文章信息
- 余建, 赵爱春, 刘长英, 梁燕梅, 朱攀攀, 蔡雨翔, 王茜龄, 李镇刚, 余茂德
- Yu Jian, Zhao Aichun, Liu Changying, Liang Yanmei, Zhu Panpan, Cai Yuxiang, Wang Xiling, Li Zhengang, Yu Maode
- 外源乙烯利与1-MCP处理对桑椹中乙烯和花青素相关代谢基因表达的影响
- Effects of Exogenous Ethylene and 1-MCP Treatments on the Expression of Genes Involved in Ethylene and Anthocyanin in Mulberry Fruit
- 林业科学, 2017, 53(2): 138-148.
- Scientia Silvae Sinicae, 2017, 53(2): 138-148.
- DOI: 10.11707/j.1001-7488.20170216
-
文章历史
- 收稿日期:2015-12-31
- 修回日期:2016-03-17
-
作者相关文章
2. 云南省农业科学院蚕桑蜜蜂研究所 蒙自 661101
2. Institute of Sericulture and Apiculture, Yunnan Academy of Agricultural Sciences Mengzi 661101
乙烯是一种重要的植物激素,已被证明作为一种信号分子参与调控果实的成熟衰老,也是果实成熟软化进程中重要的启动因子 (Ozga et al., 2003),参与调控果实成熟相关基因的转录和翻译,使果实呈现特有的成熟现象 (Wang et al., 2009)。大量研究证明,通过控制乙烯的释放可以改变果实的货架期。对不同成熟期果实施加外源乙烯可以调节其成熟进程,已在葡萄 (Vitis vinifera) (Blankenship et al., 2003)、葡萄柚 (Citrus paradisi) (Chaudhary et al., 2012; 2015) 等物种中得以证明。而添加乙烯抑制剂1-甲基环丙烯 (1-MCP),能抑制果蔬和切花后熟或衰老进程中乙烯诱导的相关生理、生化反应,明显延长贮藏寿命 (Blankenship et al., 2003; Bulens et al., 2014)。
乙烯调控果实成熟主要是通过其生物合成和信号转导来实现的。ACS(1-aminocyclopropane-1-carboxylate synthase) 和ACO(1-aminocyclopropane-1-carboxylate oxidase) 是乙烯生物合成的关键基因,改变这些基因的表达能够控制乙烯在果实中的含量,并改变果实的成熟期 (Cara et al., 2008; 刘乐等, 2009)。Oller等 (1991)将ACS反义基因导入番茄 (Lycopersicon esculentum),所得转基因番茄的乙烯合成量较对照果实降低了99%,成熟时间推迟了30~90天;Ayub等 (1996)将反义ACO基因转入甜瓜 (Cucumis melo) 中,也获得了与番茄类似的结果。
乙烯信号转导途径主要是通过ETR (ethylene receptors)、EIN2(ethylene insensitive 2)、EIN3/EIL (ethylene-insensitive 3, EIN3; EIN3-like, EIL) 和ERF (ethylene response factor) 四级元件完成。目前,对番茄、柑橘 (Citrus reticulata) 等果实乙烯受体基因研究发现,ETR各成员的表达模式以及对乙烯的响应模式存在差异 (Cara et al., 2008; Klee et al., 2002; Katz et al., 2004)。CTR1(constitutive triple response 1) 位于ETR的下游,是乙烯信号转导途径的第2个元件,CTR1基因被证实在组织的生长发育进程中与乙烯产生和果实成熟有关 (Adams-Phillips et al., 2004a; 2004b),能响应乙烯和1-MCP的处理 (El-Sharkawy et al., 2007; Dal et al., 2006)。