文章信息
- 王海华, 褚洪龙, 谢清哲, 豆青, 冯欢, 杨超, 王春燕
- Haihua Wang, Honglong Chu, Qingzhe Xie, Qing Dou, Huan Feng, Chao Yang, Chunyan Wang
- 4个蠕虫埃斯特菌株的产孢能力及对松材线虫的侵染活力
- Variation in Sporulation of Four Esteya vermicola Isolates and their Infectivity Against Pinewood Nematode
- 林业科学, 2016, 52(9): 139-146
- Scientia Silvae Sinicae, 2016, 52(9): 139-146.
- DOI: 10.11707/j.1001-7488.20160917
-
文章历史
- 收稿日期:2015-06-12
- 修回日期:2016-03-01
-
作者相关文章
Pinewood nematode(PWN), Bursaphelenchus xylophilus , is the causal agent of pine wilt disease, which is the most serious forest disease and has devastated huge areas of pine forests, causing irreparable damage to the forest ecosystems and industries in East Asia(Mota et al., 2008). As the first recorded endoparasitic fungus of PWN, Esteya vermicola was isolated from the infected PWNs in Taiwan and characterized by the production of two different types of conidiogenous cells and conidia, but only the lunate one can adhere and infect nematodes(Liou et al., 1999). After adherence, the lunate conidia germinate and form sharp infection pegs to penetrate the nematode’s cuticle and develop into hyphae. The hyphae of E. vermicola consume the content of infected nematode’s body, grow out from its cadaver, and then produce new lunate conidia for the next infection cycle(Liou et al., 1999). E. vermicola has been patented in the United States and South Korea for its high infection activity and potential as a biocontrol agent against PWN(Fang et al., 2010; Tzean et al., 2001).Recent study showed this fungus can save the pine seedlings from pine wilt disease by spraying in greenhouse(Wang et al., 2011).
The infectivity of nematophagous fungi is always influenced by the temperature, culture medium, number and species of tested nematodes, and so on(Cooke, 1963; Wang et al., 2009; Xiang et al., 2007). Moreover, different isolates of the same species always exhibit divergence and variety not only in morphological characteristics, but also in virulence and infectivity(Xiang et al., 2007). To compare the difference of isolates under the same experimental conditions will help understand the fungal diversity and variability, and screen the excellent isolate to develop it as biocontrol agent(Chu et al., 2015). There are only six isolates of E. vermicola in the world so far(Tab. 1), and four have been collected by us. In the present study, they were compared in sporulation and infectivity against PWN with different culture media in order to screen the excellent isolate for the futural application. In addition, the influences of carbon source on the growth, sporulation and infectivity of four isolates also were simply investigated and discussed. This experiment will provide more information for the biocontrol of pine wilt disease.
|
|
Two Asian(ATCC 74485, CNU 120806)and two European(CBS 115803, CBS 100821)isolates of E. vermicola were collected and used for this study(Tab. 1). They were maintained on PDA(potato dextrose agar, Becton, Dickinson and Company, France)slants at 4℃ and cultured on PDA plates at 26℃. Conidial suspensions(106 mL-1)were prepared by following the previous method(Wang et al., 2010).
1.2 Assays of E. vermicola growth and sporulation on different culture mediaAs the most usually used nature culture media for the nematophagous fungi, including E. vermicola, PDA, CMA(corn meal agar)and WA(water agar)are commonly used to serve as an example of nutrition-rich, -weak and -poor culture media, respectively(Liou et al., 1999; Wang et al., 2014). Although the specific ingredients are not very clear, their main difference in nutrition is the content of carbon source, which is richer in PDA than CMA, and almost is none in WA. In this study, the three culture media also were used for the culture of four E. vermicola isolates.
A very small piece of mycelia was transferred from the margin of 7-day-old pure culture of each E. vermicola isolates onto the center of Petri dishes(9 cm diam.)containing PDA, CMA(Becton, Dickinson and Company, France)and WA(2% agar, same with PDA and CMA)plates, respectively. After inoculation at 26℃ for 10 days, the macroscopic features of each colony were observed and photographed. In a parallel experiment, a piece of sterile cellophane(8 cm in diam.)was overlaid on each plate two days before the fungal inoculation. Prepared homogeneous conidia suspensions of each E. vermicola isolate(5 μL)were transferred onto the center of cellophane, respectively, and then incubated at 26℃ for 8 days. Subsequently, the colony on each plate was collected from the cellophane with a sterile scalpel, weighed and transferred into a 15 mL centrifuge tube containing 5 mL sterile 0.05% Tween-80 solution. After 5 min vigorous shaking with a vortex shaker(Scientific Industries, Inc., USA)to dislodge conidia from the conidiogenous cells, the conidia concentration in Tween-80 solution was determined using a haemocytometer, and finally the conidia number of each colony was calculated.
