文章信息
- 刘慧慧, 张永安, 王玉珠, 曾宝胜, 刘群, 张真
- Liu Huihui, Zhang Yong, Wang Yuzhu, Zeng Baosheng, Liu Qun, Zhang Zhen
- 美国白蛾Wnt-1基因的基因组编辑
- Genome Editing of Wnt-1 in Fall Webworm (Hyphantria cunea)
- 林业科学, 2017, 53(3): 119-127.
- Scientia Silvae Sinicae, 2017, 53(3): 119-127.
- DOI: 10.11707/j.1001-7488.20170313
-
文章历史
- 收稿日期:2016-06-14
- 修回日期:2016-10-12
-
作者相关文章
2. 中国林业科学研究院华北林业实验中心 北京 102300;
3. 中国科学院上海生命科学研究院植物生理生态研究所 昆虫发育与进化生物学重点实验室 上海 200032
2. Experimental Center of Forestry in North China, CAF Beijing 102300;
3. Key Laboratory of Insect Developmental and Evolutionary Biology Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, CAS Shanghai 200032
美国白蛾 (Hyphantria cunea) 属鳞翅目灯蛾科 (Lepidoptera:Arctiidae),是世界性检疫害虫,原产北美洲,现已广泛分布于欧亚地区。自1979年传入辽宁丹东以来,美国白蛾迅速在我国扩散蔓延。目前,在山东、河北、天津、北京、陕西等地危害严重,河南、江苏、安徽、吉林等地成为新的入侵区,给我国林业生产和生态安全带来巨大危害,每年造成巨大的经济损失 (季荣等,2003;赵铁珍等,2007;张俊杰等,2013;夏剑萍等,2015;刘俊等,2016)。目前为止,对美国白蛾的防治主要依赖于化学药剂,大量使用将导致其抗药性增强,不利于长效控制,同时给生态环境带来巨大压力,因此寻求环境友好型的害虫控制方法成为未来美国白蛾防治工作的重点。虽已有报道采用生物防治的方法,如利用天敌昆虫周氏啮小蜂 (Chouioia cunea) 和病原微生物美国白蛾NPV病毒 (杨忠岐等,2007),但因其见效慢,需要寻找其他更高效的无公害防治方法共同防治美国白蛾。近年来,释放携带显性致死基因 (release of insects carrying a dominant lethal strategy) 的RIDL技术作为新兴的无公害防治技术引起人们的关注,该技术通过遗传操作手段对昆虫进行改造获得不育雄虫,大量释放这些不育雄虫使其产生无生殖能力的后代,连续释放几代后,能有效控制害虫。此技术已在小菜蛾 (Plutella xylostella)、棉红铃虫 (Pectinophora gossypiella)、地中海实蝇 (Ceratitis capitata)、橄榄实蝇 (Bactrocera oleae) 和墨西哥实蝇 (Anastrepha ludens) 等物种中应用 (Thomas et al., 2000; Alphey et al., 2010; Martins et al., 2012; Jin et al., 2013; Ant et al., 2012)。目前此技术尚未应用于美国白蛾。
CRISPR/Cas系统作为新兴的基因组编辑工具,由sgRNA和Cas9蛋白组成,sgRNA可特异性识别基因序列,引导Cas蛋白对靶标基因的DNA双链进行定点切割 (Hsu et al., 2014)。该技术已成功应用于一些鳞翅目昆虫,如家蚕 (Bombyx mori)(Wang et al., 2013)、柑橘凤蝶 (Papilio xuthus) 和金凤蝶 (Papilio machaon)(Li et al., 2015)、斜纹夜蛾 (Spodoptera litura)(Bi et al., 2016)、小菜蛾 (Huang et al., 2016)、棉铃虫 (Helicoverpa armigera)(Wang et al., 2016)、马尾松毛虫 (Dendrolimus punctatus)(Liu et al., 2016)。为验证CRISPR/Cas9系统在美国白蛾中应用的可行性,选择昆虫翅发育相关的Wnt-1基因作为标记基因进行试验。果蝇 (Drosophila melanogaster) 的Wnt信号途径的研究比较透彻,wingless(Wnt-1) 对于果蝇翅的发育起着重要作用 (Sharma et al., 1976),其作为体节极性基因也参与一系列重要生命过程,包括体节分化 (Bolognesi et al., 2008; Fu et al., 2012; Petersen et al., 2009)、体轴发育 (Hikasa et al., 2013)、表皮的形成 (Sahai-Hernandez et al., 2012)、脑的发育 (Kobayashi et al., 2007) 和长期记忆的形成 (Tan et al., 2013) 等。在赤拟谷盗 (Tribolium castaneum) 中,Wnt signaling对于胚胎期足的发育,以及幼虫到成虫期的足与翅的重生、昆虫的变态等方面均发挥着重要功能 (Ober et al., 2006; Shah et al., 2011)。在家蚕中,Wnt-1在腹节发育和色斑形成中发挥作用 (Zhang et al., 2015)。