EIN2位于细胞膜,EIN2基因沉默会延缓果实的发育和成熟,使果实成熟相关基因和乙烯相关基因的表达受到影响 (Hu et al., 2010)。EIL基因在果实成熟、衰老进程中表达水平基本稳定 (Yokotani et al., 2003),但香蕉 (Musa nana) 中MaEIL2的表达量在果实成熟进程中呈上升趋势,可被外源乙烯诱导 (Mbéguié-A-Mbéguié et al., 2008)。
花青素是一种类黄酮物质,为植物着色的主要物质之一,其含量在果实成熟衰老过程中逐渐增加,花青素生物合成相关酶基因的表达量也有一定程度的增强。大多数植物DFR (dihydroflavonol 4-reductase) 的表达与花青素的合成呈正相关 (Nakatsuka et al., 2008),且具有底物特异性;ANS (anthocyanidin synthase) 是植物花青素生物合成途径末端的关键酶,位于DFR下游,催化无色花色素到有色花色素的转变 (Jaakola, 2013)。大量研究表明,乙烯能促进某些果实着色和花青素的合成 (Kondo et al., 1997;Zhang et al., 2009)。
桑 (Morus alba) 是一种经济价值较高的木本植物,果实桑椹已被国家列入既是食品又是药品的保健品行列 (唐忠富, 2009)。有关乙烯调控植物生长发育和果实成熟的作用机制的研究已有诸多报道,但在桑树 (Morus) 中还比较缺乏。本课题组前期对乙烯 (Liu et al., 2014; 2015) 和花青素 (Li et al., 2014) 相关基因做了初步探索,在此基础上,本试验使用乙烯利和乙烯抑制剂处理不同发育时期的桑椹,分析乙烯利和1-MCP处理对桑椹总糖含量、花青素含量、花青素生物合成相关基因和乙烯相关基因表达量的影响,探究乙烯在桑椹发育进程中的作用和乙烯相关基因的表达模式,为今后有效开发桑椹的经济价值和通过分子生物学手段调控桑椹成熟期提供理论依据。
1 材料与方法 1.1 试验材料所用材料为桑品种‘嘉陵40号’(Morus alba ‘Jialing 40’) (重庆市审定果桑品种),栽培于西南大学桑树种质资源圃。根据桑椹的发育特征和颜色的变化,于2015年4月选取‘嘉陵40号’的4年生健壮枝条上盛花期 (即桑雌花柱头左右伸开,并发白发亮时为盛花期) 后21天 (21DAF) 和26天 (26DAF) 的桑椹用于相关试验处理,每个处理重复3个枝条。
1.2 乙烯利处理桑椹分别选取大部分桑椹处于21DAF和26DAF的健壮枝条,人工去除非该时期的桑椹,分别用100 mg·L-1乙烯利 (含0.1%吐温-20) 和ddH2O (含0.1%吐温-20) 喷洒桑椹表面,喷至有液滴为止。根据桑椹处理后颜色变化程度,21DAF的桑椹在处理后48,72,81 h取样,26DAF的桑椹处理后8,24,32 h取样。液氮速冻后于-80 ℃保存备用。
1.3 1-MCP处理采摘后桑椹分别选取处于21DAF和26DAF时期的健康桑椹,带柄采摘,装入500 mL的密闭瓶中,用1-MCP (0.5 μL·L-1,SmartFreshTM) 在室温下熏蒸12 h,置于22 ℃、相对湿度90%的光照恒温培养箱内 (Xiao et al., 2013),于处理后0,12,24 h取样。液氮速冻后于-80 ℃保存备用。
1.4 RNA的提取及cDNA第1链的合成按照北京全式金公司TransZolTM Plant RNA抽提试剂盒说明书提取总RNA。分别取适量体积RNA用1%的琼脂糖凝胶电泳检测,并用紫外分光光度计检测总RNA的浓度。以总RNA为模板,参照TAKARA公司反转录酶M-MLV说明书合成cDNA第1链,于-20 ℃保存备用。
