文章信息
- Jie Chu, Junhua Zhang, Li Ma, Haidong Lu
- 楚杰, 张军华, 马莉, 路海东
- Study of the Crystallization Parameters of Bamboo Fibers with Pretreatment
- 预处理竹材的结晶度分析
- Scientia Silvae Sinicae, 2017, 53(2): 100-109.
- 林业科学, 2017, 53(2): 100-109.
- DOI: 10.11707/j.1001-7488.20170212
-
文章历史
- Received date: 2015-12-09
- Revised date: 2016-10-31
-
作者相关文章
Chemical heat treatment is a critical step for transforming a biomass to its value-added lignocellulosic biomass polymeric products (Zhao et al., 2012; Xie et al., 2015). Pretreatment can quickly remove hard dissolved lignin, leading to the physical separation of hemicellulose, increasing lignocellulose production. Currently, despite the large number of studies of the thermal chemical conversion method, general conclusions that are applicable to the specific treatment schemes have not been obtained. This is because most studies have focused on the yield of cellulose and sugar production during the process of chemical pretreatment. However, chemical pretreatment of biomasss is much more complicated, and therefore, the influence of composition changes and the effect of pretreatments on the obtained functional groups and crystallinity should be further investigated. In addition, the lignocelloses consists of complex structures, including cellulose, hemicelluloses, lignin, and a small amount of extract (Chundawat et al., 2011; Zhou et al., 2004; Merino-Pérez et al., 2014). The pretreatment solution and treatment temperature have an important effect on the variation of the content of each component. Due to the importance and high cost of the pretreatment process and the chemical conversion of functional groups, the change of the crystallinity and cellulose molecular weight during pretreatment will be subject to increasing attention from researchers in the future.
Based on the current pretreatment processing technology, Li et al.(2014) studied the development of bamboo cellulose crystallinity changes using X-ray diffraction and Rezende et al.(2011) indicated bamboo fiber crystallinity changes after radiation treatment.The results obtained by Li and Rezende agree with intensity changes of the X-ray diffraction of bamboo fiber. Abdulkhani et al.(2014) studied the trends in the bamboo crystal area changes after alkali treatment and found that these differed from the trends obtained in previous studies, with the fiber crystallinity changes of bamboo first increasing and then decreasing. Wang et al.(2008) suggested that the two important factors leading to the worse performance of bamboo are high crystallinity and the orientation structure; additionally, she found an effective method for using alkali treatment of bamboo to quickly reduce the bamboo crystallinity index. Li et al.(2010) provided pretreated bamboo to isolate cellulose for the further utilization of bioethanol.
Among the representative technologies for the pretreatment of wood biomass, Xin et al.(2015) researched the digestion absorption rate of the bamboo structure after different pretreatments with acid, alkali and sulfate; Zhang et al.(2013)and Karp et al.(2015) studied the chemical composition changes due to bamboo pretreatment; Li et al. (2013) showed that the application of dilute organic acids in the pretreatment of bamboo can be effective and clearly influenced the cell-wall structure and enzymatic digestibility of bamboo; He also provided that the pretreatment of bamboo with the same sodium hydroxide loading at higher pretreatment temperature led to greater hemicellulose and lignin removal. These investigations showed that acid and alkali pretreatment of bamboo is a more effective way to improve wood fiber content than other approaches used in the production of bioethanol and other fields (Li et al., 2014). However, to the best of our knowledge, less attention has been focused on the chemistry and molecular structure changes, with fewer studies on the mechanism causing the changes of the chemical composition of lignocellulosic biomass materials after chemical treatment. Nevertheless, a considerable amount of research has been performed on bamboo cellulose fermentation and the saccharification process under the condition of alkali treatment in the past few years. To the best of our knowledge, there are few results on cellulose crystallinity size changes. In particular, the obtained results are not consistent with the scale change rule of bamboo fiber crystallinity under the different chemical treatment conditions, such as for the use of acid alkali, and glycerol (Himmel et al., 2007).
This paper is based on four years of bamboo studies that used X-ray diffraction (XRD) with different temperatures and pretreatments with acid, alkali, and glycerol, focusing on the determination of the average degree of polymerization (DP) content of cellulose, hemicelluloses and lignin, extract and ash of pretreated bamboo; additionally, using Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction, we further investigated the influence of different heat treatment methods on the bamboo chemical composition. We used the changes of the functional groups and the mechanism of the chemical pretreatment of wooden biomass resources to reveal the rules governing the compositional changes found after the application of the different chemical treatment methods.
1 Materials and methods 1.1 MaterialsThe bamboo powder sample (Phyllostachys edulis) was acquired from USDA Forest Service, Southern Research Station in Pineville, USA, in the summer of 2015. Prior to chemical pretreatment, air-dried bamboo was milled using a hammer mill with a screen opening size of 1.0 mm. The average moisture content of the ground air-dried bamboo was 6.18% (wt). The bamboo was sealed in a zipper-lock bag and kept at 4 ℃ in a refrigerator until use.