本研究利用CRISPR/Cas9系统对Wnt-1基因进行了初步的功能研究,同时结合胚胎期Wnt-1基因转录水平和翻译水平表达模式初步推断美国白蛾胚胎发育为短胚带型和中胚带型。通过在胚胎期注射体外合成的Cas9 mRNA和Wnt-sgRNAs,检测Wnt-1基因敲除事件,统计美国白蛾的死亡率,观察对美国白蛾体节分化和附肢发育等的影响。本文首次利用CRISPR/Cas9基因组编辑技术对美国白蛾进行遗传操作,为林业害虫基因功能研究提供新的思路,同时Wnt-1基因可作为致死基因应用于美国白蛾未来的遗传防治。
1 材料与方法 1.1 昆虫饲养美国白蛾和人工饲料由中国林业科学研究院森林生态环境与保护研究所昆虫病原微生物学科组提供。在人工培养箱内饲养美国白蛾,饲养温度为 (25±1) ℃,光周期为16 h光照:8 h黑暗,相对湿度为75%。
1.2 RNA提取及cDNA合成收集产卵后4,8,12,16,18,20,24 h及2~8天的美国白蛾卵,液氮速冻后-80 ℃保存。Trizol (Invitrogen) 提取各样品的总RNA,经DNase I (Takara) 消化去除DNA后,以1 μg总RNA为模板,利用Protoscript M-MuLV First Strand cDNA Synthesis Kit (Thermo) 合成cDNA,-20 ℃保存备用。
1.3 基因克隆和蛋白质特征预测根据美国白蛾Wnt-1的转录组数据设计引物并对该基因CDS区域进行克隆,正向引物为Wnt-1-ORF-F:ATGATTGCGGCCATGTTGCGGAGC,反向引物为Wnt-1-ORF-R:CTATAAACACGTGTGCAC AAC。使用KOD-Plus (Toyobo) DNA聚合酶进行基因扩增,PCR扩增程序:94 ℃预变性3 min;94 ℃,15 s;58 ℃,15 s;68 ℃,1 min 30 s,35个循环;4 ℃保持10 min。将PCR产物连入pCR-Blunt进行测序。
利用NCBI在线网站预测该基因的开放阅读框 (http://www.ncbi.nlm.nih.gov/ gorf/gorf.html),利用ExPASy在线分析软件的Compute pI/Mw tool功能预测蛋白特点 (http://web.expasy.org/compute_pi/)。并利用在线网站预测蛋白结构 (https://www. predictprotein.org/home,http://meme-suite.org/tools/meme和http://www.ncbi.nlm. nih.gov/ Structure/cdd/wrpsb.cgi)。
1.4 实时荧光定量PCR各个时期反转录的cDNA产物作为Real-time PCR检测的模板,利用SYBR Green Realtime PCR Master Mix (Toyobo) 进行扩增,正向引物:ATGGTA TGTCTGGCTCGT,反向引物:CTGGTGATTTATGG TCTGG。核糖体蛋白基因rp32作为内参,正向引物:GCCCAGCATTGGTTATGGA,反向引物CGCTTC TTTGATGAGACACCG。反应总体系20 μL:10 μL SYBR mix,8 μL RNA-free water,0.5 μL F/R引物,1 μL DNA。反应程序为:95 ℃,10 s;然后95 ℃,15 s;60 ℃,30 s,40个循环,最后加溶解曲线。
1.5 WNT-1的免疫组化在家蚕免疫组化方法基础上进行改进 (Sweeney et al., 2012)。收集1~8天的美国白蛾卵,用固定液A (1:1 99%正庚烷heptan和10.8%甲醛formaldehyde) 固定,4 ℃保存备用。1.3%的次氯酸钠溶液处理收集的卵5~10 min,去除卵壳,PBS洗去多余的次氯酸钠;permeabilizing buffer (1×PBS,4%多聚甲醛paraformaldehyde,0.1% Triton X-100,0.1%脱氧胆酸盐deoxycholate) 固定30 min,室温下PBT洗3次;加入1×PBS,0.1% Triton X-100,0.5%牛血清蛋白bovine serum albumin,5%普通血清normal serum和1 mmol·L-1叠氮化钠sodium azide) 室温封闭1 h后加入一抗 (家蚕WNT-1抗体1:1 000),不加抗体作为对照,4 ℃冰箱孵育48 h;PBT (PBS+0.1%Triton X-100) 清洗12 h,加入封闭液室温孵育1 h后加入异硫氰酸荧光素 (FITC) 直接染色,室温孵育2 h;PBT洗3 h,olympus IX71S8F-3荧光显微镜观察并拍照。