1.5 桑椹花青素和总糖含量测定1) 桑椹花青素含量测定参考Jeong等 (2010)的方法。桑椹经低温冷冻干燥后,液氮研磨成粉末,称取0.5 g左右,加入10 mL预冷的酸性甲醇 (含1%HCl),4 ℃下避光提取12 h后,将提取液经4 000 r·min-1离心10 min。取5 mL上清,加入等体积水和氯仿,颠倒混匀,混合液经12 000 r·min-1离心1 min,吸取上层溶液测量波长530 nm处的吸光值。花青素含量 (A530·g-1)=A530×提取液体积×稀释倍数/样品质量。每个样品3次重复,并用SPSS软件进行显著性差异分析。
2) 桑椹总糖含量测定参考郑小坚等 (2002)的方法。用葡萄糖 (一水合物) 配制成0.2 mg·mL-1母液,再分别稀释成0,5,10,20,40,60,80,100 mg·L-1 8个浓度梯度,分别取1 mL与5 mL蒽酮试剂 (蒽酮:浓硫酸:ddH2O=0.6 g:100 mL:33 mL) 混合,于沸水中加热10 min,冷却后于紫外分光光度计上测定波长630 nm处的吸光值,绘制标准曲线。每个浓度设置3次重复。
准确称取桑椹干粉,加入5 mL 6 mol·L-1稀盐酸,消煮2 h,期间不时摇动,消煮完后立即加入一角勺 (约0.1 g) 活性炭,用于吸附色素,静置冷却后过滤,与5 mL蒽酮混合,沸水中加热10 min,冷却后测定波长630 nm处的吸光值。桑椹总糖含量 (mg·g-1)=标准曲线值×水解液总体积/样品质量。每个样品3次重复,并用SPSS软件进行显著性差异分析。
1.6 实时荧光定量PCR (qRT-PCR) 分析根据前期研究,选取乙烯生物合成基因MaACO2,MaACS3,信号转导相关基因MaETR1,MaETR2,MaCTR1,MaEIN2和MaEIL2 (Liu et al., 2014; 2015),花青素生物合成下游基因 (Li et al., 2014)MaDFR (KC521445) 和MaANS (JF499384),进行qRT-PCR分析。qRT-PCR引物序列如表 1,所用引物均在南京金斯瑞公司合成。使用定量PCR试剂为SYBR Premix Ex Taq Ⅱ (TaKaRa),反应体系为Premix Ex Taq Ⅱ(2×) 10 μL,10 μmol·L-1上、下游引物各0.8 μL,50×ROX Reference Dye (50×) 0.4 μL,cDNA 2 μL和无菌水6 μL。参照TaKaRa公司的SYBR Premix Ex Taq试剂盒操作说明书进行real-time PCR分析,热启动程序为95 ℃ 30 s,95 ℃ 5 s,60 ℃ 30 s,共40个循环,每个样品设3次重复取其平均值,并以MaACTIN3(李军等, 2011) (HQ163775.1) 作为内参。利用2-ΔΔCt法计算基因的相对表达量,并用SPSS进行显著性差异分析。
|
|
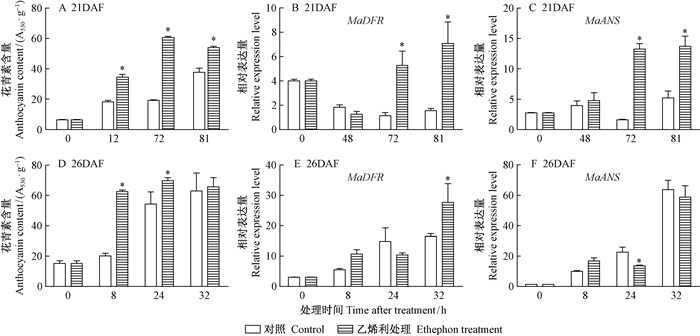
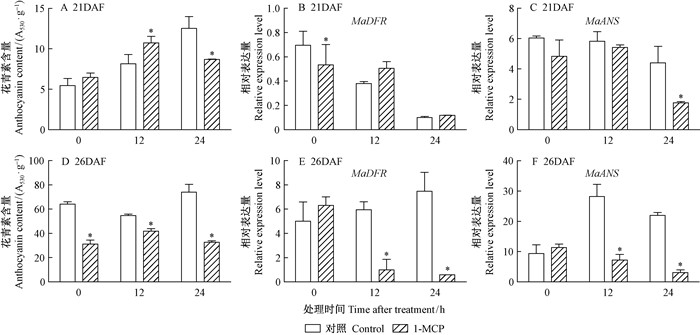
随着桑椹发育,桑椹颜色发生改变 (图 1、图 2),花青素含量也随之升高,通过乙烯利处理的桑椹花青素含量变化较对照明显 (图 3)。21DAF (盛花期后21天) 桑椹经过乙烯利处理后48,72,81 h,每1 g干质量桑椹样品的花青素含量由最初的5.48 mg逐渐上升到27.59,52.05,46.21 mg,通过ddH2O处理的桑椹中花青素含量也上升,但上升幅度较小。26DAF (盛花期后26天) 桑椹对照组和处理组中花青素含量变化趋势与21DAF处理桑椹相似。21DAF桑椹经乙烯利处理后,MaDFR和MaANS的表达量在72 h出现大幅度上升;26DAF桑椹经乙烯利处理后,MaDFR的表达量相比对照有提高,MaANS表达量没有显著差异 (图 3)。离体桑椹经乙烯抑制剂1-MCP熏蒸后,26DAF桑椹中花青素含量受到显著抑制,21DAF桑椹在处理后24 h花青素含量受到抑制;21DAF桑椹的MaDFR表达量与对照相比没有明显变化,而MaANS表达量在24 h才明显下调,26DAF桑椹的MaDFR和MaANS表达量显著低于对照处理 (图 4)。以上结果表明乙烯利及1-MCP对桑椹中花青素的积累和相关基因的表达具有调控作用。

|