H2SO4, NaOH, NaClO2, toluene, methanol, ethanol, and glacial acetic acid were used; these were purchased from Fisher Scientific (Pittsburgh, PA) and used as received. Pretreatment equipment mainly included the electronic sand bath pot and thermometer produced in JAMs experimental equipment factory (USA).
1.2 Pretreatment processingSamples were obtained by different processing methods, with the processing parameters and processes shown in Fig. 1.
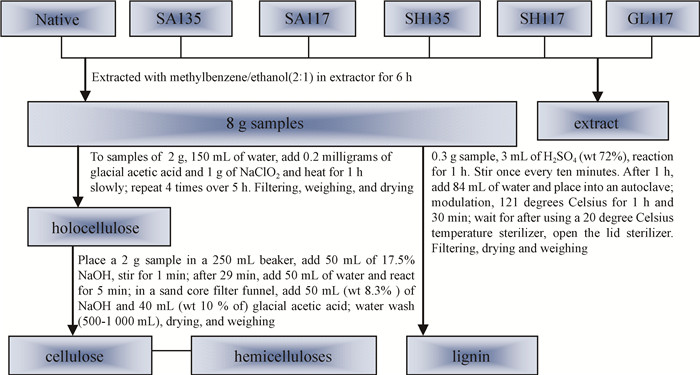
|
Fig.1 Scheme for the preparation of samples and cellulose, hemicelluloses, and lignin |
Fig. 1-5 illustrate the pretreatment processing of six bamboo samples. This includes a native sample, the sample treated by sulfuric acid at 135 ℃(SA135) and at 117 ℃(SA117), the sample treated by alkali at 135 ℃(SH135) and at 117 ℃(SH117), and the sample treated by glycerol at 117 ℃.

|
Fig.2 Dry sample under different processing conditions a. The native bamboo is original; b. The SH117 sample is pretreated with sulfuric acid at 117 ℃; c.The SH135 sample is pretreated with sulfuric acid at 135 ℃; d. The SA 117 sample is pretreated with alkali at 117 ℃; e. The SA135 sample is pretreated with alkali at 135 ℃; f. The GL117 sample is pretreated with glycerin at 117 ℃. |

|
Fig.3 Filtrate of different processing conditions |

|
Fig.4 Cellulose samples of different processing conditions |

|
Fig.5 Liquid and solid samples of lignin and ash |
The moisture content of the bamboo samples was measured by drying in an oven for 24 h at (105±2) ℃. The holocellulose and cellulose contents of the samples were determined using a Soxhlet extractor. An electric muffle furnace (programmable ramping) was used to determine the ash in the samples of bamboo. The lignin content of unpretreated and pretreated bamboo was determined according to the National Renewable Energy Laboratory (NREL) analytical procedure: determination of the structural carbohydrates and lignin in biomass.15 each test was performed for three parallel samples at the same time, with the average used as the measured value.
1.3 Measurements 1.3.1 Chemical analysisThe bamboo samples were evaluated in accordance with ASTM D1105-96 (ASTM 1996) and ASTM D-1102-84(ASTM 1990). Each evaluation was performed twice.
1.3.2 DP and molecular contentThe average degree of polymerization (DP) and molecular content were determined using the method specified by the British Standards restrictions, and the viscosity was determined according to the Part I.Cupriethylenediamine (CED) method (BS 6306: Part 1: 1982). The sample viscosity was determined based on its cupriethylenediamine viscosity [η], which was obtained using the following hydroxide (cuene) solution equation:
| $ {\rm{DP}}\;{\rm{0}}{\rm{.905 = 0}}{\rm{.75}}\left[ \eta \right]. $ | (1) |
where DP is the average degrees of polymerization and η is the intrinsic viscosity in mL·g-1. The crystallinity of the samples was determined by X-ray diffraction using an XRD-6000 instrument (Shimidzu, Japan). A capillary viscometer was used for the viscosity measurement (Shimidzu, Japan), D=(0.80±0.05) mm, with calibration using a capillary viscometer, D=(0.57±0.05) mm.
1.3.3 Fourier transform-infrared spectroscopy (FTIR)Image acquisition of Fourier infrared spectrum (FTIR). We used a NicoletiS10 Fourier trans form infrared spectrometer produced by the Thermo Nicolet company (USA) with the following test conditions: the spectral range was 4 000-400 cm-1, the spectral resolution was 4 cm-1, and the scans were performed 64 times.
1.3.4 Analysis of crystallinity by X-ray diffraction (XRD)The crystallinity of the samples was determined by X-ray diffraction (XRD) using Rigaku Ultima3 x (Louisiana State University, USA) with the scanning parameters of Ni-filtered Cu K α radiation (λ=1.540 60).