1.6 胚胎期显微注射使用显微注射平台对美国白蛾卵进行注射。将产卵2 h内的卵竖排排列到载玻片上,每列约50粒卵,卵孔朝上 (卵呈球形,卵孔位于胚胎正中间),用少量胶水固定。将注射体系装入显微注射仪的毛细玻璃管,用气泵直接注射,注射2次,注射后用胶水将注射孔封住,后用甲醛消毒,之后将载玻片放入培养皿,25 ℃黑暗培养,数天后解剖发育至黑头期的胚胎,并在显微镜下观察表型,拍照记录。
1.7 sgRNA和Cas9 mRNA的体外转录通过引物Wnt-1-sgRNA1-F1:TAATACGACTCA CTATAGGAACGACGCGGTACCGCACGTTTTAGAGCT AGAAATAGCAAGTTAA,Wnt-1-sgRNA1-F2:TAATAC GACTCACTATAGGTGCAACTGTACGTTTCACGTTTTA GAGCTAGAAATAGCAAGTTAA,分别与R-AAAAGC ACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGA CTAGCCTTATTTTAACTTGCTATT进行PCR。对照组EGFP-sgRNA 1:TAATACGACTCACTATAGGGCG AGGAGCTGTTCACCGGTTTTAGAGCTAGAAATAGCA AGTTAAAA,EGFP-sgRNA2:TAATACGACTCACTAT AGGCCACAAGTTCAGCGTGTCGTTTTAGAGCTAGAA ATAGCA AGTTAAAA同时与上述R引物进行PCR,将PCR产物连入pCR-Blunt载体,通过F-500:TTTGAGTGAGCTGATACCGCTCGC和sgRNA-R20:AAAAGCACCGACTCGGTGCC进行PCR验证,以反向插入无碱基错配质粒为模板,利用同样引物对sgRNAs序列进行扩增,将纯化后的PCR产物作为合成sgRNAs的原始模板,使用MEGAscript® T7 kit (Ambion) 试剂盒体外转录sgRNAs。Cas9质粒PTD1-Cas9由上海植生所昆虫分子实验室黄勇平课题组提供,使用mMESSAGE mMACHINETM T7 kit (Ambion) 进行体外转录,合成Cas9 mRNA (Wang et al., 2013)。
1.8 基因敲除检测为检验Cas9/sgRNA对Wnt-1基因的切割效率,收集注射后第8天G0代未发育的卵以及突变体样品,利用DNA提取试剂盒 (康为) 对收集的样品抽提基因组DNA,PCR检测和鉴定。扩增引物在靶点两侧设计,引物F:CAAACCGAGATGCGGCAG GAGTGCAA和R:GCATAGTTTGCACTTAACTTCG CAGC扩增目的序列 (图 1),PCR产物连到pCR-Blunt载体上,阳性克隆测序鉴定。将测序结果和基因组序列进行比对,观察是否有突变,找出突变碱基和突变类型。
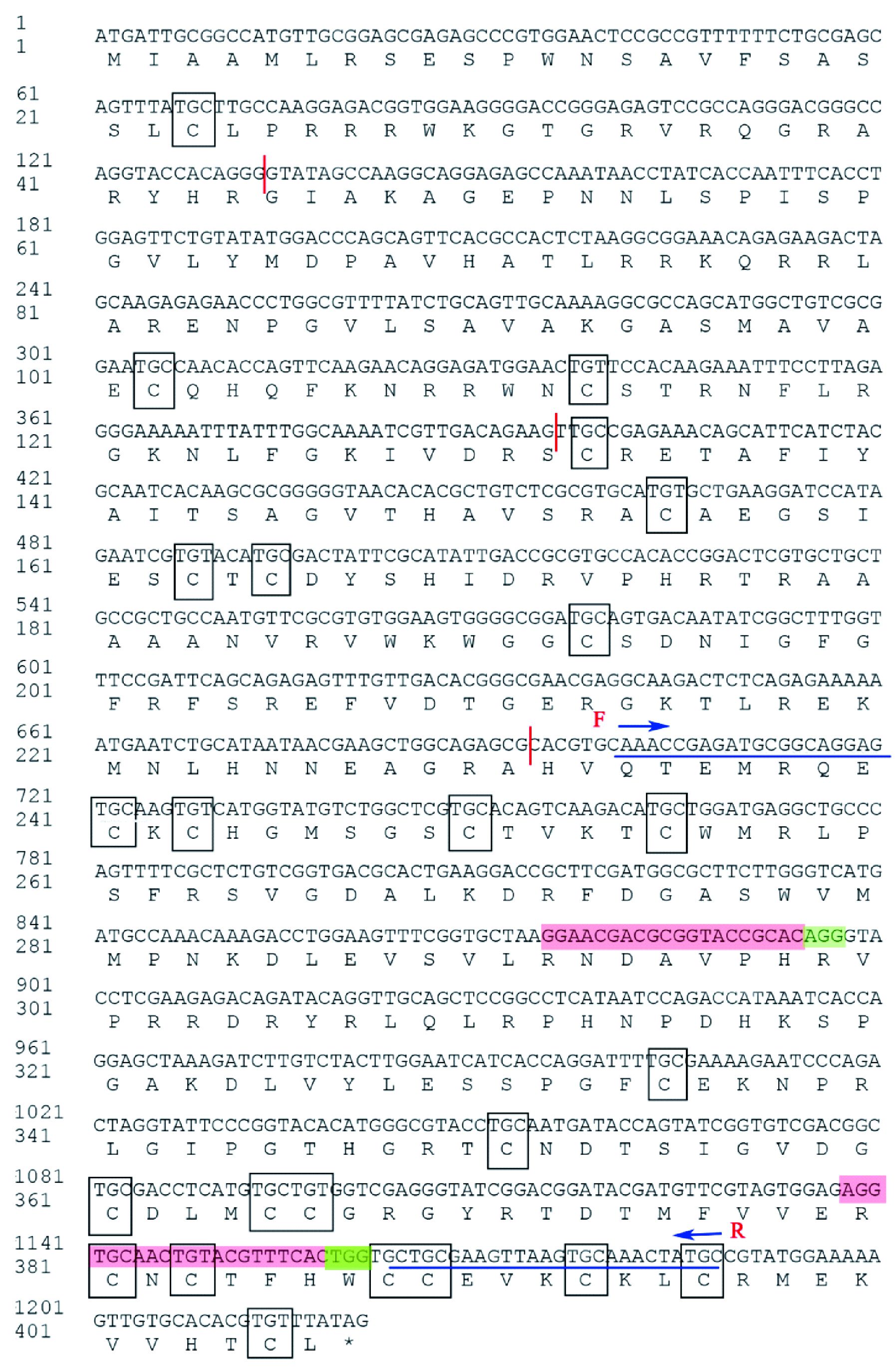
|
图 1 美国白蛾HcWnt-1基因核苷酸和氨基酸序列分析 Fig.1 Nucleic acid and deduced amino acid sequences analysis of the HcWnt-1 gene of H. cunea 星号表示终止密码子Asterisk indicates the stop codon; 方框表示半胱氨酸残基Boxed sequences show the conserved cysteine residues; 红色区域表示sgRNA的靶点, 绿色区域表示PAM结构Sequences labeled in red color represent sgRNAs targets and PAM region labeled in green color; 蓝色下划线表示靶点序列的检测引物F和R Sequences underlined are the test primers F (Forward primer) and R (Reverse primer). |
为了获得美国白蛾Wnt-1基因信息,利用NCBI已公布的美国白蛾Wingless(Genbank登陆号EU333645.1) 部分序列作为参考基因与美国白蛾转录组和基因组数据进行本地blast比对,获得接近完全匹配的基因即Wnt-1,命名为HcWnt-1。以美国白蛾蛹cDNA为模板,通过PCR直接扩增获得Wnt-1基因ORF序列,测序分析结果表明该基因核苷酸序列大小为1 221 bp,将其ORF序列与美国白蛾基因组数据进行比对,发现该基因含有4个内含子,编码407个氨基酸,预测蛋白大小为45.6 kDa,等电点为9.69。HcWNT-1蛋白结构分析表明,该基因含有Wnt家族保守结构域 (217~1 218 bp),含有螺旋-转角-螺旋DNA结合基序 (homeodomain,HD),并且高度保守的Motif分散分布于整个序列;具有Wnt家族典型特征——富含23个以上的半胱氨酸残基,HcWNT-1蛋白含24个保守的半胱氨酸残基 (图 1),结构预测结果表明这些半胱氨酸之间可以形成二硫键,进一步形成短的发夹结构,可能与蛋白活性相关。