图 1 乙烯利处理不同发育时期桑椹表型变化 Fig.1 Changes in phenotypes during development of mulberry fruit under ethephon treatment A: 21DAF (盛花期后21天) 桑椹对照处理;B: 21DAF桑椹乙烯利处理;C: 26DAF (盛花期后26天) 桑椹对照处理;D: 26DAF桑椹乙烯利处理。A: The control of 21DAF (21 days after full-bloom) mulberry fruit treated with ddH2O; B: The 21DAF mulberry fruit treated with ethephon; C: The control of 26DAF (26 days after full-bloom) mulberry fruit treated with ddH2O; D: The 26DAF mulberry fruit treated with ethephon. |
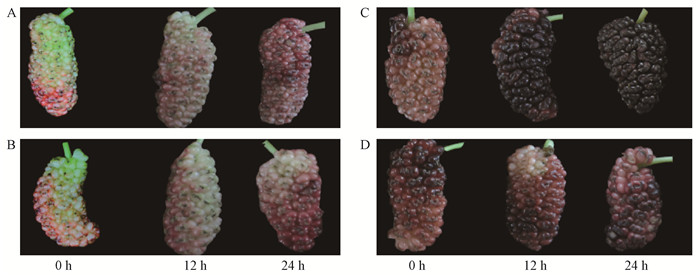
|
图 2 1-MCP处理桑椹表型变化 Fig.2 Changes in phenotypes during development of mulberry fruit under 1-MCP treatment A: 21DAF桑椹对照处理; B: 21DAF桑椹1-MCP熏蒸; C: 26DAF桑椹对照处理; D: 26DAF桑椹1-MCP熏蒸。A: The control of 21DAF mulberry fruit treated with ddH2O; B: The 21DAF mulberry fruit treated with 1-MCP; C: The control of 26DAF mulberry fruit treated with ddH2O; D: The 26DAF mulberry fruit treated with 1-MCP. |
|
图 3 乙烯利对桑椹花青素含量及其生物合成基因表达量的影响 Fig.3 Effects of ethephon treatments on anthocyanin content and the expression of anthocyanin biosynthesis related genes in mulberry 21DAF:盛花期后21天;26DAF:盛花期后26天。*,差异性显著 (P < 0.05)。下同。21DAF:21 days after full-bloom; 26DAF:26 days after full-bloom.*, Significant difference (P < 0.05). |
|
图 4 1-MCP对桑椹的花青素含量及其生物合成基因表达量的影响 Fig.4 Effects of 1-MCP treatments on anthocyanin content and the expression of anthocyanin biosynthesis related genes in mulberry fruit |
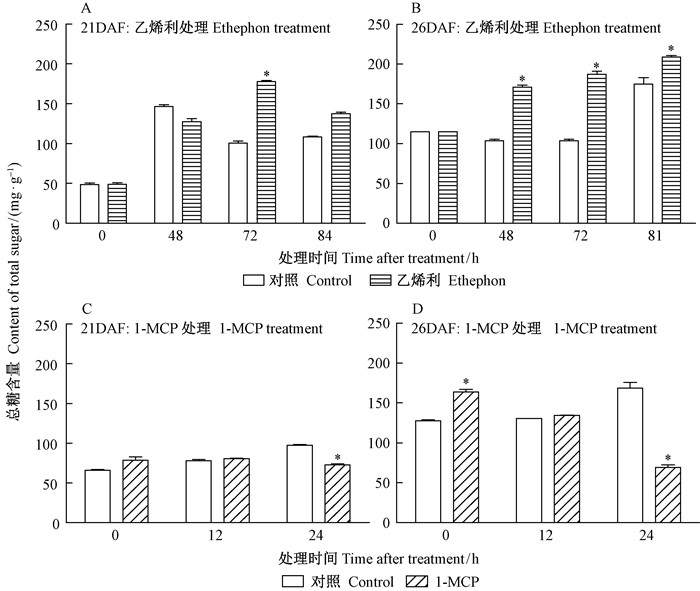
21DAF和26DAF桑椹经乙烯利处理后,总糖含量相比对照都有显著提高 (图 5A,B)。桑椹经1-MCP熏蒸后,24 h的总糖含量相比对照有所下调 (图 5C,D)。以上结果表明,乙烯利和1-MCP影响桑椹发育过程中总糖的合成效应是相反的,前者促进,后者抑制。
|
图 5 乙烯利及1-MCP对不同发育时期桑椹总糖含量影响 Fig.5 Effects of ethephon and 1-MCP treatments on total sugar content at mulberry fruit development stages |