1.3 Infectivities of four E. vermicola isolates against PWNMonoxenic PWNs were cultured and prepared as an aqueous suspension according to the previous methods(Wang et al., 2008; 2010). The infectivities of four E. vermicola isolates against PWN were investigated by following the method described by Wang et al. (2009)with some amendments. Briefly, 300 μL conidial suspensions of each isolate were spread on CMA and WA plates, respectively, and then incubated at 26℃ for 10 days. Subsequently, each plate was infested with 20 μL prepared PWN suspensions(about 500 individuals)at the center point, and continuously incubated at 28℃. Nematodes in each plate were examined at intervals of 12, 24 hours and 2, 3, 6, 9 days under the light microscope at 100-400×magnification. The adhesive rate and mortality of PWN were determined based on the percentage of nematodes attached or colonized by E. vermicola from the first encountered 100 nematodes.
1.4 Statistic analysisEach assay was replicated three times. Statistical analysis was performed using SPSS17.0 version for Windows. The data were subjected to one-way of analysis of variance(ANOVA)and means were compared by least significant difference(LSD)at the 5% level. The percent data were normalized by arcsine transformation before analysis but expressed in figures as nontransformed.
2 Results 2.1 Growth and sporulation of E. vermicola on different culture mediaIn different culture media, the four E. vermicola isolates showed obvious variations and differences in the growth speed, as well as the colonial color, shape and size(Fig. 1). Their colonies were dark green or bluish green, bigger, thicker and more compact on the nutrition-rich PDA medium, however, getting smaller, thinner, sparser and lighter along with the decrease of nutrition(Fig. 1). In addition, the isolates from the same continent(Asia or Europe)shared similar growth speed and colony macroscopic features(Fig. 1), suggesting that the geographical location has an influence on E. vermicola characteristics.
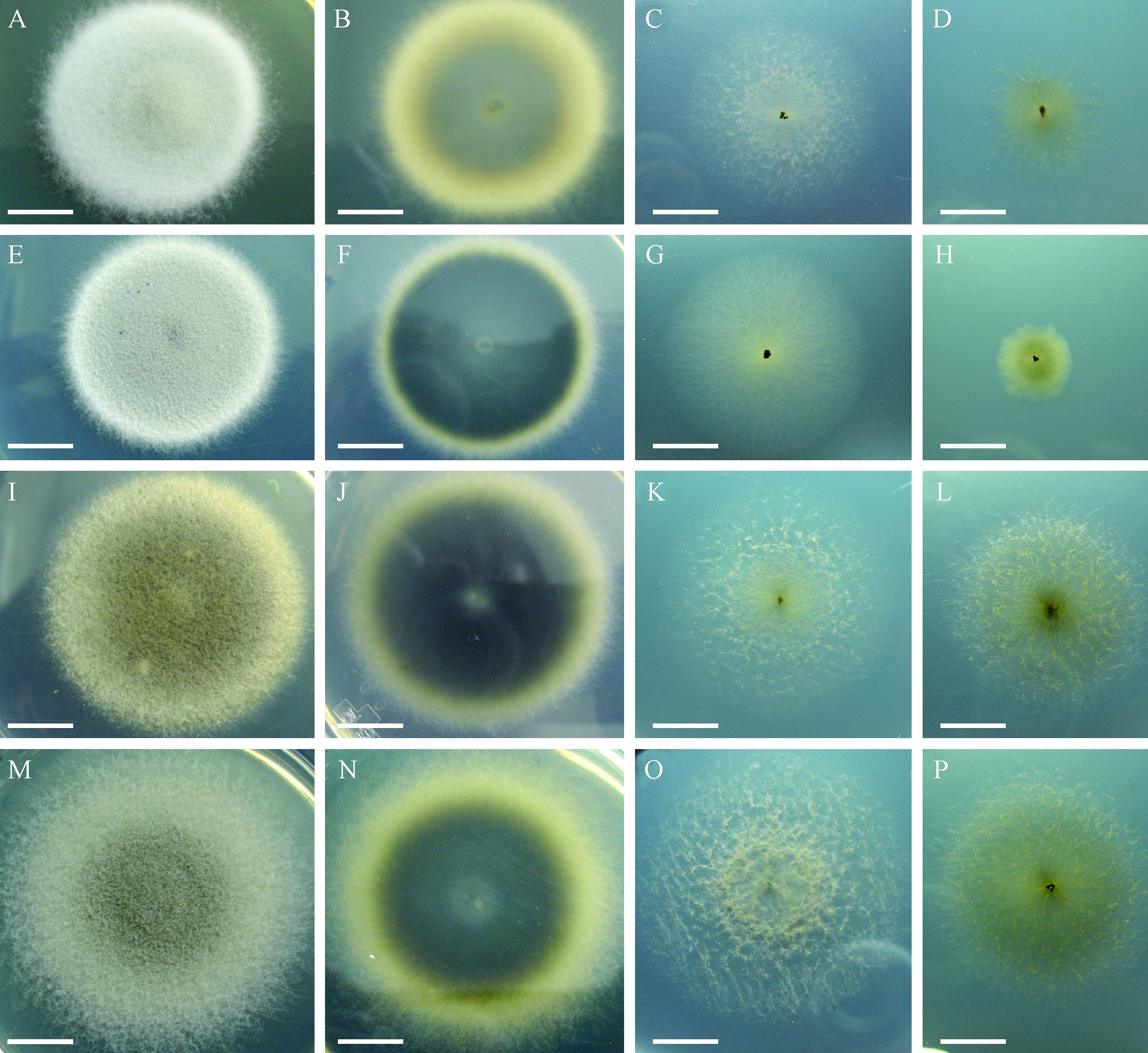
|
