也是单萜类相对含量最高,占51.77%,主要包括α -蒎烯(27.87%)、α -水芹烯(13.04%)等; 倍半萜类占30.96%,主要有石竹烯(21.78%)、大根香叶烯D(11.46%)。重度胁迫时针叶挥发性成分中,单萜类相对含量达到61.69%,主要包括α -蒎烯(32.32%)、α -水芹烯(17.55%)等; 倍半萜类占20.69%,主要是石竹烯(15.67%)。
|
|
α -蒎烯、α -水芹烯的相对含量随干旱胁迫程度加大呈“高-低-高”变化趋势,在重度胁迫时最高; 石竹烯的相对含量随干旱胁迫程度加大呈不断下降趋势; 大根香叶烯D和反-3 -己烯醇随干旱胁迫程度加大呈“低-高-低”变化趋势,均在中度胁迫时最高。
2.3 干旱胁迫下针叶内源激素的动态变化内源激素通过植物与所处外部环境的相互作用调节来适应环境胁迫,如控制植物株型、水分和营养的利用等。对马尾松幼苗针叶4种内源激素的差异性分析可知,赤霉素和生长素含量均随干旱胁迫程度加大呈逐渐降低趋势,重度胁迫时含量最低(其中赤霉素比对照下降约50%); 玉米素、脱落酸含量随干旱胁迫程度加大均呈逐渐升高趋势,重度胁迫时含量最高,分别比对照提高约5.5和4倍(图 2)。

|
图 2 干旱胁迫下马尾松针叶内源激素变化 Fig.2 Endogenous hormone at levels of drought stress in needles of masson pine seedling 不同小写字母显示在P<0.05水平同一激素不同处理间差异显著。Different lowercase letters indicate significant differences at the P<0.05 level between treatments of the same endogenous hormone. |
干旱是制约林业可持续发展的重要因素,影响树木生长(Vacchiano et al., 2012),改变有机物成分等(Pichler et al., 2007)。本研究表明,重度干旱胁迫显著抑制马尾松苗高和生物量的增长,而轻度、中度干旱胁迫则促进根冠比的增大。
挥发性物质在针叶树防御昆虫和病原体中发挥着重要作用(Bracho-Nunez et al., 2011),其中萜类常作为植物化学标记物在松属植物防御中发挥关键作用(Achotegui-Castells et al., 2013)。挥发性萜类对干旱胁迫的响应度高,对植物抵抗逆境胁迫具有重要意义(Gargallo-Garriga et al., 2014)。本研究表明,马尾松具有较高的调节能力,并可分配针叶内不同的次生代谢产物,该结果对于马尾松逆境生态学研究具有重要意义。马尾松针叶挥发性物质中α -蒎烯含量最高,其次是大根香叶烯D、β -蒎烯和石竹烯(Ioannou et al., 2014),本研究的挥发性物质组成与以往研究结果相近。本研究中单萜类和倍半萜类是各个干旱胁迫处理的主要挥发性物质,且2类物质比例在不同胁迫处理下呈动态变化,其中单萜类含量随着干旱胁迫程度加大而上升,与前人研究结果较为一致(Simpraga et al., 2011)。本研究结果显示,不同干旱胁迫下马尾松挥发性物质数量不变,干旱胁迫仅改变了不同组分的相对含量,这说明挥发性物质的生物合成主要受遗传控制,环境因素影响其含量变化。