21DAF桑椹的乙烯生物合成基因 (MaACO2,MaACS3) 和信号转导基因 (MaETR1,MaETR2,MaCTR1,MaEIN2,MaEIL2) 经乙烯利处理后,表现为3种不同的表达模式 (图 6)。MaACO2,MaACS3以及MaCTR1经乙烯利处理后,表达量先上调后下调,48 h时表达量最高,72 h和81 h均降低;MaETR1,MaEIN2,MaEIL2经乙烯利处理后,表达量在0~48 h下调,72 h升高,81 h再次下调;而MaETR2表达模式与MaETR1相反,为先升高再降低,81 h表达量升到最高。使用乙烯利处理后,与ddH2O相比,乙烯信号转导途径的基因都受到显著上调,对乙烯利有显著的响应。

|
图 6 乙烯利对21DAF桑椹乙烯生物合成及信号转导相关基因的影响 Fig.6 Effects of ethephon treatments on ethylene biosynthesis and signal transduction related gene in the 21DAF mulberry fruits |
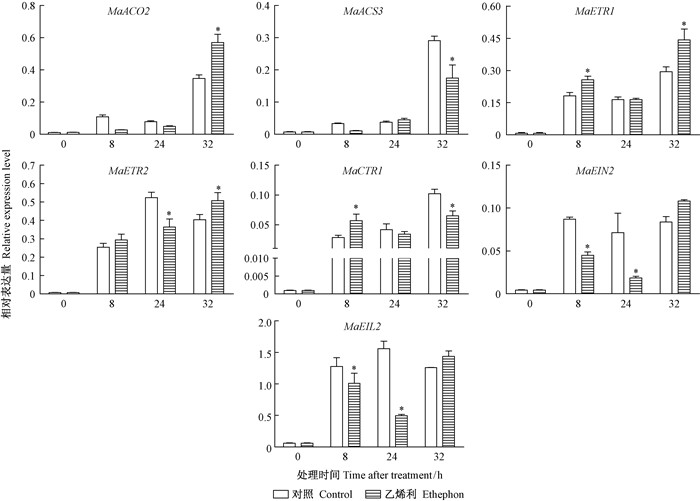
26DAF桑椹经乙烯利和ddH2O处理后,乙烯生物合成及信号转导基因的表达量都在处理后8 h显著上调 (图 7),MaACO2,MaACS3,MaETR1和MaCTR1的表达量在处理后32 h达到最高,MaETR2和MaEIL2在处理后24 h达到最高,而MaEIN2的表达量在处理后8 h趋于稳定。各个基因对乙烯利处理表现出了不同的响应模式。MaACO2,MaEIN2和MaEIL2表现出相似的表达模式,其表达量在处理后8~24 h下调,在处理后32 h上调;MaACS3的表达量经过乙烯利处理后有所下调;MaETR1,MaETR2和MaCTR1的表达在处理后8~24 h略有上调,处理后32 h MaETR1和MaETR2上调,而MaCTR1下调。
|
图 7 乙烯利对26DAF桑椹乙烯生物合成及信号转导相关基因的影响 Fig.7 Effects of ethephon treatments on ethylene biosynthesis and signal transduction related gene in the 26DAF mulberry fruits |
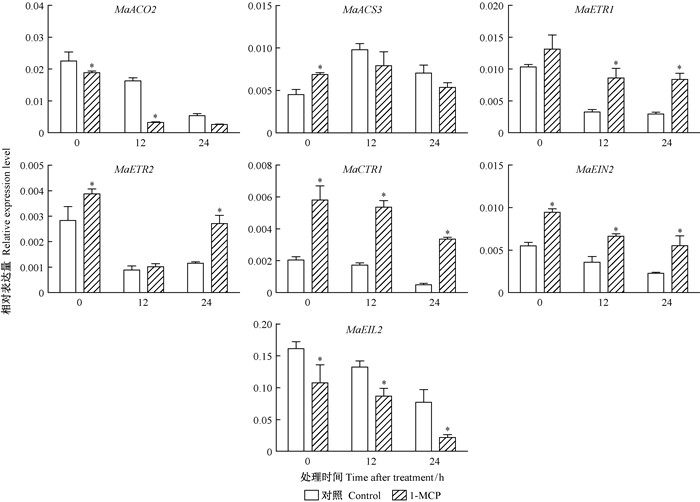
21DAF桑椹中,各个基因对1-MCP熏蒸呈现不同的响应模式。其中MaACS3在0 h表达量上调,其他时段均受到抑制;MaACO2和MaEIL2的表达量受到1-MCP熏蒸的抑制,而MaETR1,MaETR2,MaCTR1,MaEIN2却在1-MCP熏蒸后上调 (图 8)。
|
图 8 1-MCP对21DAF采后桑椹乙烯生物合成及信号转导相关基因的影响 Fig.8 Effects of 1-MCP treatments on ethylene biosynthesis and signal transduction related gene in the 21DAF mulberry fruits |
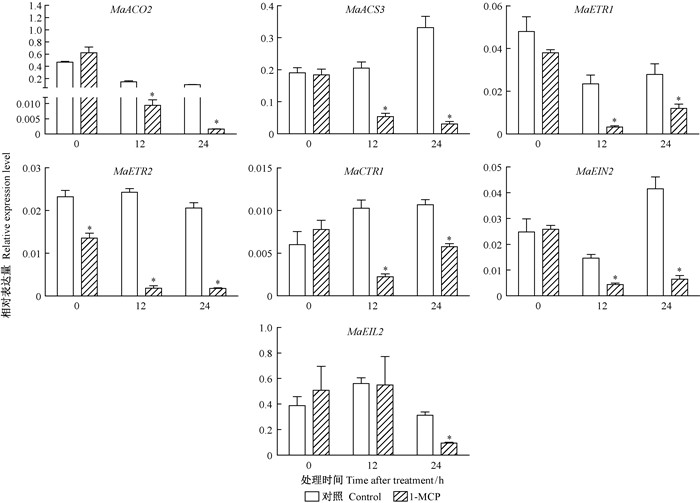
1-MCP熏蒸26DAF采后桑椹对乙烯合成与信号转导各基因的表达量大多有抑制作用,尤其对12 h和24 h表达量的抑制效应更为显著 (图 9)。
|
图 9 1-MCP对26DAF采后桑椹乙烯生物合成及信号转导相关基因的影响 Fig.9 Effects of 1-MCP treatments on ethylene biosynthesis and signal transduction related gene in the 26DAF mulberry fruits |
桑椹是一种营养丰富、风味独特的果品,其生产和商业化发展长久以来受到其货架期短、易脱落、易腐烂和不耐储藏的生理特征的困扰。先前研究表明,桑椹在盛花期26天 (26DAF) 前可溶固形物含量、乙烯释放速率和呼吸速率较为稳定,而26DAF后骤变,且对乙烯及其抑制剂具有响应作用,认为乙烯对桑椹的发育起重要调控作用,但对其具体的调控机制还不是很清楚 (Liu et al., 2014; 2015;刘长英等,2014)。