2 Results and discussion 2.1 Changes in the chemical composition of pretreated bambooFig. 6 shows the main chemical components obtained for different pretreatment conditions. The holocellulose content decreases in the order of SH117> SH135 > SA117 > SA135 > GL117 > NA; the cellulose content change decreases in the order of SH135 > SH117 > SA117 > SA135 > GL117 > NA; the amount of lignin removal decreases in the order of SH117 > SH135 > SA135 > > SH117 > GL117 > NA; and the degree of hemicellulose degradation decreases in the order of SH135>GL117>SA117> SA135 > SH117.
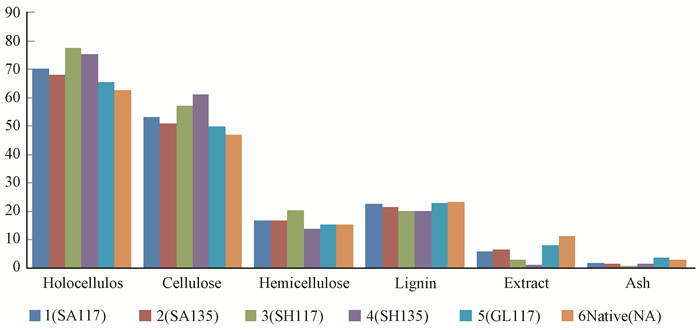
|
Fig.6 Main chemical components obtained with the use of different pretreatment conditions |
The results obtained by pretreatment with sodium hydroxide are much better than those obtained after sulfuric acid pretreatment at both 117 ℃ and 135 ℃; in particular, the cellulose yield showed a clear increase after the sodium hydroxide pretreatment at 135 ℃, with a 29.6% higher average ratio than that of the native material. However, pretreatment with glycerin was found to be not ideal. A remarkable lignin removal effect was also observed for the sodium hydroxide pretreatment at 117 ℃, with the average removal rate reaching 14.69%.
2.3 Degree of polymerizationTo investigate the cellulose depolymerization behavior in the pretreatment, the viscosity of the sample was determined and the related parameters were calculated. The obtained η values were in the 103.88-132.62 range, and the obtained DP values were in the 336-396 range (Tab. 1).
|
|
Inspection of the data from Tab. 1 shows that for all of the pretreatments, relatively low DP values are obtained, the reason might that pretreatment processing could change the super molecular structure of plant fiber raw material, that lead to changes for cellulose crystalline region size, on the other hand, better pretreatment can be part of the degradation of lignin and hemicellulose, increasing accessibility, which is benefit to improve the efficiency of plant cellulose enzyme hydrolysis.The DP value of the native sample is mainly higher that maybe due to the low value deformation of the cellulose crystal lattice in the processing.
2.4 Analysis of bamboo samples by Infrared spectroscopyThe obtained FTIR spectra in the 4 000-400 cm-1 range and the changes in the absorption peak of the functional group are shown in Fig. 7 and Fig. 8.
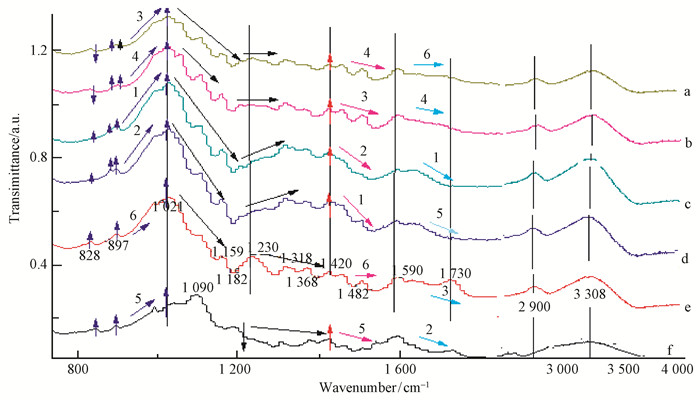
|
Fig.7 Compositional changes in the infrared spectra of pretreated and native bamboo
a:SA117; b:SA135; c: SH117; d: SH135; e: NATIVE; f:GL117 Note: |
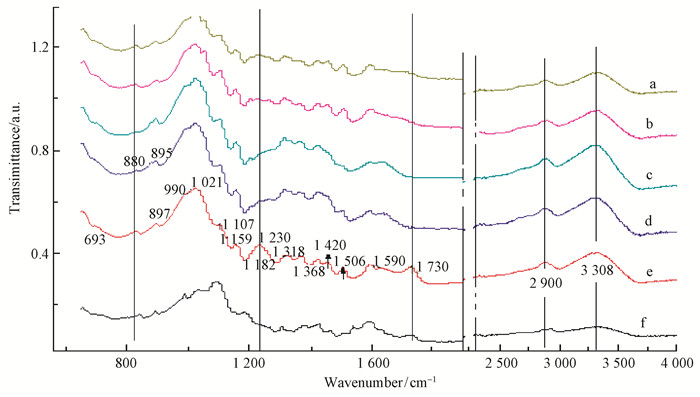
|
Fig.8 Infrared spectra of pretreatment and native bamboo a:SA117; b:SA135; c: SH117; d: SH135; e: NATIVE; f:GL117. |
The absorbance band at 897 cm-1 is the characteristic peak of ß glucose anhydride and the C-H bond bending vibration of cellulose (Li, 2003). While the absorbance band at 897 cm-1 exhibits only one peak in the native spectrum, it can be divided into two peaks at 880 and 895 cm-1for the SH117, SH135, SA135, and SA117 samples. The absorption peak at 828 cm-1(Fig. 7) was enhanced, indicating the change in cellulose content after pretreatment. After acid treatment, the crystallization of water O-H weakened the cellulose peak at 828 