文章信息
- 李安鑫, 吕建雄, 蒋佳荔
- Li Anxin, Lü Jianxiong, Jiang Jiali
- 木材细胞壁结构及其流变特性研究进展
- A Review of Wood Cell Wall Structure and Its Rheological Property
- 林业科学, 2017, 53(12): 136-143.
- Scientia Silvae Sinicae, 2017, 53(12): 136-143.
- DOI: 10.11707/j.1001-7488.20171215
-
文章历史
- 收稿日期:2017-01-07
- 修回日期:2017-08-10
-
作者相关文章
流变学(rheology)主要研究材料在应力/应变、温度、湿度等条件下与时间因素有关的变形规律和机制,以研究材料的黏弹性为主要内容。木材是一种生物高分子聚合物材料,其对应力的响应同时体现弹性固体和黏性流体的双重特性,即黏弹性。木材黏弹性主要研究木材在应力作用下所发生的与时间因素有关的变形规律和机制,以蠕变研究为典型代表。蠕变是在恒定应力作用下木材应变随时间增加而增大的现象,木材发生蠕变时时,其质际承载结构是细胞壁,细胞壁的壁层构造和化学组分对木材宏观黏弹行为有着极其重要的影响(Salmén et al., 2009;Navi et al., 2009)。Eder等(2006)首次联合利用厚度为200 μm的木材组织切片和单根纤维拉伸技术,分别从组织和细胞壁水平上揭示了S2层微纤丝角对木材力学松弛行为的影响。Zhang等(2012)首次采用原位纳米压痕技术研究了木材细胞壁的黏弹行为。此外,对于木材单根纤维(本文特指针叶材的管胞和阔叶材的木纤维)而言,深入了解其黏弹性及湿热软化机制,对于实现木纤维/塑料复合材料的高效设计具有重要意义,并可为高效节能的制浆造纸工艺设计提供科学依据。因此,将木材流变学研究从宏观引向微观尺度,从细胞壁水平上揭示木材结构与黏弹性之间的关系及其影响因子,才能真正掌握木材黏弹行为的作用机制。然而,在我国木材科学领域,目前关于细胞壁水平的木材黏弹行为研究较少,尤其是围绕细胞壁结构与黏弹性关系的系统研究更少。
鉴于此,本文从归纳木材细胞壁构造和化学组分的最新研究成果入手,围绕微纤丝角和化学组分对木材细胞壁黏弹行为的影响规律与作用机制进行综述,总结开展木材细胞壁黏弹性研究的测试方法和技术手段,并且提出今后进一步开展这方面工作的建议与设想。
1 木材细胞壁结构 1.1 细胞壁S2层超微构造微纤丝在细胞壁各层的沉积主要取决于细胞的几何尺寸、纤维素分子链的数量以及微纤丝之间的横向距离(Emons et al., 1998;2000)。由于S2层最厚,占细胞壁质量的80%以上,其对木材宏观性质具有决定性影响,因此S2层一直是木材细胞壁结构研究的关键与热点。
随着现代显微技术的不断发展,透射电子显微镜(TEM)、原子力显微镜(AFM)和小角X-射线散射仪(SAXS)的应用,木材细胞壁S2层的超微构造逐渐被揭示。人们认识到,细胞壁纤维素聚集体(cellulose aggregate)的尺寸分布范围为3.5~30 nm(Fahlén et al., 2003;2005;Bardage et al., 2004;Jungnikl et al., 2007),平均尺寸为16~20 nm,具有吸湿性,与水分子作用会引起横向尺寸发生较大变化,因此可推测纤维素聚集体内存在部分结晶的纤维素或无定形的半纤维素(Salmén et al., 2006a)。Andersson等(2015)研究了银杏(Ginkgo biloba)木材沿髓心至树皮方向细胞壁S2层纤维素的微晶尺寸分布,结果发现纤维素微晶的平均宽度为3.1~3.2 nm,长度为27.5~30.0 nm。Åkerholm等(2001;2003)利用动态傅里叶红外光谱(FTIR)证明了针叶材的纤维素与葡甘露聚糖之间存在紧密连接,在外力作用下2个组分的分子性能显示二者之间有较强的交互作用。Joseleau(2007)研究表明,对于阔叶材,半纤维素包括低取代度的木聚糖和高取代度的木聚糖2种类型,其中低取代度的木聚糖首先沉积于微纤丝上并与之形成紧密连接,从而增加了微纤丝的聚合度。Bardage等(2004)利用快速冷冻和深度蚀刻技术(RFDE)揭示了针叶材管胞S2层的微纤丝沿细胞轴向呈波浪形聚集态分布,相邻的纤维素聚集体之间形成纺锤形状,其横向直径为3~14 nm(图 1),证实了之前Boyd(1982)所提出的木材细胞壁微纤丝排列呈纺锤状的设想。正是根据S2层的这种结构特点,可以对“湿热处理或脱部分Matrix物质(全部由半纤维素和木质素组成)处理会引起纤维素聚合度增加” (Duchesne et al., 2000;Hult et al., 2001;Fahlén et al., 2003)的现象做出解释:沿细胞壁轴向,纤维素聚集体之间被Matrix物质填充,经过破坏半纤维素的湿热处理和脱Matrix物质处理后,相邻纤维素聚集体之间的距离减小、接触面积增大,从而使纤维素聚合度增加(Salmén,2006)。关于纤维素聚集体的空间排列方式,目前的观点认为是沿细胞轴向呈同心圆的层状排列(Fahlé et al., 2005;Salmén et al., 2006a)。