乙烯调控植物果实的发育和成熟,主要是通过其生物合成途径和信号转导来实现的,乙烯对果实调控的分子机制也已在番茄、西瓜 (Citrullus lanatus) 和美味猕猴桃 (Actinidia deliciosa var. deliciosa cv. Hayward) 等物种中得以验证 (Cara et al., 2008; Wechter et al., 2008; Yin et al., 2008)。基于本文研究结果和前期对34DAF桑椹处理的结果 (表 2),对乙烯调控桑椹发育及成熟的机制进行了探讨。均被乙烯利上调,但在34DAF处理的桑椹中却受到了乙烯的抑制,试验结果与无花果 (Ficus carica) 的研究 (Owino et al., 2006) 一致。以上试验结果表明乙烯生物合成基因在跃变前后参与了乙烯的合成,在果实发育后期受到了乙烯的反馈调控。此外,在桑椹不同发育阶段,MaACO2和MaACS3都受到了1-MCP的抑制,而且减缓了桑椹的成熟进程。1-MCP与乙烯分子结构相似,能够与乙烯受体结合,从而阻断乙烯的传递,使得乙烯生理效应无法完成,也间接证明了乙烯对于桑椹成熟的重要性。
|
|
在乙烯信号转导途径中,MaETR1,MaETR2,MaEIN2和MaEIL2等发挥正调控作用的基因在不同发育时期桑椹中都受到了乙烯的上调,暗示这些基因对于桑椹的发育起调控作用。MaETR1,MaETR2的表达差异,主要体现在MaETR2的表达量在26DAF对乙烯无明显响应,而MaETR1在26DAF表达量受到乙烯上调,分别与成熟柑橘果实采后贮藏过程中CsETR1和CsERS1的表达模式 (Katz et al., 2004) 相似。根据果实成熟过程中乙烯生成量的变化,可分为乙烯系统Ⅰ和乙烯系统Ⅱ(Mcmurchi et al., 1972),在柑橘中CsETR1可能参与系统Ⅰ乙烯生理效应发挥,CsERS1则调节果实对乙烯的敏感性。因此,MaETR1很可能在桑椹成熟早期发挥作用,而MaETR2可能在桑椹成熟后期才发挥作用。MaCTR1基因在26DAF和34DAF桑椹中受到乙烯的抑制,MaCTR1在桑椹发育和成熟过程中,扮演负调控因子的作用,通过基因工程提高该基因的表达,可为今后改良桑树果实的成熟期提供一个很好的思路。此外,本文还分析了乙烯信号转导基因对1-MCP的响应机制,发现参与乙烯信号转导的各个元件受到了1-MCP的抑制,1-MCP可以阻止桑椹中乙烯信号转导和传递,从而达到抑制桑椹的成熟。
关于乙烯如何促进果实花青素的积累有2种观点:一种认为乙烯直接促进花青素相关基因的表达从而促进花青素的积累 (Kondo et al., 1997);另一种则认为乙烯首先促进果实成熟,从而促进花青素的积累 (潘增光等, 1995; Zhang et al., 2009)。本试验中,乙烯上调21DAF和26DAF桑椹中花青素生物合成相关基因的表达,促进花青素和总糖含量的积累;而1-MCP则下调花青素生物合成相关基因的表达,抑制花青素和总糖含量的积累。表明乙烯对桑椹发育各时期中花青素的积累具有调控作用,但其分子机制还有待今后深入研究。
4 结论乙烯利处理能够诱导桑椹乙烯生物合成和花青素生物合成相关基因的上调表达,对乙烯信号转导基因也有一定调控作用,且能促进桑椹花青素和总糖含量的积累,并能加快桑椹的发育进程。1-MCP熏蒸能抑制乙烯信号转导各元件基因的表达,阻止乙烯信号转导的传递,且抑制桑椹中花青素和总糖含量的积累。
| [] |
李军, 赵爱春, 王茜龄, 等. 2011. 三个桑树肌动蛋白基因的克隆与组织表达分析. 作物学报, 37(4): 641–649.
( Li J, Zhao A C, Wang X L, et al. 2011. Molecular cloning and tissues expression analysis of three Actin genes from mulberry (Morus alba). Acta Agronomica Sinica, 37(4): 641–649. DOI:10.3724/SP.J.1006.2011.00641 [in Chinese] ) |
| [] |
刘乐, 饶景萍, 常晓晓, 等. 2009. 丙烯和1-MCP处理对采后柿果实ACS和ACO基因表达的影响. 中国农业科学, 42(6): 2092–2097.