2.2 胚胎期Wnt-1基因及其蛋白的表达谱为了解胚胎期Wnt-1的表达模式,对不同时期的胚胎进行取样,并进行相对定量分析。结果表明,Wnt-1在胚胎早期表达量迅速提高,24 h表达水平达到峰值,随后逐渐下降,144 h出现另一个峰值,随后又迅速下降 (图 2)。

|
图 2 HcWnt-1在美国白蛾胚胎不同发育时期的表达模式 Fig.2 Expression of HcWnt-1 during embryonic development in H. cunea |
利用家蚕WNT-1蛋白多克隆抗体分别对美国白蛾24~192 h胚胎进行免疫染色,观察HcWNT-1蛋白胚胎期表达模式。对照不能观察到胚胎,说明异硫氰酸荧光素 (FITC) 对胚胎染色无影响 (图 3H)。免疫组化结果表明,24 h时,美国白蛾已形成早期胚带,HcWNT-1蛋白主要集中于前端区域即原头区 (图 3I);随着胚胎的发育,HcWNT-1蛋白表达沿着前后体轴由头部向尾部逐渐延伸,此时体节从前端往腹部逐渐增加,且体节分化不明显;48 h,HcWNT-1蛋白主要集中于尾部的端部表达,除端部外其他部位可观察到微弱表达 (图 3J);随着胚胎的分化,头和胸部体节的附肢原基出现,而腹部尚未出现附肢原基,72 h为原足期。HcWNT-1蛋白沿着附肢近远端轴向端部延伸,同时在尾部高表达 (图 3K);随着附肢的分化,头、胸和腹部的附肢原基增长,体节分化完成,为多足期;96 h,HcWNT-1蛋白主要集中在附肢端部表达 (图 3L);144 h HcWNT-1蛋白表达主要集中附肢和体节处表达,该时期附肢发育快速完成,身体开始反转 (图 3M);192 h,胚胎发育完成,形成成熟的头、胸和腹节,而HcWNT-1蛋白表达也消失殆尽 (图 3N)。
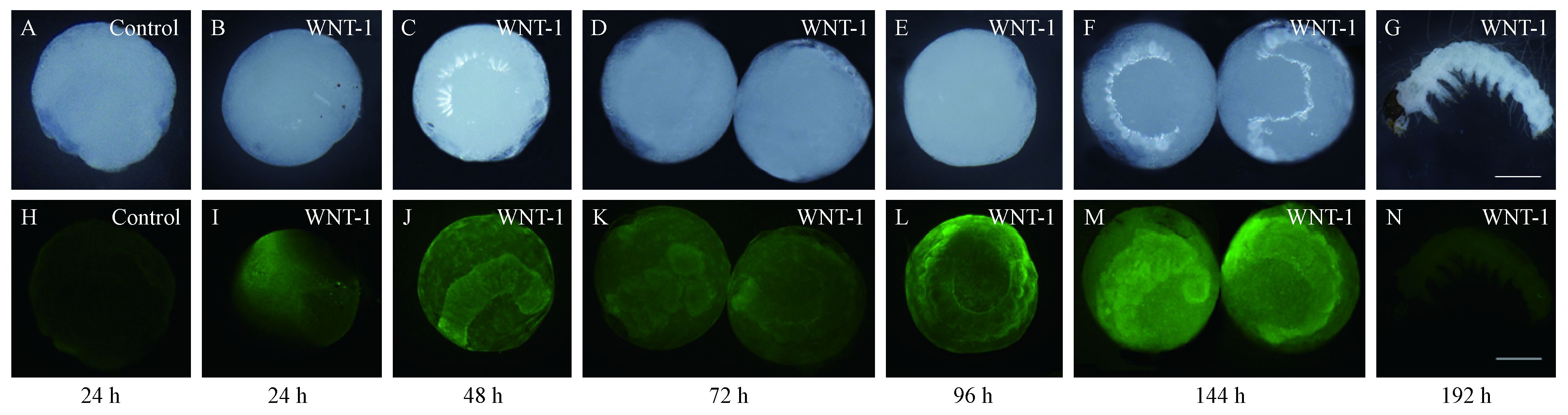
|
图 3 免疫组化验证美国白蛾HcWNT-1蛋白胚胎时期表达模式 Fig.3 Expression pattern of HcWNT-1 at early embryonic stages as revealed by immunohistochemistry A-G:白光White light; H-N:绿光Green light; A, H:对照Control, B-G, I-N:胚胎发育不同时间点WNT-1抗体免疫染色WNT-1 immunostaining in embryos at different times after fertilization showing FITC fluorescence in embryos. |
为检验CRISPR/Cas9系统对美国白蛾的基因编辑效率,胚胎期直接注射HcWnt-1-sgRNAs和Cas9 mRNA。当注射300 ng·μL-1 EGFP sgRNA/Cas9 mRNA和水作为对照,1 000颗卵的孵化率均达85%以上,无表型变化。注射300 ng·μL-1 HcWnt-1-sgRNAs和300 ng·μL-1 Cas9 mRNA,1 000颗卵的胚胎死亡率高达99.8%,大部分个体不能发育至黑头期,解剖结果显示未发育至黑头期的个体不能完成胚胎发育,个别能够发育至黑头期的胚胎出现体节缺失 (5头)、足缺失 (6头)、头部畸形 (3头) 等表型 (图 4,表 1),这表明CRISPR/Cas9系统可以对美国白蛾进行高效基因组编辑,同时也表明HcWnt-1基因在美国白蛾胚胎发育阶段,尤其在早期胚胎发育和分化中起着决定性作用。

|
图 4 Wnt-1-sgRNAs/Cas9 mRNA诱导美国白蛾Wnt-1突变的胚胎期表型 Fig.4 Embryonic phenotypes in H.cunea resulting from Wnt-1-sgRNAs/Cas9 mRNA injection A:注射EGFP sgRNAs和Cas9 mRNA Control injected with EGFP-sgRNAs/Cas9 mRNA; B-D:胚胎期突变体表型Severely affected embryo resulting from Wnt-1-sgRNA/Cas9 mRNA injection (B:足缺失, 胸节聚合Compacted thoracic segments with thoracic legs missing; C:头畸形, 第8-12腹节缺失Deformed head, missing A8-A12 abdominal segments;D:头畸形, 胸足缺失Malformed head, missing thoracic legs). |
|
|