cm-1(Shao et al., 2008; Abdulkhani et al., 2014). Other characteristic absorption peaks of the cellulose are found at 2 900 cm-1 and 3 308 cm -1; these peaks change slightly and exhibit no other obvious absorption peak near the band. The relative ranking for the change in the cellulose was obtained as SH135 > SH117 > SA117> SA135 > GL117, namely, the order of the 1, 2, 3, 4, and 5 (Fig. 7); this is consistent with the standard method for the determination of cellulose content increase presented above in Fig. 6.
The absorbance bands at 1 730 cm-1and 897 cm-1 are the characteristic peaks for the acetyl and hydroxyl groups of hemicelluloses (Liu et al., 2008; Singh et al., 2014). As shown in Fig. 3, the strong absorbance peak at 1 730 cm-1 in spectrum of native bamboo become less pronounced after different pretreatments and disappeared completely for the SH135 and SH117 samples, confirming the degradation of hemicelluloses. Additionally, a C-O-C stretching vibration appears at 1 021 cm-1 and with a steep fall in absorption at 1 159 cm-1, indicates the increased degree of hemicelluloses' degradation (Shimokawa et al., 2009). Based on the slope from 1 021 cm-1 to 1 182 cm-1, the ranking of the changes in the hemicelluloses' content is SH135 > GL117 > SA117 > SA135 > SH117, as can also be seen in the spectrum of C=O stretching and OH deformation vibration at 1 090 cm-1. For glycerol (GL117), the absorption peak decline at 1 230 cm-1 showed the high degree of hemicelluloses degradation; however, the origin of this effect could not be determined (Fig. 8).
The analysis of the spectra is more complicated for the functional groups of lignin. Usually, the functional groups of lignin are benzene and benzene with C-C bonds combined with functional groups; a stretching vibration C-C absorption peak is found at approximately 1 420 cm-1 (Sun et al., 2010; Singh et al., 2014; Gabov et al., 2014) .Quite strong absorbance bands are present at 1 230 cm-1, which are due to the diaryl ether bond of the native sample that become weaker for the pretreated sample. In addition, from 1 420 cm-1 to 1 482 cm-1, the absorption peak also exhibits a steep fall (Fig. 7). These feature demonstrate the presence of a remarkable lignin removal effect after pretreatment.
Based on the changes of the characteristic band of lignin, it can be speculated that the strength of lignin removal is ranked as SH117 > SH135 > SA135 >>SH117 > GL117 > NA, which is in agreement with the previous test results.
For glycerol pretreatment, an obvious change of hemicelluloses with glycerol pretreatment was observed, with no significant removal of lignin and only incremental changes in the cellulose content; elucidation of the origin of this effect requires further study.
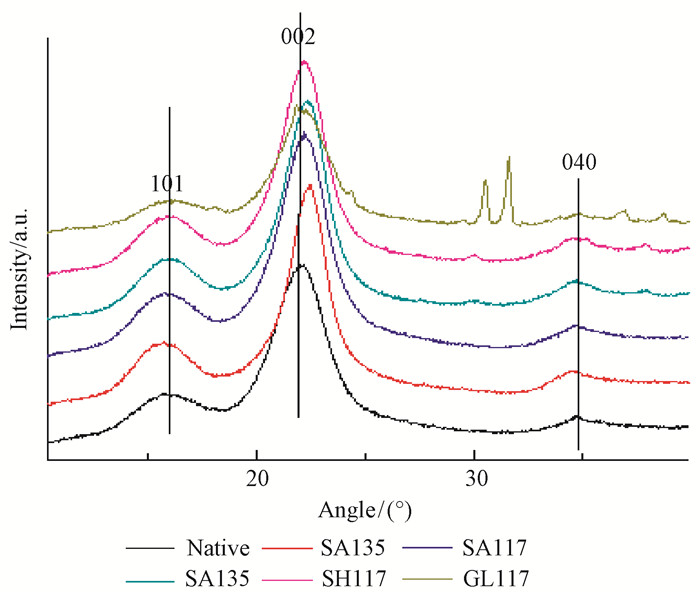
2.5 Analysis of the bamboo samples by XRDAccording to the diffraction spectroscopy characteristics of bamboo fiber, the 002 peak and the 004 peak indicate the length and width of the cellulose crystal area, respectively. In most cases, the 040 peak is not obvious, and we therefore calculated the diffraction intensity and position of the 002 peak to discuss the changes in the cellulose crystallization area. We use the Scherer formula (Segal et al., 1959) to calculate D, d and CrI as follows.