Fig.1 Colonies of four E. vermicola isolates in different culture media(10 days, 26℃) A, B: CBS 100821 on PDA plate and its reverse; C, D: CBS 100821 on CMA and WA plates, respectively; E, F: CBS 115803 on PDA plate and its reverse: G, H: CBS 115803 on CMA and WA plates, respectively; I, J: CNU 120806 on PDA plate and its reverse; K, L: CNU 120806 on CMA and WA plates, respectively; M, N: ATCC 74485 on PDA plate and its reverse; O, P: ATCC 74485 on CMA and WA plates, respectively. Bar=2.5 cm. A, B: CBS 100821 on PDA plate and its reverse; C, D: CBS 100821 on CMA and WA plates, respectively; E, F: CBS 115803 on PDA plate and its reverse: G, H: CBS 115803 on CMA and WA plates, respectively; I, J: CNU 120806 on PDA plate and its reverse; K, L: CNU 120806 on CMA and WA plates, respectively; M, N: ATCC 74485 on PDA plate and its reverse; O, P: ATCC 74485 on CMA and WA plates, respectively. Bar=2.5 cm. |
As to the four E. vermicola isolates, the colonial diameters and weights, as well as the total conidia numbers were the significantly biggest on nutrition-rich PDA, followed by on nutrition-weak CMA, and the smallest on nutrition-poor WA medium(P<0.05)(Fig. 2). Moreover, the numbers of their lunate conidia were significantly higher(P<0.05)on PDA than on CMA and WA media. On the contrary, the proportions of their lunate infective conidia were significantly increased(P<0.01)with the decrease of nutrition except for that of CNU 120806, which was significantly higher(P<0.01)on PDA than on CMA and WA media(Fig. 2C). These results suggested the carbon nutrition of culture media had a markedly positive correlation with the growth and sporulation of E. vermicola, while had a negative correlation with the proportion of lunate infective conidia. Therefore, the best culture medium for the growth and sporulation of E. vermicola was PDA, but WA was the best one for the higher proportion of lunate infective conidia.
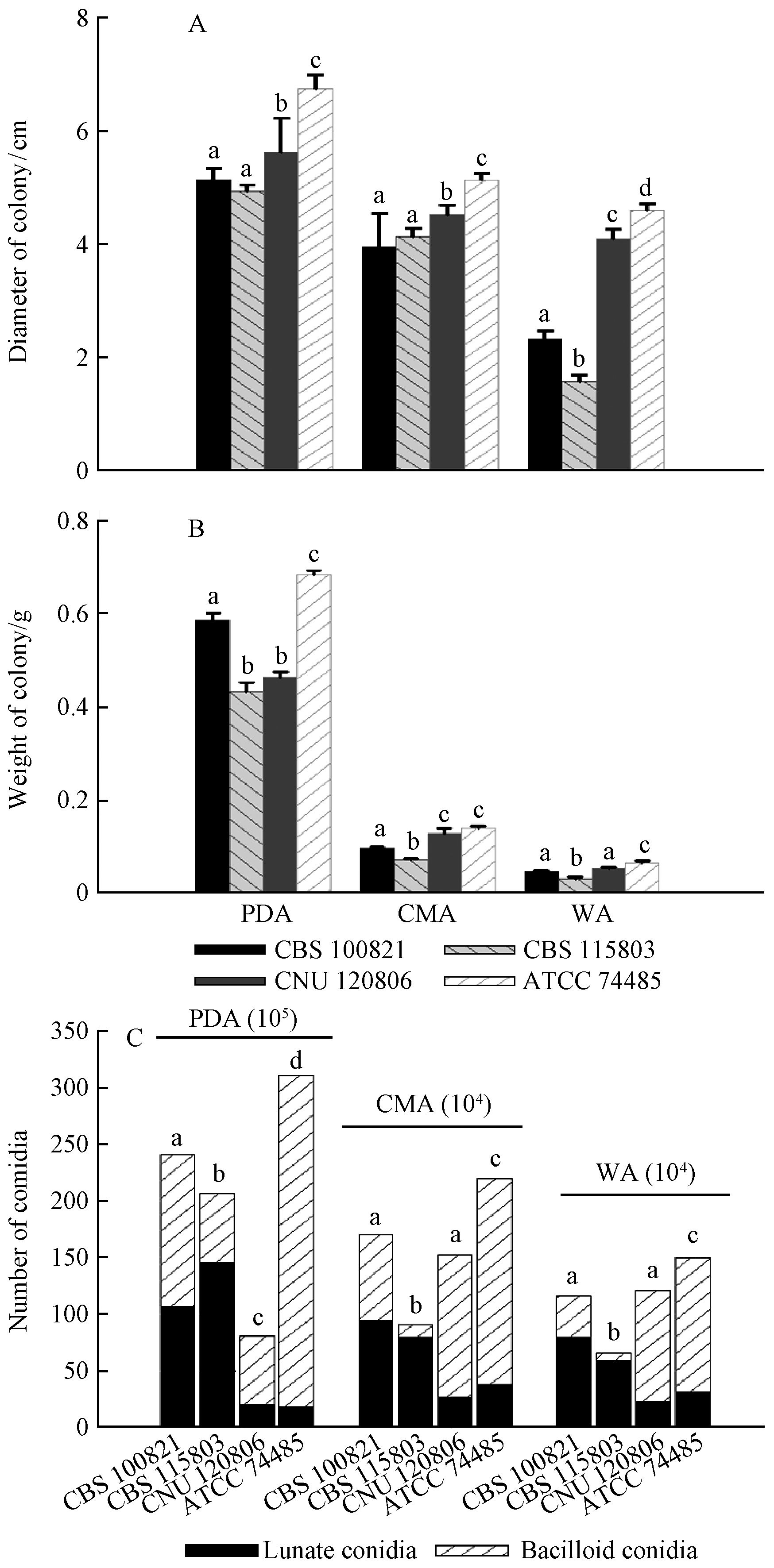
|