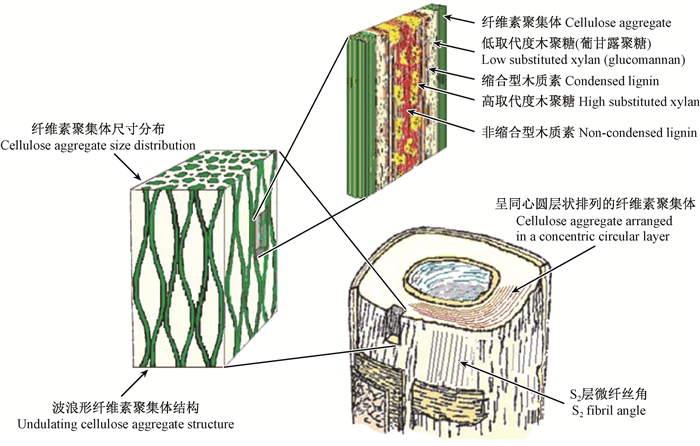
|
图 1 针叶材管胞壁结构示意(Salmén et al., 2009) Figure 1 Schematic illustration of tracheid wall structure of softwood |
诸多研究表明,木材细胞壁S2层中不同类型木质素是以特定方式与不同种类的半纤维素相结合的(Lawoko et al., 2005;Joseleau et al., 2005)。Joseleau等(2005)采用免疫标记法研究了位于纤维素聚集体之间的Matrix中半纤维素和木质素的结构排列,在细胞壁形成的不同阶段,观察到了非缩合型木质素(包括愈创木基结构和紫丁香基结构)、缩合型木质素(愈创木基结构)和多种木聚糖的沉积,提出了“缩合型木质素和低取代度木聚糖的早期沉积会将Matrix与纤维素聚集体更紧密地联系在一起”的观点。Lawoko等(2005)对云杉(Picea asperata)的木质素-碳水化合物复合体(LCC)进行了化学分析,发现高缩合型木质素与葡甘露聚糖之间、低缩合型木质素与木聚糖之间均存在化学连接。对于阔叶材,低取代度木聚糖在纤维素与缩合型木质素之间起连接作用,而高取代度木聚糖与非缩合型木质素之间的结合更为紧密。对于针叶材,低取代度木聚糖的位置被葡甘露聚糖取代,如图 1所示。至于纤维素聚合体内部的半纤维素与微纤丝之间是否存在交联作用以及交联的方式,目前仍不清楚(Terashima et al., 2004)。由此可见,木材细胞各化学组分之间的连接与相互作用极其复杂,其共同决定了细胞壁的物理力学性能(Salmén et al., 1998)。
1.2 细胞壁的化学组分木材细胞壁主要由纤维素、半纤维素和木质素构成。针对单个细胞而言,可视为由微纤丝(全部由纤维素构成)与Matrix(全部由木质素和半纤维素构成)组成。对于纤维素,过去30年来研究取得的最大进展是对其Iα和Iβ晶型结构的认识(Atalla et al., 1984)。迄今为止,关于2种晶型结构的差异是否会对纤维素的力学性质产生影响仍不清楚。据报道,结晶纤维素的刚度约为134 GPa(Nishino et al., 1995);然而,纤维素作为细胞壁的骨架物质,其有效刚度仍亟待研究。此外,对于纤维素聚集体的结晶度与刚度之间的关系也亟待阐明。对于半纤维素和木质素,由于处于分离状态和原位状态时其分子结构和空间排列均存在很大差异,因此,木材细胞壁半纤维素和木质素单一组分的力学参数测定有赖于细胞壁原位测试技术的进步(Salmén et al., 2009)。Takeichi等(2013)利用原位测试技术获得日本柳杉(Cryptomeria japonica)细胞壁木质素的拉伸弹性模量为2.8 GPa。
纤维素、半纤维素和木质素之间的键合方式对木材细胞壁性能有重要影响。迄今为止,未发现纤维素与木质素之间存在直接交联。而具有双亲性的半纤维素:一方面,通过氢键与纤维素之间建立物理连接;另一方面,与木质素之间既存在物理连接,同时也存在化学连接(酯键、醚键、苷键等共价键)。Westbye等(2007)研究证实了木质素与木聚糖之间通过共价键连接,揭示了木聚糖同时沉积于木质素和纤维素表面。有研究指出,葡甘露聚糖的脱除,能增强木质素与纤维素之间的交联,可揭示木质素对细胞壁轴向刚度的贡献(Salmén et al., 2016)。由此可见,木材细胞壁是通过半纤维素将刚性的、亲水性的纤维素与黏性的、疏水性的木质素联系在一起,从而维持细胞壁的整体性(图 1)。
2 木材细胞壁的黏弹性围绕木材细胞壁黏弹性的研究,考察的对象主要有3类:1)拉伸模式下厚度不大于200 μm的组织切片(Kojima et al., 2004;2005;Roszyk et al., 2010;2012);2)拉伸模式下的单根纤维(Eder et al., 2006;Dong et al., 2010;Olsson et al., 2014);3)单个细胞壁横截面的原位压痕(Zhang et al., 2012;Meng et al., 2015)。所涉及的研究内容主要是从材料内因(微纤丝角、化学组分)和环境外因(主要是水分)2个角度探讨木材细胞壁黏弹性的响应机制。本文分别以“细胞壁S2层微纤丝角”和“细胞壁的化学组分”对木材细胞壁黏弹性的影响为主线,同时将水分通过改变细胞壁结构进而影响其黏弹性的研究结果穿插其中,归纳了近年来木材细胞壁流变学的研究进展和取得的主要结论。
2.1 细胞壁S2层微纤丝角细胞壁S2层微纤丝角对木材细胞壁的蠕变行为有显著影响。诸多研究表明,木材细胞壁纵向拉伸蠕变变形量随着微纤丝角的增加而增大(Kojima et al., 2004;2005;Dong et al., 2010;Roszyk et al., 2010;2012)。可通过木材细胞壁的复合结构理论来解释这一现象:木材细胞壁可视为由Matrix与纤维素微纤丝构成,其中,Matrix全部由半纤维素和木质素构成,其黏性大,对木材细胞壁的蠕变变形起主要贡献;而纤维素微纤丝具有高刚度,包裹于Matrix中,作为其增强相存在。当微纤丝角很小时,微纤丝沿细胞轴向的刚度分量大,制约了Matrix沿轴向的蠕变变形;随着微纤丝角增大,微纤丝沿细胞轴向的刚度分量减小,即对Matrix沿轴向蠕变变形的约束力减弱,使得木材细胞的轴向蠕变变形量增加(Engelund et al., 2011;Roszyk et al., 2013)。