| $ D = \frac{{k\lambda }}{{{\rm{Bhkl}}\cdot{\rm{cos}}\theta }}; $ | (2) |
| $ d = \frac{\lambda }{{2{\rm{sin}}\theta }}; $ | (3) |
| $ {C_{{\rm{rⅠ}}}} = \frac{{{I_{002}} - {I_{{\rm{am}}}}}}{{{I_{002}}}} \times 100\% . $ | (4) |
where D is the length or width of the crystalline region; D is the crystal layer spacing (nm); λ is the incident wavelength (0.154 nm); Bhkl is the diffraction peak half width; θ is the diffraction angle; and k is diffraction constant, 0.89; CrI is the relative degree of crystallinity (%); I002 is the great strength of the 002 diffraction angle; and Iam is the background diffraction scattering intensity when 2θ is equal to 180°.
Six different test samples were prepared based on the use of different alkali and glycerol media and heating temperatures; XRD scans showed obvious changes of the 002 peak position and intensity (Fig. 9).
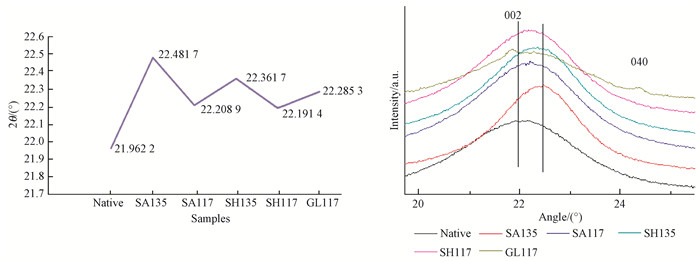
|
Fig.9 002 crystal plane diffraction peak position changes |
Examination of Fig. 9 shows that the angle of diffraction (2θ), that is, the position of the 002 crystal plane diffraction peak, changed significantly, and the peak exhibits an obvious offset after different pretreatments. This phenomenon shows that the lattice parameters of the crystalline region and the interplanar spacing decrease (Foreman et al., 1993; Mansfield et al., 1999; Liu et al., 2008).The effect of the chemical pretreatment is beneficial, as the decreased bamboo crystal spacing improves the dimensional stability of bamboo and provides a strong reference value for composite processing. These results consistent with previous research (Tamura et al., 2003). The change rate of chemical pretreatment at 135 ℃ is significantly higher than that at 117 ℃.
2.6 Analysis of various crystallization parameters of the different pretreatmentsBased on the 002 crystal crystallization index and Eqns. (2), (3), and (4), the crystallization parameters can be obtained for different pretreatment conditions.
Fig. 10 shows the change of the crystalline region width D. The width of the crystalline region increases after different pretreatments, showing that the grain size of the crystalline region decreased. Examination of the data shows that the rate of change rate is significantly higher for pretreatment at 135 ℃ than at 117 ℃. However, no obvious differences were observed between the SA135, SH135, SA117 and SH117 samples. This indicated that the pretreatment temperature has a stronger influence on the grain size of the crystalline region. After glycerol pretreatment, a significantly smaller grain size was found.
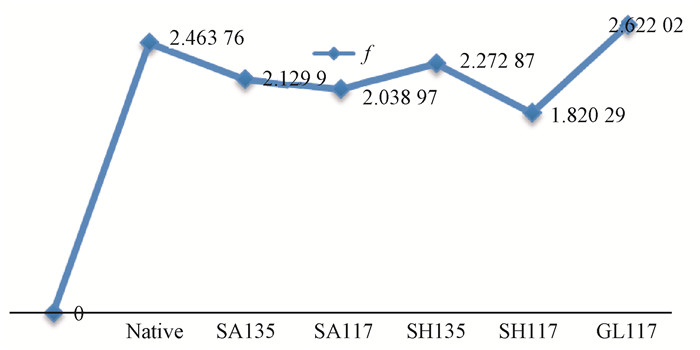
|
Fig.10 Width variation for the 002 crystal plane |
Obtaining the fine grain structure is the most effective approach for improving the strength and toughness of bamboo, therefore, the improvement of the degree of crystalline parameters through chemical heat treatment has important implications for the enhanced use and performance of bamboo.