3.2 针叶挥发性物质的主要代谢途径马尾松针叶挥发性物质的生物合成主要通过4种生化途径(图 3):脂肪氧合酶途径(GLV-s)合成的绿叶挥发物(Hatanaka,1993)、莽草酸途径合成的芳香挥发物(Pare et al., 1996)、赤霉素途径(MEP)合成的单萜类化合物(Pichersky et al., 2006; Rajabi Memari et al., 2013)和甲羟戊酸途径(MVA)合成的倍半萜类化合物(Rajabi Memari et al., 2013; Rosenkranz et al., 2013)。任何生物或非生物胁迫都可能改变植物挥发性物质的释放速率,改变其比例(Holopainen et al., 2010; Niinemets et al., 2013),如叶片损伤增加植物倍半萜类的释放(Theis et al., 2009),本研究与以往研究结果一致。萜类物质具有独特的化感活性,往往在较低浓度即能表现出很强的抑制作用,萜类物质在植物的相互作用中起着重要作用,如自毒作用、植物防御和环境胁迫等(Yang et al., 2008; Chen et al., 2011; Singh et al., 2015)。
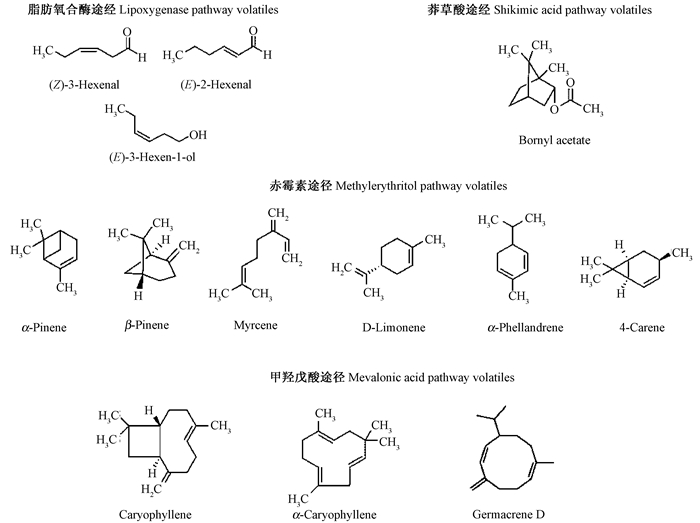
|
图 3 马尾松针叶内挥发性物质的分子结构 Fig.3 Molecular structures of plant volatiles in needles of masson pine seedling |
生长素对干旱胁迫响应主要是针对吲哚乙酸(IAA)的研究。IAA是公认的根源信号物质,参与多种植物生长过程(Shi et al., 2014),但针对干旱胁迫下植物生长素含量的变化研究相对较少。有研究认为干旱初期植物叶片的瞬时IAA含量增加,但随着干旱胁迫的持续其含量大幅下降(Wang et al., 2008)。本研究各干旱胁迫处理的马尾松针叶IAA含量是在较长时间胁迫后测定,随着干旱程度的加大其含量逐渐下降。
赤霉素(GA)在植物叶的分化、光形态建成等过程中扮演重要角色。本研究结果显示,马尾松针叶赤霉素含量随着干旱胁迫程度加大呈逐渐降低趋势,重度胁迫时含量最低。GA含量降低是马尾松应对干旱条件的生态策略,其含量降低可诱导芽休眠以应对干旱(Zawaski et al., 2014)。
脱落酸(ABA)被称为胁迫激素,逆境条件下植物内源激素ABA变化是研究植物抗逆的热点。ABA在根和叶中都能合成(Thompson et al., 2007),有研究认为干旱胁迫下植物根合成ABA并转移到叶,导致气孔关闭,抑制植物生长,从而使植物适应干旱胁迫(Park et al., 2015)。本研究结果显示,随着干旱胁迫加大针叶ABA含量呈逐渐升高趋势。
玉米素(ZT)是植物体内主要的细胞分裂素之一,其含量变化是植物对环境胁迫的响应(Ha et al., 2012)。前人研究认为干旱胁迫下植物叶片ZT含量升高(Diego et al., 2012; Liu et al., 2016)。本研究结果显示,针叶ZT随着干旱胁迫程度加大含量逐渐升高且变化幅度最大,马尾松针叶内源ZT是最具代表性的干旱胁迫信号激素,其变化机制有待进一步研究。
4 结论1) 顶空固相微萃取(HS-SPME)方法,需要样品量少,对苗木伤害小,检测快速方便,灵敏度高,成本低,可以作为挥发性物质的提取方法。本研究建立的方法,可以快速测定9个主要马尾松挥发性萜类成分,GC-MS是适合于鉴定马尾松针叶挥发性物质的一种手段。
2) 干旱胁迫时马尾松幼苗针叶挥发性物质种类稳定,每个针叶样品均含有丰富的单萜类和倍半萜类物质。针叶通过转化单萜类与倍半萜类含量的比例来响应干旱胁迫,α -蒎烯和α -水芹烯是单萜类含量的主要成分,倍半萜类主要由石竹烯和大根香叶烯D组成。
3) 干旱胁迫时马尾松幼苗针叶中ZT与ABA含量迅速升高,可作为重要的干旱胁迫信号,诱导植物体一系列生理生化变化以应对干旱胁迫。同时,内源IAA和GA含量降低,与其他逆境响应因子协调马尾松生理反应,避免干旱伤害。
| [] |
丁贵杰, 周志春, 王章荣, 等. 2006. 马尾松纸浆用材林培育与利用. 北京, 中国林业出版社: 1-33.