为检验Wnt-1基因敲除效率,对对照 (EGFP),(图 5B 1-2)、美国白蛾40头未发育胚胎 (第8天)(图 5B 3-6) 和突变体胚胎 (图 5B 7-10) 进行PCR检测,选择菌落PCR阳性克隆直接测序,每组送样2个。结果表明8组克隆中有5组检测到突变,突变率为62.5%。Wnt-1基因敲除会造成该基因片段删除,最大删除片断为423 bp,除了主带以外可以同时观察到其他短片段,并且出现条带弥散现象。将PCR产物连入T载体进行测序,发现Wnt-1基因在2个靶点处均存在片段删除,证明该基因被成功敲除 (图 5C,D)。
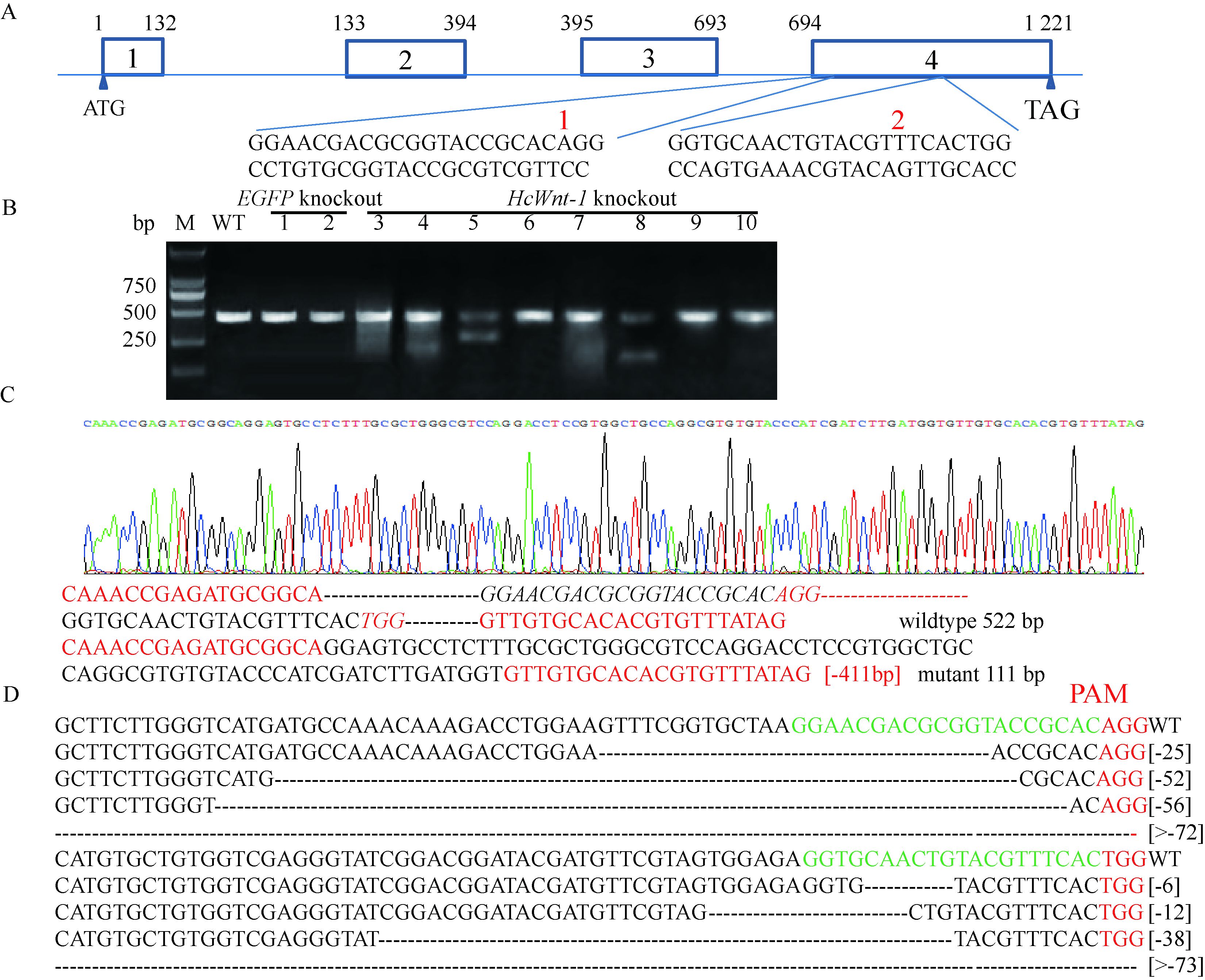
|
图 5 HcWnt-1靶点设计图及突变检测 Fig.5 Schematic diagram of HcWnt-1 sgRNA targets and mutation events A:在HcWnt-1第4个外显子处设计2个靶点Schematic representation of Wnt-1 sgRNAs targeting sites; B:PCR产物电泳图Representative electrophoretogram of PCR products sequencing (1, 2:对照Control, 注射EGFP sgRNA/Cas9 mRNA Injection of EGFP sgRNA/Cas9 mRNA; 4-10: HcWnt-1突变体HcWnt-1 mutants); C: Cas9诱导的嵌合体色谱图Representative chromatograms of chimera individual induced by sgRNA/Cas9; D: HcWnt-1 G0代胚胎突变体突变类型Various deletion genotypes in G0 injected embryos. |
昆虫翅是昆虫分类学上一个重要进化特征,昆虫翅的进化有助于其扩散,并且极大地提高了昆虫的生存和繁衍的能力,在迁飞昆虫的研究中尤其重要,同时翅斑的进化与昆虫生物学行为相关,对于昆虫翅的研究一直以来是科学家们关注的焦点 (Allen et al., 2011;Derks et al., 2015;Lin et al., 2016;Nishikawa et al., 2015;liver et al., 2011)。美国白蛾具有强大的飞行能力,Wnt-1基因的研究可以拓展人们对于Wnt-1信号途径的认识,同时对美国白蛾飞行机制也有更深入了解。本试验在美国白蛾胚胎期直接注射Cas9 mRNA与sgRNA靶向敲除Wnt-1,测序检验发现突变体的Wnt-1基因存在定点突变,说明CRISPR/Cas9系统可对美国白蛾进行高效基因组编辑。同样,CRISPR/Cas9体系在其他鳞翅目昆虫中也可以进行基因的高效编辑,如家蚕、柑橘凤蝶和金凤蝶、斜纹夜蛾、小菜蛾和马尾松毛虫,G0代突变率均高达90%(Li et al., 2015a; Wang et al., 2013;Bi et al., 2016;Huang et al., 2016;Liu et al., 2017)。因此,CRISPR/Cas9体系可以普遍适用于非模式物种特定基因的基因组编辑。敲除Wnt-1导致美国白蛾胚胎期致死,致死率高达99.8%,该结果与家蚕和马尾松毛虫Wnt-1基因功能缺失的表型一致 (Zhang et al., 2015; Liu et al., 2017),并且在这2个物种中验证该基因导致的胚胎期致死属于剂量依赖型的胚胎死亡,这些结果说明在鳞翅目中Wnt-1基因功能保守,并且在胚胎的发育和分化过程中发挥重要作用。由于突变体不能发育至成虫阶段,Wnt-1基因参与美国白蛾翅形成机制以及其参与美国白蛾其他生命活动的分子机制尚不清楚,需要对该信号途径及机制进行进一步的探索,该通路的研究将为美国白蛾致死品系的获得奠定基础,并为美国白蛾遗传防治提供新的思路。
HcWnt-1基因在美国白蛾胚胎发育过程中扮演着重要的角色。RT-PCR和免疫组化可以直观反映美国白蛾的Wnt-1基因和WNT-1蛋白时空表达模式,有利于揭示美国白蛾的胚胎发育类型。RT-PCR结果表明HcWnt-1基因在美国白蛾胚胎早期大量表达,24 h达到第1个峰值,免疫组化结果表明HcWNT-1蛋白在整个胚带,特别是在原头区高表达,说明Wnt-1基因在早期胚带形成,尤其在胚带前端发育发挥关键作用;随后HcWnt-1 mRNA表达量水平逐渐降低,而HcWNT-1蛋白的表达沿着前后体轴逐渐向尾部延伸,并且在尾部高表达,说明HcWnt-1对美国白蛾体节形成起着关键作用;随着附肢和体节的形成,HcWNT-1蛋白沿着附肢的近远端轴逐渐向端部延伸,mRNA表达水平该时期出现第2个峰值,说明HcWnt-1在附肢的发育进程中发挥重要功能;随后胚胎形成逐渐成熟,mRNA表达水平和WNT-1蛋白表达水平降低。美国白蛾HcWnt-1基因表达模式与短胚带型和中间胚胎型昆虫类似。在短胚带型模式赤拟谷盗中,胚盘阶段首次检测到Wnt-1(wg) 基因表达,随着胚盘从前往后延伸,在胚胎晚期阶段,可以在腹部检测到表达 (Nagy et al., 1994)。在中间胚胎型昆虫中,胚盘中部可以检测到Wnt-1/wg转录本,随着体节分化逐渐向腹后延伸 (Nakao, 2010)。初步推断美国白蛾胚胎发育类型符合短胚带型和中间胚带型特征。