The change of the half peak width (f) of the crystalline region indicated the grain size of the crystalline region: a smaller peak width (f) implies that the grain size is smaller as well (Nishimiya et al., 1998; Inari et al., 2007). Fig. 11 further illustrates the change of the crystalline region grain size. The half peak width (f) is lower for pretreatment at 117 ℃ than at 135 ℃; this is especially pronounced for treatment with alkali at 117 ℃, where the half peak width of the crystallization area is minimum, showing that alkali treatment will be the most effective approach for obtaining tiny crystalline bamboo particles.
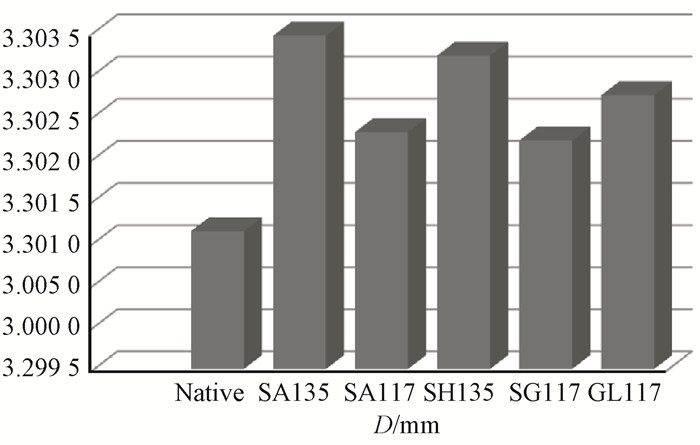
|
Fig.11 FWHM variation for the 002 crystal plane |
Fig. 12 shows the changes of the crystalline region layer spacing (d), with the layer spacing dimension exhibited a trend of significant decrease after pretreatment and a more obvious concentration at 135 ℃ compared to that at 117 ℃. Investigation of the origin of this effect showed that adsorption on the cell walls of bamboo was gradually enhanced and that the oligosaccharide in the crystalline region of cellulose surface molecular chain was dissolved (Yang et al., 2008). That due to the interaction between hemicelluloses and a weak base, a peeling reaction may occur, leading to decreased layer spacing (Foreman et al., 1993).
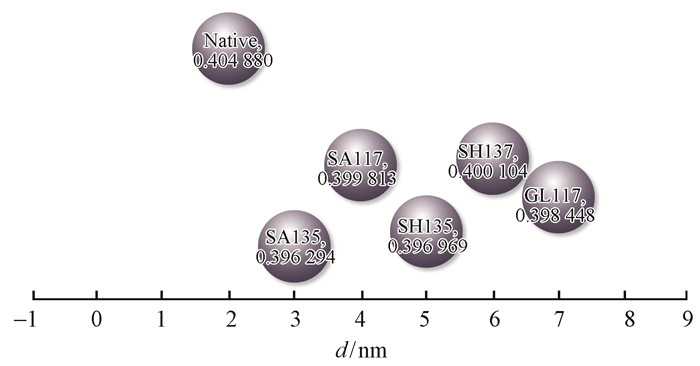
|
Fig.12 Layer spacing variation for the 002 crystal plane diffraction peak |
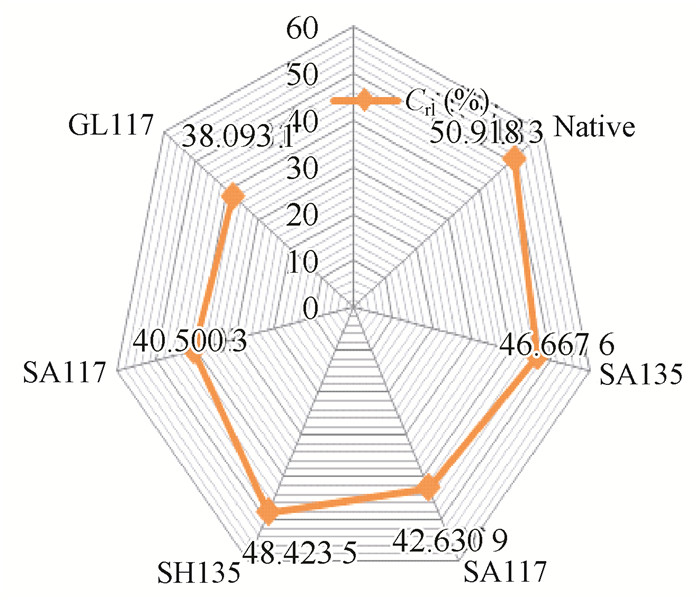
As shown in Fig. 13, the 002 peak is high and sharp, with the degree of crystallinity increasing significantly after pretreatment. Fig. 14 showed the trend of a first reduced and then increased intensity, motivating the present research to achieve a consistent increase of crystallinity of pretreated bamboo. Bamboo crystallinity declined at 117 ℃; however, the diffraction peaks become sharp; when the temperature rose to 135 ℃, the diffraction peaks increased gradually. The results showed that after pretreatment with sulfuric acid at 135 ℃, the bamboo crystallinity fell by 5% and the relative crystallinity increased by 3%; by contrast, the bamboo crystallinity fell by 15% and the relative crystallinity increased by 9% after pretreatment with alkali at 135 ℃; after heat treatment, the crystallinity increased and the most obviously decreased pretreatment was with glycerol, where two irregular peaks appeared near the 040 peak; the elucidation of the reason for this effect requires further study.