( Ding G J, Zhou Z C, Wang Z R, et al. 2006. Cultivation and utilization of pulpwood stand for Pinus massoniana. Beijing, China Forestry Publishing House: 1-33. [in Chinese] ) |
| [] | Achotegui-Castells A, Llusia J, Hoder J A, et al. 2013. Needle terpene concentrations and emissions of two coexisting subspecies of Scots pine attacked by the pine processionary moth (Thaumetopoea pityocampa). Acta Physiologiae Plantarum, 35(10): 3047–3058. DOI:10.1007/s11738-013-1337-3 |
| [] | Bracho-Nunez A, Knothe N M, Costa W R, et al. 2011. Root anoxia effects on physiology and emissions of volatile organic compounds (VOC) under short-and long-term inundation of trees from Amazonian floodplains. Springerplus, 9(1): 1–16. |
| [] | Chen F, Tholl D, Bohlmann J, et al. 2011. The family of terpene synthase in plants: a mid size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant Journal, 66(1): 212–229. DOI:10.1111/tpj.2011.66.issue-1 |
| [] | Diego N D, Pérez-Alfocea F, Cantero E, et al. 2012. Physiological response to drought in radiata pine: phytohormone implication at leaf level. Tree Physiology, 32(4): 435–449. DOI:10.1093/treephys/tps029 |
| [] | Dixon R A. 2001. Natural products and plant disease resistance. Nature, 411(6839): 843–847. DOI:10.1038/35081178 |
| [] | Fan F H, Ding G J. Wen X P. 2016. Proteomic analyses provide new insights into the responses of Pinus massoniana seedlings to phosphorus deficiency. Proteomics, 16(3): 504–515. DOI:10.1002/pmic.v16.3 |
| [] | Gargallo-Garriga A, Sardans J, Pérez-Trujillo M, et al. 2014. Opposite metabolic responses of shoots and roots to drought. Scientific Reports, 4: 6829. DOI:10.1038/srep06829 |
| [] | Ha S, Vankova R, Yamaguchi-Shinozaki K, et al. 2012. Cytokinins: metabolism and function in plant adaptation to environmental stresses. Trends in Plant Science, 17(3): 172–179. DOI:10.1016/j.tplants.2011.12.005 |
| [] | Hatanaka A. 1993. The biogeneration of green odour by green leaves. Phytochemistry, 34(5): 1201–1218. DOI:10.1016/0031-9422(91)80003-J |
| [] | Holopainen J K, Gershenzon J. 2010. Multiple stress factors and the emission of plant VOCs. Trends in Plant Science, 15(3): 176–184. DOI:10.1016/j.tplants.2010.01.006 |
| [] | Hu W J, Chen J, Liu T W, et al. 2014. Proteome and calcium-related gene expression in Pinus massoniana needles in response to acid rain under different calcium levels. Plant and Soil, 380(1): 285–303. |
| [] | Ioannou E, Koutsaviti A, Tzakou O, et al. 2014. The genus Pinus: a comparative study on the needle essential oil composition of 46 pine species. Phytochemistry Reviews, 13(4): 741–768. DOI:10.1007/s11101-014-9338-4 |
| [] | Kim H, Lee B, Yun K W. 2013. Comparison of chemical composition and antimicrobial activity of essential oils from three pinus species. Industrial Crops & Products, 44: 323–329. |
| [] | Kojima M, Kamada-Nobusada T, Komatsu H, et al. 2009. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant and Cell Physiology, 50(7): 1207–1214. |
| [] | Laothawornkitkul J, Paul N D, Vickers C E, et al. 2008. Isoprene emissions influence herbivore feeding decisions. Plant, Cell and Environment, 31(10): 1410–1415. DOI:10.1111/pce.2008.31.issue-10 |
| [] | Liu Y, Liang H Y, Lü X K, et al. 2016. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiology & Biochemistry, 100: 113–129. |
| [] | Loreto F, Schnitzler J P. 2010. Abiotic stresses and induced BVOCs. Trends in Plant Science, 15(3): 154–166. DOI:10.1016/j.tplants.2009.12.006 |
| [] | Niinemets U, Kannaste A, Copolovici L. 2013. Quantitative patterns between plant volatile emissions induced by biotic stresses and the degree of damage. Frontiers in Plant Science, 4(9): 1–15. |