微纤丝角不同是造成早材与晚材、幼龄材与成熟材、应力木与正常材之间蠕变存在差异的主要原因(Dong et al., 2010;Brémaud et al., 2013;Sharma et al., 2015)。Dong等(2010)研究了挪威云杉(Picea abies)早材与晚材、成熟材与幼龄材的单根纤维蠕变行为,结果表明:早材的微纤丝角比晚材大,早材的蠕变变形量大于晚材;幼龄材的微纤丝角大于成熟材,是幼龄材蠕变变形量较大的原因。Roszyk等(2012)研究了欧洲赤松(Pinus sylvestris)蠕变变形量与微纤丝角之间的关系,揭示了当微纤丝角为10°~18°时,蠕变变形量与微纤丝角之间呈线性正相关;当微纤丝角高于18°时,蠕变变形量显著增大。Brémaud等(2013)比较研究了正常材和应压木的动态刚度与阻尼性质,证实了应压木的微纤丝角大于正常材,使得前者具有较低的动态刚度和较高的阻尼。围绕微纤丝角对应力木与正常材细胞壁应力松弛行为的影响,Eder等(2006)以挪威云杉应压木和正常材为试验材料,联合利用厚度为200 μm的木材组织切片和单根纤维拉伸技术开展应力松弛测定,揭示了与具有较小微纤丝角(10°~20°)的正常材组织切片及单根纤维相比,具有较大微纤丝角(40°~ 45°)的应压木组织切片和单根纤维的应力松弛行为更显著。
此外,木材细胞壁的水分变化也会引起微纤丝角发生改变,进而影响细胞壁的蠕变行为。诸多研究表明,木材细胞壁纵向拉伸蠕变变形量随着平衡含水率的增加而增大(Kojima et al., 2005;Roszyk et al., 2010;2012;Engelund et al., 2012)。原因在于:当水分子进入木材细胞壁时,会切断细胞壁聚合物分子之间的氢键连接,进而与非结晶纤维素、半纤维素和木质素的羟基、羧基、羰基等极性基团之间形成新的氢键。一方面,加大了分子链之间的距离, “自由体积”增加,使得分子链的延展性增强;另一方面,Matrix发生各向同性的吸湿润胀,会对微纤丝产生横向作用力,引起微纤丝角增大,促使微纤丝与Matrix之间发生剪切滑移,最终使得细胞壁的轴向蠕变变形量增加(Navi et al., 2002;Placet et al., 2007;Engelund et al., 2011;Toba et al., 2013)。Kojima等(2005)以日本柳杉早材组织切片为研究对象,在不同微纤丝角条件下探讨了纤维饱和点以下的平衡含水率对细胞壁纵向拉伸蠕变性能的影响,结果表明:当微纤丝角很小(12.0°)时,含水率增加对细胞壁纵向拉伸蠕变的影响甚微,这是因为此时的微纤丝对Matrix发生纵向吸湿润胀的束缚力大,制约了细胞壁的纵向蠕变变形;当微纤丝角增大(20.4°,29.8°、44.1°)时,微纤丝对Matrix发生纵向吸湿润胀的约束力减弱,此时含水率增加会引起细胞壁纵向拉伸蠕变变形量明显增大。
当木材细胞壁中的水分处于动态变化时,在恒定外力下所产生的蠕变变形量比同载荷高平衡含水率下的蠕变变形量要大得多,这就是机械吸湿蠕变现象(Olsson et al., 2007;2014;Dong et al., 2010;Roszyk et al., 2010;Violaine et al., 2015)。当发生机械吸湿蠕变时,木材往往在较低的应力水平、较短的时间内就发生破坏。然而,关于木材单根纤维是否存在机械吸湿蠕变现象曾存在争议:Sedlachek等(1994;1995)以采用化学分离法获得的木材单根纤维为研究对象,没有观察到机械吸湿蠕变现象;Salmén等(2006b)、Navi等(2006)和Meylan(2006)通过机械分离法获得了细胞次生壁中天然化学组分保持完整的木材单根纤维(Burgert et al., 2005),蠕变试验结果表明,单根纤维在相对湿度循环变化条件下的蠕变变形量要比在高恒定相对湿度下的蠕变变形量明显增大,从而证明了木材单根纤维具有机械吸湿蠕变效应。由此可认为,单根纤维是否存在机械吸湿蠕变现象取决于纤维的分离方式。Roszyk等(2010)在7%~25%含水率范围内研究了微纤丝角对木材组织切片轴向机械吸湿蠕变的影响,结果发现:当微纤丝角为12°~18°时,机械吸湿蠕变变形量差异很小;当微纤丝角超过18°时,机械吸湿蠕变变形量随微纤丝角的增大明显增加。此外,随着微纤丝角增大,细胞壁的弹性变形稍有降低,而塑性变形显著增加,这说明微纤丝角的变化主要影响木材机械吸湿蠕变的塑性变形部分。
2.2 细胞壁的化学组分木材细胞壁的纤维素、半纤维素、木质素以及抽提物的性质及其在细胞壁中的作用均存在较大差异,分别研究其在细胞壁中的相对含量、分布方式、结构特点与木材细胞壁黏弹性之间的关系,有利于从本质上认识木材黏弹性的发生和演变机制。