Wnt-1基因在美国白蛾体节分割和附肢发育过程中发挥重要功能。Wnt-1基因敲除会导致多元的表型,如头部畸形、体节缺失、附肢缺失等,与RT-PCR结果和免疫组化结果一致,与马尾松毛虫和家蚕Wnt-1基因敲除表型一致,推测Wnt-1基因在鳞翅目昆虫中参与的信号通路途径相似 (Zhang et al., 2015;Liu et al., 2017)。Wnt-1基因敲除会导致头部畸形的突变体表型,在昆虫和动物中也可见Wnt-1参与头部形成过程的报道,比如眼的发育,端脑和间脑发育等 (Rossi et al., 2007; Fridrich et al., 2003; Bally-Cuif et al., 1995; Lekven et al., 2003)。在果蝇中,时序性调节Wingless(Wnt-1) signalling对于触角和上颌骨分化起决定性作用 (Lebreton et al., 2008)。在赤拟谷盗中,Wnt/β-catenin signalling对于头部发育发挥重要功能 (Benton et al., 2013; Bolognesi et al., 2008; Fu et al., 2012),说明Wnt-1基因在美国白蛾头部分化中发挥重要功能。Wnt-1基因敲除同样会影响美国白蛾腹部体节的形成,该表型与家蚕、果蝇和马尾松毛虫的结果一致 (Zhang et al., 2015;Larsen et al., 2003;Liu et al., 2017);然而,在其他昆虫如双蟋蟀 (Gryllus bimaculatus)、乳草长蝽 (Oncopeltus fasciatus) 和赤拟谷盗中,敲除Wnt-1并不会造成体节数量的减少,但是敲除Wnt信号通路其他基因,如双蟋蟀中敲除Arm,乳草长蝽中敲除Pan,赤拟谷盗中敲除Wnt-8均会造成体节缺失和体节转换 (Angelini et al., 2005; Miyawaki et al., 2004; Bolognesi et al., 2008)。同样,Wnt-1在动物附肢进化的多元化上至关重要,Wnt-1敲除会导致美国白蛾胸足和腹足缺失,该基因在全变态类昆虫中发挥相似功能,如Wnt-1参与鞘翅目 (Coleoptera),鳞翅目 (Lepidoptera),膜翅目 (Hymynoptera) 和双翅目 (Diptera) 的胚胎后期附肢发育 (Siegfried et al., 1994; Sato et al., 2008; Shah et al., 2011; Zhang et al., 2015)。然而在一些不完全变态昆虫中,Wnt-1并不会影响昆虫附肢发育,如双蟋蟀 (Angelini et al., 2005) 和美洲大蠊 (Periplaneta americana) (Chesebro et al., 2013)。美国白蛾的Wnt-1基因由407个氨基酸组成,具有Wnt信号通路家族的典型特征——富含24个半胱氨酸残基,与已报道各物种WNT-1功能肽序列特征一致,笔者推测Wnt信号通路在昆虫中相对保守,但在功能上存在差异,这些结果支持上述猜测,即Wnt signalling决定美国白蛾等后生动物的分节机制。
4 结论美国白蛾符合短胚带型和中间胚带型的胚胎发育类型,其中HcWnt-1基因在其胚胎发育过程中发挥重要功能,为美国白蛾未来遗传防治提供候选基因。同时CRISPR/Cas9体系成功在美国白蛾中实现,为该害虫功能基因研究奠定基础,也为其他林业害虫的功能基因研究提供重要的参考依据。
| [] |
季荣, 谢宝瑜, 李欣海, 等. 2003. 外来入侵种——美国白蛾的研究进展. 昆虫知识, 40(1): 13–18.
( Ji R, Xie B Y, Li X H, et al. 2003. Research progress on the invasive species, Hyphantria cunea. Eentomological Knowledge, 40(1): 13–18. DOI:10.7679/j.issn.2095-1353.2003.004 [in Chinese] ) |
| [] |
刘俊, 叶利芹, 成聪, 等. 2016. 江苏省2015年林业有害生物发生情况及2016年发生趋势预测. 金陵科技学院学报, 32(1): 64–67.
( Liu J, Ye L Q, Cheng C, et al. 2016. The occurrence of forestry pests in 2015 and its occurrence trend prediction in 2016 in Jiangsu Province. Journal of Jinling Institute of Technology, 32(1): 64–67. [in Chinese] ) |
| [] |
夏剑萍, 梅爱华, 陈亮, 等. 2015. 美国白蛾入侵湖北省的危险性分析. 湖北林业科技, 44(1): 28–40.