( Liu L, Rao J P, Chang X X, et al. 2009. Effects of propylene and 1-methylcyclopropene on expressions of ACC synthase and ACC oxidase genes in persimmon fruits. Scientia Agricultura Sinica, 42(6): 2092–2097. [in Chinese] ) |
| [] |
刘长英, 李军, 赵爱春, 等. 2014. 桑椹发育中花青素、叶绿素含量变化及相关基因的表达分析. 林业科学, 50(9): 59–66.
( Liu C Y, Li J, Zhao A C, et al. 2014. Changes of anthocyanin and chlorophyll content, and expression levels of related genes during development process of mulberry fruit. Scientia Silvae Sinicae, 50(9): 59–66. [in Chinese] ) |
| [] |
潘增光, 范辉, 束怀瑞. 1995. 苹果果实花青素形成与乙烯释放的关系. 植物生理学通讯, 31(5): 338–340.
( Pan Z G, Fan H, Shu H R. 1995. The relationship between anthocyanidin content and rate of ethylene production in apple fruits. Plant Physiology Communications, 31(5): 338–340. [in Chinese] ) |
| [] |
唐忠富. 2009. 桑资源的药用开发与利用. 蚕桑通报, 39(1): 6–8.
( Tang Z F. 2009. Exploitation and utilization of mulberry, Morus alba, in the medicine. Bulletin of Sericulture, 39(1): 6–8. [in Chinese] ) |
| [] |
郑小坚, 陆小平. 2002. 桑树组织总糖含量蒽酮比色法测定技巧. 广西蚕业, 39(1): 8–9.
( Zheng X J, Lu X P. 2002. The techniques of determinating total sugar content by anthrone colorimetry in mulberry. Guangxi Sericulture, 39(1): 8–9. [in Chinese] ) |
| [] | Adams-Phillips L, Barry C, Giovannoni J. 2004a. Signal transduction systems regulating fruit ripening. Trends in Plant Science, 9(7): 331–338. DOI:10.1016/j.tplants.2004.05.004 |
| [] | Adams-Phillips L, Barry C, Kannan P, et al. 2004b. Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Molecular Biology, 54(3): 387–404. DOI:10.1023/B:PLAN.0000036371.30528.26 |
| [] | Ayub R, Guis M, Ben A M, et al. 1996. Expression of ACC oxidase antisense gene inhibits ripening of cantaloupe melon fruits. Nature Biotechnology, 14(7): 862–866. DOI:10.1038/nbt0796-862 |
| [] | Blankenship S M, Dole J M. 2003. 1-methylcyclopropene:a review. Postharvest Biology and Technology, 28(1): 1–25. DOI:10.1016/S0925-5214(02)00246-6 |
| [] | Bulens I, van de Poel B, Hertog M L, et al. 2014. Dynamic changes of the ethylene biosynthesis in 'Jonagold' apple. Physiologia Plantarum, 150(2): 161–173. DOI:10.1111/ppl.2014.150.issue-2 |
| [] | Cara B, Giovannoni J J. 2008. Molecular biology of ethylene during tomato fruit development and maturation. Plant Science, 175(1/2): 106–113. |
| [] | Chaudhary P R, Jayaprakasha G K, Patil B S. 2015. Ethylene degreening modulates health promoting phytochemicals in Rio Red grapefruit. Food Chemistry, 188: 77–83. DOI:10.1016/j.foodchem.2015.04.044 |
| [] | Chaudhary P, Jayaprakasha G K, Porat R, et al. 2012. Degreening and postharvest storage influences 'Star Ruby' grapefruit (Citrus paradisi Macf.) bioactive compounds. Food Chemistry, 135(3): 1667–1675. DOI:10.1016/j.foodchem.2012.05.095 |
| [] | Dal C V, Rizzini F M, Botton A, et al. 2006. The ethylene biosynthetic and signal transduction pathways are differently affected by 1-MCP in apple and peach fruit. Postharvest Biology and Technology, 42(2): 125–133. DOI:10.1016/j.postharvbio.2006.06.008 |
| [] | El-Sharkawy I, Kim W S, El-Kereamy A, et al. 2007. Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.). Journal of Experimental Botany, 58(13): 3631–3643. DOI:10.1093/jxb/erm213 |
| [] | Hu Z L, Deng L, Chen X Q, et al. 2010. Co-suppression of the EIN2-homology gene LeEIN2 inhibits fruit ripening and reduces ethylene sensitivity in tomato. Russian Journal of Plant Physiology, 57(4): 554–559. DOI:10.1134/S102144371004014X |
| [] | Jaakola L. 2013. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Science, 18(9): 477–483. DOI:10.1016/j.tplants.2013.06.003 |
| [] | Jeong S W, Das P K, Jeoung S C, et al. 2010. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiology, 154(3): 1514–1531. DOI:10.1104/pp.110.161869 |