木材单根纤维受纵向拉伸时,细胞壁中的纤维素为主要承载物质,其在细胞各壁层的取向与结晶度对细胞壁的弹性有显著影响(Bergander et al., 2003)。Gierlinger等(2006)利用拉曼光谱技术分析了纵向拉伸过程中单根纤维细胞壁内部分子键的变形情况,发现纤维素的特征峰发生了明显偏移,而木质素的特征峰未发生明显变化,从而证实了纤维素是木材单根纤维纵向拉伸时的主要承载物质,对细胞壁的纵向刚度起决定作用。类似地,Salmén等(2004)通过动态傅里叶红外光谱技术揭示了细胞壁主要通过纤维素的C—O—C骨架震动来承载和抵抗纵向变形。此外,纤维素结晶区的宽度对细胞壁的阻尼性质有较大影响(Hori et al., 2002)。有研究指出,半纤维素和木质素也可以增加细胞壁的刚性,但贡献度低于纤维素(Scheller et al., 2010;Salmén et al., 2016)。
木材细胞壁的黏性主要由木质素和半纤维素共同决定,尤其是细胞壁的横向性能(Bergander et al., 2003;Salmén,2004;Sharma et al., 2015)。木质素含量、类型或性质的不同会引起木材细胞壁黏弹性的差异,例如,与正常材相比,应压木具有较高的缩合型木质素含量,因此后者的阻尼仅为前者的66%(Brémaud et al., 2013)。Sharma等(2015)研究指出,木质素和木聚糖的含量对应压木的阻尼性质有显著影响。在有水分参与的情况下,由于木质素和半纤维素的吸湿能力不同,二者对木材细胞壁黏弹性的影响存在差异:木质素的吸湿性弱,受水分影响小,随着脱木质素程度的增加,细胞壁中的吸着点增加,吸湿能力增强,使得机械吸湿蠕变明显增大(Zhang et al., 2006a;2006b;2007;Olsson et al., 2014);半纤维素的吸湿性强,木聚糖和葡甘露聚糖的部分脱除对木材单根纤维机械吸湿蠕变的影响不显著(Olsson et al., 2014),但完全脱除半纤维素会引起木材单根纤维机械吸湿蠕变显著降低(Fioravanti et al., 2006;Navi et al., 2009)。
此外,近年来的一些研究指出,细胞壁中的抽提物会影响其黏弹性,引起细胞壁的刚性增加、黏滞性降低,对于细胞壁的湿热软化是不利因素(Bag et al., 2011;Song et al., 2014)。
3 木材细胞壁黏弹性的测试技术动态力学分析技术(DMA)和纳米压痕技术(nanoindentation)的发展,为从组织切片、单根纤维和细胞壁原位测定等不同层次上研究木材结构与黏弹性之间的关系提供了新的技术手段。
利用动态力学分析技术可以实现组织切片或单根纤维的轴向拉伸,获得动态或静态黏弹性参数。当单根纤维受到拉伸作用时,基于细胞壁S2层中的微纤丝沿细胞轴呈“Z螺旋型”取向(Meylan et al., 1978),微纤丝往往会沿轴向发生旋转,引起细胞壁中的分子变形(Salmén et al., 2004;Gierlinger et al., 2006)。具体表现(图 2)为:在拉伸应力作用下,细胞壁S2层的微纤丝角变小(MFA1<MFA0),同时,纤维素分子链中2个葡萄糖分子之间的“C—O—C”键被拉长(L1>L0)。联合利用单根纤维拉伸技术和光谱分析技术(偏光显微镜、激光共聚焦显微镜、X-射线散射/衍射技术等),可以从微纤丝角、晶格和分子键的变化等方面揭示细胞壁在轴向拉伸过程中黏弹性变形的演变机制和分子机制(Kamiyama et al., 2005;Kölln et al., 2005;Marjan et al., 2007;Peura et al., 2007;Thygesen et al., 2007)。
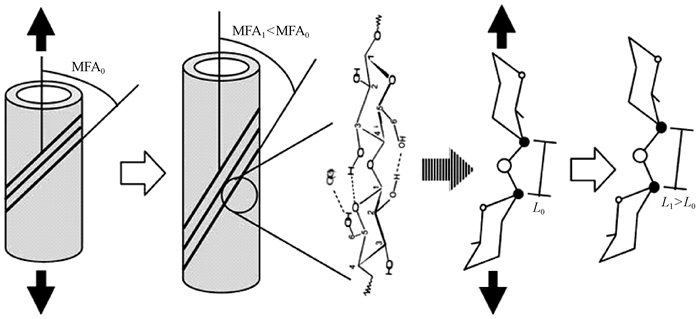
|
图 2 拉伸应力下细胞壁S2层微纤丝C—O—C键的分子变形示意(Salmén et al., 2009) Figure 2 Schematic illustration of molecular deformation of C—O—C bridge in microfibril in S2 layer of cell wall under tensile stress |
近年来,利用纳米压痕技术可以实现在亚微米水平下直接测量木材细胞壁的黏弹性质(Zhang et al., 2012;Meng et al., 2015),主要内容包括静态恒定应力作用下的蠕变现象及动态交变应力作用下的滞后现象和力学损耗。Zhang等(2012)利用纳米压痕技术测定了苦油楝(Carapa procera)木材细胞壁蠕变柔量与时间的关系曲线、贮存模量和损耗因子与载荷频率的关系曲线,揭示了木材细胞壁的蠕变速率与应力水平呈正相关,压痕深度与载荷频率对木材细胞壁的贮存模量和损耗因子有显著影响。Meng等(2015)采用配备环境湿度控制装置的纳米压痕仪测定了火炬松(Pinus taeda)木材细胞壁在不同平衡含水率(0%、6%、18%和110%)下的蠕变行为,并分别利用Burgers模型和Maxwell模型对蠕变曲线进行拟合分析,揭示了木材细胞壁的蠕变柔量随着平衡含水率的增加而增大,阐明了水分所引起的细胞壁结构变化对其蠕变性能的影响。