|
Fig.13 crystal strength of crystal plane variation |
|
Fig.14 The value of crystal plane variation |
Due to the stronger cell wall adsorption, following the alkali pretreatment for bamboo, the oligosaccharide in the crystalline region of the cellulose surface molecular chain is dissolved and the reactive amorphous zone reactive is exposed. Moreover, the interaction between hemicellulose and a weak base led to decreased crystallinity because of the swelling of the cellulose matrix and the increase in the amorphous area (Yang et al., 2008). Similarly, this led to a relative reduction in the shaped area of the cellulose, making an additional contribution to the further decrease of crystallinity. Hemicelluloses of bamboo hydrolysis are used to produce acetic acid involving the degradation of microfibrils in the amorphous region and in the setting area of cellulose (Yang et al., 2007) for the pretreatment temperature of 135 ℃, resulting in the decrease of the cellulose crystallinity.
Additionally, the density of the bamboo crystalline region increased after pretreatment that caused the enhancement of the diffraction peak.
3 ConclusionsComparison of the hemicellulose degradation degree and rate of lignin removal obtained with FTIR shows that the yield of cellulose is increasing; for the lignin removal efficiency and hemicelluloses after different pretreatments, the effect of pretreatment with sodium hydroxide is relatively better than that with sulfuric acid under the same conditions, with an obvious enhancement of cellulose output with sodium hydroxide at 135 ℃, as shown by the 29.6% increase in the average ratio relative to native bamboo; for sodium hydroxide treatment at 117 ℃, the removal of lignin reaches to 14.69%. Investigations of the cellulose depolymerization behavior in the pretreatment shows that t all of the pretreatments, relatively low DP values are obtained, the reason might that pretreatment processing could change the super molecular structure of plant fiber raw material, that lead to changes for cellulose crystalline region size, on the other hand, better pretreatment can be part of the degradation of lignin and hemicellulose, increasing accessibility. The yield of holocellulose and cellulose increased significantly after pretreatment, with a stronger effect observed for dilute alkali (NaOH) than for dilute acid (H2SO4) and glycerin (glycerin). The XRD results exhibit a high and sharp peak, with an obvious enhancement of the diffraction peak intensity and large changes in the angle of the 002 peak after pretreatment.
| [] | Abdulkhani A, Hosseinzadeh J, Ashori A, et al. 2014. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polymer Testing, 35 : 73–79. DOI:10.1016/j.polymertesting.2014.03.002 |
| [] | Chundawat S P S, Beckham G T, Himmel M E, et al. 2011. Deconstruction of lignocellulosic biomass to fuels and chemicals. Chem Biomol Eng, 2 : 121–145. DOI:10.1146/annurev-chembioeng-061010-114205 |
| [] | Foreman D W, Jakes K A. 1993. X-Ray diffractometric measurement of microcrystallite size, unit cell dimensions and crystallinity:application to cellulosic marine textiles. Textile Res J, 63(8) : 455–464. DOI:10.1177/004051759306300805 |
| [] | Gabov K, Gosselink R J A, Smeds A I, et al. 2014. Characterization of lignin extracted from birch wood by a modified hydrotropic process. Agric Food Chem, 62(44) : 10759–10767. DOI:10.1021/jf5037728 |
| [] | Himmel M E, Ding S Y, Johnson D K, et al. 2007. Biomass recalcitrance:engineering plants and enzymes for biofuels. Prod Sci, 315(5813) : 804–807. |
| [] | Inari G N, Petrissans M, Gerardin P. 2007. Chemical reactivity of heat-treated wood. Wood Science and Technology, 41(2) : 157–168. DOI:10.1007/s00226-006-0092-7 |
| [] | Karp E M, Resch M G, Donohoe B S, et al. 2015. Alkaline pretreatment of switchgrass. ACS Sustainnble Chem Eng, 3 : 1479–1491. DOI:10.1021/acssuschemeng.5b00201 |
| [] | Li J. 2003. Wood spectroscopy. Beijing: Science Press: 104-110. |