| [] | Pare P W, Tumlinson J H. 1996. Plant volatile signals in response to herbivore feeding. Florida Entomologist, 79(2): 93–103. DOI:10.2307/3495807 |
| [] | Park S Y, Peterson F C, Mosquna A, et al. 2015. Agrochemical control of plant water use using engineered abscisic acid receptors. Nature, 520(7548): 545–548. DOI:10.1038/nature14123 |
| [] | Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology, 14(3): 290–295. DOI:10.1016/j.pbi.2011.02.001 |
| [] | Pichersky E, Noel J, Dudareva N. 2006. Biosynthesis of plant volatiles: nature's diversity and ingenuity. Science, 311(5762): 808–811. DOI:10.1126/science.1118510 |
| [] | Pichler P, Oberhuber W. 2007. Radial growth response of coniferous forest trees in an inner Alpine environment to heat-wave in 2003. Forest Ecology and Management, 242(2): 688–699. |
| [] | Rajabi Memari H, Pazouki L, Niinemets U. 2013. The biochemistry and molecular biology of volatile messengers in trees//Niinemets U, Monson R K. Biology, controls and models of tree volatile organic compound emissions. Berlin: Springer, 47-93. |
| [] | Rosenkranz M, Schnitzler J P. 2013. Genetic engineering of BVOC emissions from trees// Niinemets U, Monson R K. Biology, controls and models of tree volatile organic compound emissions. Berlin:Springer, 95-118. |
| [] | Ross J J, Weston D E, Davidson S E, et al. 2011. Plant hormone interactions: how complex are they? Physiologia Plantarum, 141(4): 299-309. Physiologia Plantarum, 141(4): 299–309. DOI:10.1111/ppl.2011.141.issue-4 |
| [] | Sack L. Holbrook N M. 2006. Leaf hydraulics. Annual Review of Plant Biology, 57: 361–381. DOI:10.1146/annurev.arplant.56.032604.144141 |
| [] | Santner A. Estelle M. 2009. Recent advances and emerging trends in plant hormone signalling. Nature, 459(7250): 1071–1078. DOI:10.1038/nature08122 |
| [] | Shi H, Chen L, Ye T, et al. 2014. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiology and Biochemistry, 82(3): 209–217. |
| [] | Singh B, Sharma R A. 2015. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech, 5(2): 129–151. DOI:10.1007/s13205-014-0220-2 |
| [] | Simpraga M, Verbeeck H, Demarcke M, et al. 2011. Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmospheric Environment, 45(30): 5254–5259. DOI:10.1016/j.atmosenv.2011.06.075 |
| [] | Theis N, Kesler K, Adler L S. 2009. Leaf herbivory increases floral fragrance in male but not female Cucurbita pepo subsp. texana (Cucurbitaceae) flowers. American Journal of Botany, 96(5): 897–903. DOI:10.3732/ajb.0800300 |
| [] | Thompson A J, Mulholland B J, Jackson A C, et al. 2007. Regulation and manipulation of ABA biosynthesis in roots. Plant Cell & Environment, 30(1): 67–78. |
| [] | Vacchiano G, Garbarino M, Mondino E B, et al. 2012. Evidences of drought stress as a predisposing factor to Scots pine decline in Valle d'Aosta (Italy). European Journal of Forest Research, 131(4): 989–1000. DOI:10.1007/s10342-011-0570-9 |
| [] | Wang C, Yang A, Yin H, Zhang J. 2008. Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. Journal of Integrative Plant Biology, 50(4): 427–434. DOI:10.1111/jipb.2008.50.issue-4 |
| [] | Wang X, Zheng L, Li N, et al. 2013. Long-term effects of simulated acid rain stress on a staple forest plant Pinus massoniana Lamb: a proteomic analysis. Trees, 27(1): 297–309. DOI:10.1007/s00468-012-0799-z |
| [] | Yang G Q, Wan F H, Liu W X, et al. 2008. Influence of two allelochemicals from Ageratina adenophora Sprengel on ABA, IAA and ZR contents in roots of upland rice seedlings. Alleopathy Journal, 21(2): 253–262. |
| [] | Zawaski C, Busov V B. 2014. Roles of gibberellin catabolism and signaling in growth and physiological response to drought and short-day photoperiods in Populus trees. PLoS One, 9(1): e86217. DOI:10.1371/journal.pone.0086217 |
 2017, Vol. 53
2017, Vol. 53