4 总括与科学问题随着微观力学表征技术的不断发展,有关木材细胞壁结构与黏弹性关系的研究也在逐渐深化。然而总体来看,目前国内围绕木材细胞壁流变学的研究尚未系统开展;国际上近20年在该领域取得的最突出进展仅为:微纤丝角对木材细胞壁黏弹性影响的显著性不断被研究者所证实(Salmén et al., 2009)。根据上述关于木材细胞壁结构及其流变学的研究现状分析,综合学科发展和研究趋势,本文提出一些亟待解决的科学问题和深化研究的建议。
木材细胞壁黏弹性宏观性能是细胞壁微观分子结构的反映,准确认识和掌握木材细胞壁结构才能对其黏弹行为的发生和演变机制做出合理解释。因此,围绕木材细胞壁结构的深化研究,应集中在:1)释明木材细胞生长过程中的微纤丝取向、纤维素结晶区与非结晶区比例的分子控制机制;2)阐明木材细胞壁次生壁Matrix的空间组织排列方式、纤维素聚合体与Matrix之间相互作用的力学行为表达;3)揭示木材细胞壁中半纤维素的含量、种类以及木质素类型对细胞壁黏弹性的影响机制。在此基础上,将环境外因(温度、湿度)和载荷类型(静态/动态、拉/压/弯)纳入研究体系,系统揭示“湿-热-力”协同作用下木材细胞壁的机械吸湿蠕变行为规律和响应机制。另外,多种测试技术手段的联合运用,相关学科研究方法及理论模型的引入,如有限元法和复合材料的研究方法的应用,构建可以解释木材细胞壁黏弹特性的物理和数学模型,也势在必行。
Åkerholm M, Salmén L. 2001. Interactions between wood polymers studied by dynamic FT-IR spectroscopy[J]. Polymer, 42(3): 963-969. DOI:10.1016/S0032-3861(00)00434-1 |
Åkerholm M, Salmén L. 2003. The oriented structure of lignin and its viscoelastic properties studied by static and dynamic FT-IR spectroscopy[J]. Holzforschung, 57(5): 459-465. |
Andersson S, Wang Y, Pönni R, et al. 2015. Cellulose structure and lignin distribution in normal and compression wood of the maidenhair tree (Ginkgo biloba L.)[J]. Journal of Integrative Plant Biology, 57(4): 388-395. DOI:10.1111/jipb.12349 |
Atalla R H, VanderHart D L. 1984. Native cellulose:a composite of two distinct crystalline forms[J]. Science, 223(4633): 283-285. DOI:10.1126/science.223.4633.283 |
Bag R, Beaugrand J, Dole P, et al. 2011. Viscoelastic properties of woody hemp core[J]. Holzforschung, 65(2): 239-247. |
Bardage S, Donaldson L, Tokoh C, et al. 2004. Ultrastructure of the cell wall of unbeaten norway spruce pulp fibre surfaces[J]. Nordic Pulp and Paper Research Journal, 19(4): 448-482. DOI:10.3183/NPPRJ-2004-19-04-p448-452 |
Bergander A, Salmén L. 2003. Cell wall properties and their effects on the mechanical properties of fibers[J]. Radiation Protection Dosimetry, 103(2): 103-109. DOI:10.1093/oxfordjournals.rpd.a006120 |
Boyd J D. 1982. An anatomical explanation for visco-elastic and mechano-sorptive creep in wood, and effects of loading rate on strength//New perspectives in wood anatomy. Springer Netherlands, 171-222. http://link.springer.com/content/pdf/10.1007/978-94-017-2418-0_8.pdf
|
Brémaud I, Ruelle J, Thibaut A, et al. 2013. Changes in viscoelastic vibrational properties between compression and normal wood:role of microfibril angle and of lignin[J]. Holzforschung, 67(1): 75-85. |