( Xia J P, Mei A H, Chen L, et al. 2015. Risk analysis of Hyphantria cunea invasion in Hubei Province. Hubei Forestry Science and Technology, 44(1): 28–40. [in Chinese] ) |
| [] |
杨忠岐, 张永安. 2007. 重大外来入侵害虫——美国白蛾生物防治技术研究. 昆虫知识, 44(4): 465–471.
( Yang Z Q, Zhang Y A. 2007. Researches on techniques for biocontrol of the fall webworm, Hyphantria cunea, a severe invasive insect pest to China. Chinese Bulletin of Entomology, 44(4): 465–471. DOI:10.7679/j.issn.2095-1353.2007.107 [in Chinese] ) |
| [] |
张俊杰, 董琴, 赵涵博, 等. 2013. 中国大陆美国白蛾的侵入分布、危害与防治概述. 吉林林业科技, 42(3): 27–47.
( Zhang J J, Dong Q, Zhao H B, et al. 2013. A summary of distribution, harm and control measures of Hyphantria cunea in mainland China. Journal of Jilin Forestry Science and Technology, 42(3): 27–47. [in Chinese] ) |
| [] |
赵铁珍, 高岚, 柯水发, 等. 2007. 美国白蛾入侵部分非经济损失评估案例分析. 北京林业大学学报, 29(3): 171–177.
( Zhao T Z, Gao L, Ke S F, et al. 2007. Case study on the evaluation of non-economic loss after Hyphantria cunea's invading China. Journal of Beijing Forestry University, 29(3): 171–177. [in Chinese] ) |
| [] | Allen C E, Zwaan B J, Brakefield P M. 2011. Evolution of sexual dimorphism in the Lepidoptera. Annu Rev Entomol, 56: 445–464. DOI:10.1146/annurev-ento-120709-144828 |
| [] | Alphey L, Benedict M, Bellini R, et al. 2010. Sterile-insect methods for control of mosquito-borne diseases:an analysis. Vector Borne Zoonotic Dis, 10(3): 295–311. DOI:10.1089/vbz.2009.0014 |
| [] | Angelini D R, Kaufman T C. 2005. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev Biol, 283(2): 409–423. DOI:10.1016/j.ydbio.2005.04.034 |
| [] | Ant T, Koukidou M, Rempoulakis P. 2012. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol, 10(51): 1–8. |
| [] | Bally-Cuif L, Cholley B, Wassef M. 1995. Involvement of WNT-1 in the formation of the mes/metencephalic boundary. Mechanisms of Development, 53(1): 23–34. DOI:10.1016/0925-4773(95)00421-1 |
| [] | Benton M A, Akam M, Pavlopoulos A. 2013. Cell and tissue dynamics during Tribolium embryogenesis revealed by versatile fluorescence labeling approaches. Development, 140(15): 3210–3220. DOI:10.1242/dev.096271 |
| [] | Bi H L, Xu J, Tan A J, et al. 2016. CRISPR/Cas9-mediated targeted gene mutagenesis in Spodoptera litura. Insect Sci, 23(3): 469–77. DOI:10.1111/ins.2016.23.issue-3 |
| [] | Bolognesi R, Farzana L, Fischer T D. 2008. Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol, 18(20): 1624–1629. DOI:10.1016/j.cub.2008.09.057 |
| [] | Brisson J A. 2010. Aphid wing dimorphisms:linking environmental and genetic control of trait variation. Philos Trans R Soc Lond B Biol Sci, 365(1540): 605–616. DOI:10.1098/rstb.2009.0255 |
| [] | Chesebro J E, Pueyo J I, Couso J P. 2013. Interplay between a Wnt-dependent organiser and the Notch segmentation clock regulates posterior development in Periplaneta americana. Biol Open, 2(2): 227–237. DOI:10.1242/bio.20123699 |
| [] | Derks M F, Smit S, Salis L, et al. 2015. The genome of Winter Moth (Operophtera brumata) provides a genomic perspective on sexual dimorphism and phenology. Genome Biol Evol, 7(8): 2321–2332. DOI:10.1093/gbe/evv145 |
| [] | Fridrich M. 2003. Evolution of insect eye development first insights from fruit fly, grasshopper and flour beetle. Integr Comp Biol, 43(4): 508–521. DOI:10.1093/icb/43.4.508 |
| [] | Fu G, Condon K C, Epton M J. 2007. Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol, 25(3): 353–357. DOI:10.1038/nbt1283 |
| [] | Fu J, Posnien N, Bolognesi R. 2012. Asymmetrically expressed axin required for anterior development in Tribolium. PNAS, 109(20): 7782–7786. DOI:10.1073/pnas.1116641109 |
| [] | Hikasa H, Sokol S Y. 2013. Wnt signaling in vertebrate axis specification. Cold Spring Harb Perspect Biol, 5(1): a007955. DOI:10.1101/cshperspect.a007955 |
| [] | Hsu P D, Lander E S, Zhang F. 2014. Development and applications of CRISPR-Cas9 for genome engineering. Cell, 157(6): 1262–1278. DOI:10.1016/j.cell.2014.05.010 |
| [] | Huang Y P, Chen Y, Zeng B, et al. 2016. CRISPR/Cas9 mediated knockout of the abdominal-A homeotic gene in the global pest, diamondback moth (Plutella xylostella). Insect Biochem Mol Biol, 75: 98–106. DOI:10.1016/j.ibmb.2016.06.004 |
| [] | Ian M Slaymaker L G, Bernd Z. 2016. Rationally engineered Cas9 nucleases with improved specificity. Science, 351(6268): 84–88. DOI:10.1126/science.aad5227 |
| [] | Jin L, Walker A S, Fu G, et al. 2013. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synth Biol, 2(3): 160–166. DOI:10.1021/sb300123m |
| [] | Kobayashi C, Saito Y, Ogawa K. 2007. Wnt signaling is required for antero-posterior patterning of the planarian brain. Dev Biol, 306(2): 714–724. DOI:10.1016/j.ydbio.2007.04.010 |
| [] | Larsen C W, Hirst E, Alexandre C. 2003. Segment boundary formation in Drosophila embryos. Development, 130(23): 5625–5635. DOI:10.1242/dev.00867 |