| [] | Li M F, Fan Y M, Xu F, et al. 2010. Ind Crop Prod. Cold sodium hydroxide/urea based pretreatment of bamboo for bioethanol production:characterization of the cellulose rich fraction, 32(3) : 551–559. |
| [] | Li Z Q, Jiang Z H, Fei B H, et al. 2014. Bioresour Technol. Comparison of bamboo green, timber and yellow in sulfite, sulfuric acid and sodium hydroxide pretreatments for enzymatic saccharification, 151 : 91–99. |
| [] | Liu Y P, Hu H. 2008. Fibers Polym. X-ray diffraction study of bamboo fibers treated with NaOH, 9(6) : 735–739. |
| [] | Mansfield S D, Mooney C, Saddler J N. 1999. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol Progr, 15(5) : 804–816. DOI:10.1021/bp9900864 |
| [] | Merino-Pérez O, Martínez-Palou R, Labid J, et al. 2014. Microwave-assisted pretreatment of lignocellulosic biomass to produce biofuels and value-added products. Biofuels and Biorefineries, 3 : 197–224. |
| [] | Nishimiya K, Hata T, Imamura Y, et al. 1998. Analysis of chemical structure of wood charcoal by X-ray photoelectron spectroscopy. Journal of Wood Science, 44(1) : 56–61. DOI:10.1007/BF00521875 |
| [] | Rezende C A, de Lima M A, Maziero P, et al. 2011. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnology for Biofuels, 4 : 1–18. DOI:10.1186/1754-6834-4-1 |
| [] | Segal L, Creely J J, Martin A E, et al. 1959. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Res J, 29(10) : 786. |
| [] | Shao S, Wen G, Jin Z. 2008. Changes in chemical characteristics of bamboo (Phyllostachys pubescens) components during steam explosion. Wood Sci Technol, 42 : 439–451. DOI:10.1007/s00226-008-0183-8 |
| [] | Shimokawa T, Ishida M, Yoshida S, et al. 2009. Effects of growth stage on enzymatic saccharification and simultaneous saccharification and fermentation of bamboo shoots for bioethanol production. Bioresour Technol, 100(24) : 6651–6654. DOI:10.1016/j.biortech.2009.06.100 |
| [] | Singh S B, Bharadwaja S T P, Yadav P K, et al. 2014. Mechanistic investigation in ultrasound-assisted (alkaline) delignification of Parthenium hysterophorus biomass. Ind Eng Chem Res, 53 : 14241–14252. |
| [] | Sun Y, Lin L. 2010. Hydrolysis behavior of bamboo fiber in formic acid reaction system. J Agric Food Chem, 58 : 2253–2259. DOI:10.1021/jf903731s |
| [] | Tamura N, MacDowell A A, Spolenak R, et al. 2003. Scanning X-ray microdiffraction with submicrometer white beam for strain/stress and orientation mapping in thin films. J Synchrotron Rad, 10 : 137–143. DOI:10.1107/S0909049502021362 |
| [] | Wang G, Li W, Li B, et al. 2008. TG study on pyrolysis of biomass and its three components under syngas. Fuel, 87(4/5) : 552–558. |
| [] | Xie J, Hse C Y, Shupe T F, et al. 2015. Physicochemical characterization of lignin recovered from microwave-assisted delignified lignocellulosic biomass for use in biobased materials. Appl Polym Sci, 132(40) : 42635. |
| [] | Xin D L, Yang Z, Liu F, et al. 2015. Comparision of aqueous ammonnia and dilute acid pretreatment of bamboo fractions:struture properties and enzymatic hydrolysis. Bioresour. Technol, 175 : 529–536. DOI:10.1016/j.biortech.2014.10.160 |
| [] | Yang H P, Yan R, Chen H P, et al. 2007. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel, 86(12/13) : 1781–1788. |
| [] | Yang Z, Xu S, Ma X, et al. 2008. Characterization and acetylation behavior of bamboo pulp. Wood Science and Technology, 42(8) : 621–632. DOI:10.1007/s00226-008-0194-5 |
| [] | Zhang D S, Yang Q, Zhu J Y, et al. 2013. Sulfite (SPORL) pretreatment of switchgrass for enzymatic saccharification. Bioresour. Technol, 129 : 127–134. DOI:10.1016/j.biortech.2012.11.031 |
| [] | Zhao Y, Yan N, Feng M. 2012. Polyurethane foams derived from liquefied mountain pine beetle-infested barks. Appl Polym Sci, 123(5) : 2849–2858. DOI:10.1002/app.v123.5 |
| [] | Zhou J, Zhang L, Cai J. 2004. Behavior of cellulose in NaOH/urea aqueous solution characterized by light scattering and viscometry. J Polym Sci Pol Phys, 42(2) : 347–353. DOI:10.1002/(ISSN)1099-0488 |
 2017, Vol. 53
2017, Vol. 53