Burgert I, Eder M, Frühmann K, et al. 2005. Properties of chemically and mechanically isolated fibres of spruce (Picea abies[L.] Karst.). Part 3:Mechanical characterisation[J]. Holzforschung, 59(3): 354-357. |
Dong F, Olsson A M, Salmén L. 2010. Fibre morphological effects on mechano-sorptive creep[J]. Wood Science and Technology, 44(3): 475-483. DOI:10.1007/s00226-009-0300-3 |
Duchesne I, Daniel G. 2000. Changes in surface ultrastructure of Norway spruce fibres during kraft pulping-visualization by field emission-SEM[J]. Nordic Pulp and Paper Research Journal, 15(1): 54-61. DOI:10.3183/NPPRJ-2000-15-01-p054-061 |
Eder M, Burgert I, Stanzl-Tschegg S. 2006. Relaxation experiments on wood fibres and tissues[J]. Proceedings of the Third International Conference of the European Society of Wood Mechanics: 141-147. |
Emons A M C, Mulder B M. 1998. The making of the architecture of the plant cell wall:how cells exploit geometry[J]. Proceedings of the National Academy of Sciences, 95(12): 7215-7219. DOI:10.1073/pnas.95.12.7215 |
Emons A M, Mulder B M. 2000. How the deposition of cellulose microfibrils builds cell wall architecture[J]. Trends in Plant Science, 5(1): 35-40. DOI:10.1016/S1360-1385(99)01507-1 |
Engelund E T, Svensson S. 2011. Modelling time-dependent mechanical behaviour of softwood using deformation kinetics[J]. Holzforschung, 65(2): 231-237. |
Engelund E T, Salmén L. 2012. Tensile creep and recovery of norway spruce influenced by temperature and moisture[J]. Holzforschung, 66(8): 959-965. |
Fahlén J, Salmén L. 2003. Cross-sectional structure of the secondary wall of wood fibers as affected by processing[J]. Journal of Materials Science, 38(1): 119-126. DOI:10.1023/A:1021174118468 |
Fahlén J, Salmén L. 2005. Pore and matrix distribution in the fibre wall revealed by atomic force microscopy and image analysis[J]. Biomacromolecules, 6(1): 433-438. DOI:10.1021/bm040068x |
Fioravanti M, Sodini N, Navi P. 2006. Investigation of the influence of hemicelluloses on time dependent behaviour of wood. Proceedings of the International Conference on Integrated Approach to Wood Structure, Behaviour and Application, 190-195.