| [] | Lebreton G, Faucher C, Cribbs D L. 2008. Timing of Wingless signalling distinguishes maxillary and antennal identities in Drosophila melanogaster. Development, 135(13): 2301–2309. DOI:10.1242/dev.017053 |
| [] | Lekven A C, Buckles G R, Kostakis N. 2003. Wnt1 and Wnt10b function redundantly at the zebrafish midbrain-hindbrain boundary. Dev Biol, 254(2): 172–187. DOI:10.1016/S0012-1606(02)00044-1 |
| [] | Li X, Fan D, Zhang W. 2015. Outbred genome sequencing and CRISPR/Cas9 gene editing in butterflies. Nat Commun, 6(8212): 1–10. |
| [] | Lin X, Xu Y, Yao Y, et al. 2016. JNK signaling mediates wing form polymorphism in brown planthoppers (Nilaparvata lugens). Insect Biochem Mol Biol, 73: 55–61. DOI:10.1016/j.ibmb.2016.04.005 |
| [] | Liu H, Liu Q, Zhou X, et al. 2017. Genome editing of Wnt-1, a gene associated with segmentation, via CRISPR/Cas9 in the Pine Caterpillar Moth, Dendrolimus punctatus. Front Physiol, 7(666): 1–7. DOI:10.3389/fphys.2016.00666 |
| [] | Martins S, Naish N, Walker A, et al. 2012. Germline transformation of the diamondback moth, Plutella xylostella L., using the piggyBac transposable element. Insect Mol Biol, 21(4): 414–421. DOI:10.1111/imb.2012.21.issue-4 |
| [] | Miyawaki K, Mito T, Sarashina I. 2004. Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev, 121(2): 119–130. DOI:10.1016/j.mod.2004.01.002 |
| [] | Nagy L M, Carroll S. 1994. Conservation of wingless patterning functions in the short-germ embryos of Tribolium castaneum. Nature, 367(3): 460–463. |
| [] | Nakao H. 2010. Characterization of Bombyx embryo segmentation process:expression profiles of engrailed, even-skipped, caudal, and Wnt1/wingless homologues. J Exp Zool B Mol Dev Evol, 314(3): 224–231. |
| [] | Nishikawa H, Iijima T, Kajitani R, et al. 2015. A genetic mechanism for female-limited Batesian mimicry in Papilio butterfly. Nat Genet, 47(4): 405–409. DOI:10.1038/ng.3241 |
| [] | Ober K A, Jockusch E L. 2006. The roles of wingless and decapentaplegic in axis and appendage development in the red flour beetle, Tribolium castaneum. Dev Biol, 294(2): 391–405. DOI:10.1016/j.ydbio.2006.02.053 |
| [] | Oliver J C, Monteiro A. 2011. On the origins of sexual dimorphism in butterflies. Proc Biol Sci, 278(1714): 1981–1988. DOI:10.1098/rspb.2010.2220 |
| [] | Petersen C P, Reddien P W. 2009. Wnt signaling and the polarity of the primary body axis. Cell, 139(6): 1056–1068. DOI:10.1016/j.cell.2009.11.035 |
| [] | Rogers B T, Kaufman T C. 1996. Structure of the insect head as revealed by the EN protein pattern in developing embryos. Development, 122(11): 3419–3432. |
| [] | Rossi E, Siwiec F, Yan C Y. 2007. Pattern of Wnt ligand expression during chick eye development. Braz J Med Biol Res, 40(10): 1333–1338. DOI:10.1590/S0100-879X2006005000155 |
| [] | Sahai-Hernandez P, Castanieto A, Nystul T G. 2012. Drosophila models of epithelial stem cells and their niches. Wiley Interdiscip Rev Dev Biol, 1(3): 447–457. DOI:10.1002/wdev.v1.3 |
| [] | Sato K, Matsunaga T M, Futahashi R. 2008. Positional cloning of a Bombyx wingless locus flugellos (fl) reveals a crucial role for fringe that is specific for wing morphogenesis. Genetics, 179(2): 875–885. DOI:10.1534/genetics.107.082784 |
| [] | Shah M, Namigai E K, Suzuki Y. 2011. The role of canonical Wnt signaling in leg regeneration and metamorphosis in the red flour beetle Tribolium castaneum. Mech Dev, 128(7-10): 342–358. DOI:10.1016/j.mod.2011.07.001 |
| [] | Sharma R P, Chopra V L. 1976. Effect of the Wingless (wg1) mutation on wing and haltere development in Drosophila melanogaster. Dev Biol, 48(2): 461–465. DOI:10.1016/0012-1606(76)90108-1 |
| [] | Siegfried E, Wilder E L, Perrimon N. 1994. Components of wingless signaling in Drosophila. Nature, 367(6458): 76–80. DOI:10.1038/367076a0 |
| [] | Sweeney S T, Hidalgo A, de Belle J S, et al. 2012. Antibody staining of the central nervous system in adult Drosophila. Cold Spring Harb Protoc(2): 235–238. |
| [] | Tan Y, Yu D, Busto G U. 2013. Wnt signaling is required for long-term memory formation. Cell Reports, 4(6): 1082–1089. DOI:10.1016/j.celrep.2013.08.007 |
| [] | Thomas D D, Donnelly C A, Wood R J, et al. 2000. Insect population control using adominant, repressible, lethal genetic system. Science, 287(5462): 2474–2476. DOI:10.1126/science.287.5462.2474 |
| [] | Wang Y, Li Z, Xu J. 2013. The CRISPR/Cas system mediates efficient genome engineering in Bombyx mori. Cell Res, 23(12): 1414–1416. DOI:10.1038/cr.2013.146 |
| [] | Wang J, Zhang H, Wang H, et al. 2016. Functional validation of cadherin as a receptor of Bt toxin Cry1Ac in Helicoverpa armigera utilizing the CRISPR/Cas9 system. Insect Biochem Mol Biol, 76: 11–17. DOI:10.1016/j.ibmb.2016.06.008 |
| [] | Zhang Z, Aslam A F, Liu X, et al. 2015. Functional analysis of Bombyx Wnt1 during embryogenesis using the CRISPR/Cas9 system. J Insect Physiol, 79: 73–79. DOI:10.1016/j.jinsphys.2015.06.004 |
 2017, Vol. 53
2017, Vol. 53