| [] | Katz E, Lagunes P M, Riov J, et al. 2004. Molecular and physiological evidence suggests the existence of a system-like pathway of ethylene production in non-climacteric citrus fruit. Planta, 219(2): 243–252. DOI:10.1007/s00425-004-1228-3 |
| [] | Klee H, Tieman D. 2002. The tomato ethylene receptor gene family:Form and function. Physiologia Plantarum, 115(3): 336–341. DOI:10.1034/j.1399-3054.2002.1150302.x |
| [] | Kondo S, Inoue K. 1997. Abscisic acid (ABA) and 1-aminocyclopropane-1-carboxylic acid (ACC) content during growth of 'Satohnishiki' cherry fruit, and the effect of ABA and ethephon application on fruit quality. Journal of Horticultural Science, 72(2): 221–227. DOI:10.1080/14620316.1997.11515509 |
| [] | Li J, Lü R H, Zhao A C, et al. 2014. Isolation and expression analysis of anthocyanin biosynthetic genes in Morus alba L. Biology Plantarum, 58(4): 618–626. DOI:10.1007/s10535-014-0450-5 |
| [] | Liu C Y, Lü R H, Zhao A C, et al. 2014. Characterization and expression profiles of MaACS and MaACO genes from mulberry (Morus alba L.). Journal of Zhejiang University-Science B, 15(7): 611–623. DOI:10.1631/jzus.B1300320 |
| [] | Liu C Y, Zhao A C, Zhu P P, et al. 2015. Characterization and expression of genes involved in the ethylene biosynthesis and signal transduction during ripening of mulberry fruit. PLoS ONE, 10(3): e0122081. DOI:10.1371/journal.pone.0122081 |
| [] | Mbéguié-A-Mbéguié D, Hubert O, Fils-Lycaon B, et al. 2008. EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande naine). Physiologia Plantarum, 133(2): 435–448. DOI:10.1111/j.1399-3054.2008.01083.x |
| [] | Mcmurchi E J, Mcglasso W B, Eaks I L. 1972. Treatment of fruit with propylene gives information about biogenesis of ethylene. Nature, 237(5352): 235–236. DOI:10.1038/237235a0 |
| [] | Nakatsuka T, Haruta K S, Pitaksutheepong C, et al. 2008. Identification and characterization of R2R3-MYB and bHLH transcription factors regulating anthocyanin biosynthesis in gentian flowers. Plant and Cell Physiology, 49(12): 1818–1829. DOI:10.1093/pcp/pcn163 |
| [] | Oller P W, Wong L M, Tayfor L P, et al. 1991. Reversible inhibition of tomato fruit senescence by antisense RNA. Science, 254(5030): 437–439. DOI:10.1126/science.1925603 |
| [] | Owino W O, Manabe Y, Mathooko F M, et al. 2006. Regulatory mechanisms of ethylene biosynthesis in response to various stimuli during maturation and ripening in fig fruit (Ficus carica L.). Plant Physiol Biochem, 44(5/6): 335–342. |
| [] | Ozga J A, Reinecke D M. 2003. Hormonal interactions in fruit development. Journal of Plant Growth Regulation, 22(1): 73–81. DOI:10.1007/s00344-003-0024-9 |
| [] | Wang A, Yamakake J, Kudo H, et al. 2009. Null mutation of the MdACS3 gene, coding for a ripening-specific 1-aminocyclopropane-1-carboxylate synthase, leads to long shelf life in apple fruit. Plant Physiology, 151(1): 391–399. DOI:10.1104/pp.109.135822 |
| [] | Wechter W P, Levi A, Harris K R, et al. 2008. Gene expression in developing watermelon fruit. BMC Genomics, 9: 275. DOI:10.1186/1471-2164-9-275 |
| [] | Xiao Y Y, Chen J Y, Kuang J F, et al. 2013. Banana ethylene response factors are involved in fruit ripening through their interactions with ethylene biosynthesis genes. Journal of Experimental Botany, 64(8): 2499–2510. DOI:10.1093/jxb/ert108 |
| [] | Yin X R, Chen K S, Allan A C, et al. 2008. Ethylene-induced modulation of genes associated with the ethylene signaling pathway in ripening kiwifruit. Journal of Experimental Botany, 59(8): 2097–2108. DOI:10.1093/jxb/ern067 |
| [] | Yokotani N, Tamura S, Nakano R, et al. 2003. Characterization of a novel tomato EIN3-like gene (LeEIL4). Journal of Experimental Botany, 54(393): 2775–2776. DOI:10.1093/jxb/erg308 |
| [] | Zhang M, Yuan B, Leng P. 2009. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. Journal of Experimental Botany, 60(6): 1579–1588. DOI:10.1093/jxb/erp026 |
 2017, Vol. 53
2017, Vol. 53