|
Gierlinger N, Schwanninger M, Reinecke A, et al. 2006. Molecular changes during tensile deformation of single wood fibers followed by Raman microscopy[J]. Biomacromolecules, 7(7): 2077-2081. DOI:10.1021/bm060236g |
Hori R, Müller M, Watanabe U, et al. 2002. The importance of seasonal differences in the cellulose microfibril angle in softwoods in determining acoustic properties[J]. Journal of Materials Science, 37(20): 4279-4284. DOI:10.1023/A:1020688132345 |
Hult E L, Larsson P T, Iversen T. 2001. Cellulose fibril aggregation-an inherent property of kraft pulps[J]. Polymer, 42(8): 3309-3314. DOI:10.1016/S0032-3861(00)00774-6 |
Joseleau J P, Ruel K. 2005. Deposition of hemicelluloses and lignins during secondary wood cell wall assembly[J]. New Knowledge in Wood Quality: 103-113. |
Joseleau J P. 2007. Micro-scale approaches for wood cell wall analysis and structure//Entwistle K, Harris P, Walker J. The compromised wood workshop Univ. Canterbury, ISBN 0-473-12768-8, 113-124. https://www.researchgate.net/publication/278813078_Micro-scale_approaches_for_wood_cell_wall_analysis_and_structure
|
Jungnikl K, Paris O, Fratzl P, et al. 2007. The implication of chemical extraction treatments on the cell wall nanostructure of softwood[J]. Cellulose, 15(3): 407-418. |
Kamiyama T, Suzuki H, Sugiyama J. 2005. Studies of the structural change during deformation in Cryptomeria japonica by time-resolved synchrotron small-angle X-ray scattering[J]. Journal of Structural Biology, 151(1): 1-11. DOI:10.1016/j.jsb.2005.04.007 |
Kojima Y, Yamamoto H. 2004. Effect of microfibril angle on the longitudinal tensile creep behavior of wood[J]. Journal of Wood Science, 50(4): 301-306. |
Kojima Y, Yamamoto H. 2005. Effect of moisture content on the longitudinal tensile creep behavior of wood[J]. Journal of Wood Science, 51(5): 462-467. DOI:10.1007/s10086-004-0676-5 |
Kölln K, Grotkopp I, Burghammer M, et al. 2005. Mechanical properties of cellulose fibres and wood. Orientational aspects in situ investigated with synchrotron radiation[J]. Journal of Synchrotron Radiation, 12(6): 739-744. DOI:10.1107/S0909049505011714 |
Lawoko M, Henriksson G, Gellerstedt G. 2005. Structural differences between the lignin-carbohydrate complexes present in wood and in chemical pulps[J]. Biomacromolecules, 6(6): 3467-3473. DOI:10.1021/bm058014q |
Marjan S G, Navi P. 2007. Experimental observations and micromechanical modeling of successive-damaging phenomenon in wood cells' tensile behavior[J]. Wood Science and Technology, 41(1): 69-85. DOI:10.1007/s00226-006-0094-5 |
Meylan B A, Butterfield B G. 1978. Helical orientation of the microfibrils in tracheids, fibres and vessels[J]. Wood Science and Technology, 12(3): 219-222. DOI:10.1007/BF00372867 |
Meylan B. 2006. Characterization and modeling of the thermo-hydro-mechanical behavior of isolated wood fibres. Master thesis in Material and Science and Engineering, Ecole Poly-technique Fédérale de Lausanne, Lausanne, Switzerland.
|
Meng Y, Xia Y, Young T M, et al. 2015. Viscoelasticity of wood cell walls with different moisture content as measured by nanoindentation[J]. RSC Advances, 5: 47538-47547. DOI:10.1039/C5RA05822H |
Navi P, Pittet V, Plummer C J G. 2002. Transient moisture effects on wood creep[J]. Wood Science and Technology, 36(6): 447-462. DOI:10.1007/s00226-002-0157-1 |
Navi P, Meylan B, Plummer C J G. 2006. Role of hydrogen bonding in wood stress relaxation under humidity variation. In International Conference on Integrated Approach to Wood Structure, Behavior and Application, Joint Meeting of ESWM and COST Action E35.
|
Navi P, Stefanie S T. 2009. Micromechanics of creep and relaxation of wood. A review cost action E35 2004-2008:wood machining-micromechanics and fracture[J]. Holzforschung, 63(2): 186-195. |
Nishino T, Takano K, Nakamae K. 1995. Elastic modulus of the crystalline regions of cellulose polymorphs[J]. Journal of Polymer Science Part B:Polymer Physics, 33(11): 1647-1651. DOI:10.1002/polb.1995.090331110 |
Olsson A M, Salmén L, Eder M, et al. 2007. Mechano-sorptive creep in wood fibres[J]. Wood Science and Technology, 41(1): 59-67. DOI:10.1007/s00226-006-0086-5 |
Olsson A M, Salmén L. 2014. Mechano-sorptive creep in pulp fibres and paper[J]. Wood Science and Technology, 48(3): 569-580. DOI:10.1007/s00226-014-0624-5 |
Peura M, Kölln K, Grotkopp I, et al. 2007. The effect of axial strain on crystalline cellulose in Norway spruce[J]. Wood Science and Technology, 41(7): 565-583. DOI:10.1007/s00226-007-0141-x |
Placet V, Passard J, Perré P. 2007. Viscoelastic properties of green wood across the grain measured by harmonic tests in the range 0-95 C:hardwood vs. softwood and normal wood vs. reaction wood[J]. Holzforschung, 61(5): 548-557. |
Roszyk E, Moliński W, Jasińska M. 2010. The effect of microfibril angle on hygromechanic creep of wood under tensile stress along the grains[J]. Wood Research, 55(3): 13-24. |
Roszyk E, Mania P, Moliński W. 2012. The influence of microfibril angle on creep of scotch pine wood under tensile stress along the grains[J]. Wood Research, 57(3): 347-358. |
Roszyk E, Kwiatkowski T, Moliński W. 2013. Mechanical parameters of pine wood in individual annual rings under tensile stress along the grains in dry and wet state[J]. Wood Research, 58(4): 571-580. |
Salmén L, Olsson A M. 1998. Interaction between hemicelluloses, lignin and cellulose:structure-property relationships[J]. Journal of Pulp and Paper Science, 24(3): 99-103. |
Salmén L. 2004. Micromechanical understanding of the cell-wall structure[J]. Comptes Rendus Biologies, 327(9): 873-880. |
Salmén L, Åkerholm M, Hinterstoisser B. 2004. Two-dimensional Fourier transform infrared spectroscopy applied to cellulose and paper[J]. Polysaccharides:Structural Diversity and Functional Versatility: 159-187. |
Salmén L. 2006. Ultra-structural arrangement and rearrangement of the cellulose aggregates within the secondary cell wall. Proceedings of the Fifth Plant Biomechanics Conference, STFI-Packforsk, Stockholm, 215-220.
|
Salmén L, Fahlén J. 2006a. Reflections on the ultrastructure of softwood fibres[J]. Cell Chem Technol, 40: 181-185. |
Salmén L, Olsson A M, Eder M, et al. 2006b. Role of hydrogen bonding in wood stress relaxation under humidity variation. Proceedings of International Conference on Integrated Approach to Wood Structure, Behaviour and Application, Joint Meeting of ESWM and COST Action E35, 87-91.
|
Salmén L, Burgert I. 2009. Cell wall features with regard to mechanical performance. A review COST Action E35 2004-2008:wood machining-micromechanics and fracture[J]. Holzforschung, 63(2): 121-129. |
Salmén L, Stevanic J S, Olsson A M. 2016. Contribution of lignin to the strength properties in wood fibres studied by dynamic FTIR spectroscopy and dynamic mechanical analysis (DMA)[J]. Holzforschung, 70(12): 1155-1163. |
Scheller H V, Ulvskov P. 2010. Hemicelluloses[J]. Annual Review of Plant Physiology, 61(1): 263-289. |
Sedlachek K M, Ellis R L. 1994. The effect of cyclic humidity on the creep of single fibres of southern pine. Moisture-induced Creep Behaviour of Paper and Board, STFI USDA Stockholm, 22-49.
|
Sedlachek K M. 1995. The effect of hemicelluloses and cyclic humidity on the creep of single fibres. PhD thesis, Institute of Paper Science and Technology, Atlanta, University of Georgia Tech, Atlanta, GA, USA. https://www.researchgate.net/publication/27522948_The_effect_of_hemicelluloses_and_cyclic_humidity_on_the_creep_of_single_fibers
|
Sharma M, Brennan M, Chauhan S S, et al. 2015. Wood quality assessment of Pinus radiata saplings by dynamic mechanical analysis[J]. Wood Science and Technology, 49(6): 1239-1250. DOI:10.1007/s00226-015-0769-x |
Song K, Yin Y, Salmén L, et al. 2014. Change in the properties of wood cell walls during the transformation from sapwood to heartwood[J]. Journal of Material Science, 49(4): 1734-1742. DOI:10.1007/s10853-013-7860-1 |
Takeichi Y, Yoshida M, Kitano K, et al. 2013. In situ measurement of tensile elastic moduli of individual component polymers with a 3D assembly mode in wood cell walls[J]. Journal of Wood Science, 59(2): 104-111. DOI:10.1007/s10086-012-1315-1 |
Terashima N, Awano T, Takabe K, et al. 2004. Formation of macromolecular lignin in ginkgo xylem cell walls as observed by field emission scanning electron microscopy[J]. Comptes Rendus Biologies, 327(9): 903-910. |
Thygesen L G, Eder M, Burgert I. 2007. Dislocations in single hemp fibres-investigations into the relationship of structural distortions and tensile properties at the cell wall level[J]. Journal of Materials Science, 42(2): 558-564. DOI:10.1007/s10853-006-1113-5 |
Toba K, Yamamoto H. 2013. On the mechanical interaction between cellulose microfibrils and matrix substances in wood cell wall:effects of chemical pretreatment and subsequent repeated dry-and-wet treatment[J]. Journal of Wood Science, 59(5): 359-366. DOI:10.1007/s10086-013-1347-1 |
Violaine G-R, Cisse O, Placet V, et al. 2015. Creep behaviour of single hemp fibres. Part Ⅱ:Influence of loading level, moisture content and moisture variation[J]. Journal of Materials Science, 50(5): 2061-2072. DOI:10.1007/s10853-014-8768-0 |
Westbye P, Köhnke T, Glasser W, et al. 2007. The influence of lignin on the self-assembly behavior of xylan rich fractions from birch (Betula pendula)[J]. Cellulose, 14(6): 603-613. DOI:10.1007/s10570-007-9178-0 |
Zhang W, Tokumoto M, Takeda T, et al. 2006a. Effects of delignifying treatments on mechano-sorptive creep of wood Ⅰ:instantaneous and total compliance of radial specimens[J]. Journal of the Japan Wood Research Society, 52(1): 19-26. DOI:10.2488/jwrs.52.19 |
Zhang W, Tokumoto M, Takeda T, et al. 2006b. Effects of delignifying treatments on mechano-sorptive creep of wood Ⅲ:MS creep of longitudinal specimens[J]. Journal of the Japan Wood Research Society, 52(1): 206-214. |
Zhang W, Tokumoto M, Takeda T. 2007. Effects of temperature on mechano-sorptive creep of delignified wood[J]. Journal of Wood Science, 53(3): 187-191. DOI:10.1007/s10086-006-0858-4 |
Zhang T, Bai S L, Zhang Y F, et al. 2012. Viscoelastic properties of wood materials characterized by nanoindentation experiments[J]. Wood Science and Technology, 46(5): 1003-1016. DOI:10.1007/s00226-011-0458-3 |
 2017, Vol. 53
2017, Vol. 53