Fig.2 Influence of culture media on the growth and sporulation of four E. vermicola isolates A, B. Diameters and weights of 8-day-old colonies; C. Numbers of two types of conidia. Each bar represents mean±SE. Bars with a different letter above are significantly different(P<0.05). A, B. Diameters and weights of 8-day-old colonies; C. Numbers of two types of conidia. Each bar represents mean±SE. Bars with a different letter above are significantly different(P<0.05). |
Regardless of culture media, the colonial diameter was the significantly biggest for ATCC 74485(P<0.01), followed by CNU 120806(P<0.05), and the smallest for CBS 100821 and CBS 115803(P<0.01), while the colonial weight was the significantly heaviest(P<0.05)for ATCC 74485 and the lightest(P<0.05)for CBS 115803. On PDA medium, CNU 120806 was significantly bigger(P<0.01)than CBS 100821 in the colonial diameter, but was significantly lighter(P<0.05)in the colonial weight resulting from its thinner and looser hyphae (Fig. 2A, B). In general, ATCC 74485 showed the significantly fastest(P<0.01)growth speed, while CBS 115803 was the lowest(P<0.05)one. The total conidia numbers, proportion and number of lunate infective conidia were also significantly different(P<0.05)on different culture media among the four E. vermicola isolates(Fig. 2C). On nutrition-rich PDA medium, ATCC 74485 produced the significantly most(P<0.01)conidia, followed by CBS 100821, CBS 115803, while CNU 120806 produced the significantly least(P<0.01)conidia. However, the number of lunate conidia was significantly highest(P<0.05)for CBS 115803, followed by CBS 100821, and the least(P<0.01)for ATCC 74485 and CNU 120806. The proportion of lunate conidia was the significantly highest(P<0.01)for CBS 115803(70.5%), followed by CBS 100821(44.2%), CNU 120806(25%), and the least(P<0.01)for ATCC 74485(5.9%). On nutrition-poor CMA and WA media, the conidia number was the most(P<0.01)for ATCC 74485, followed by CBS 100821 and CNU 120806, and the least(P<0.01)for CBS 115803. However, CBS 115803 and CBS 100821 produced significantly more(P<0.01)lunate conidia than ATCC 74485 and CNU 120806 did, and did not show significantly difference(P>0.05)between them(Fig. 2C). Furthermore, the proportion of lunate conidia was the most(P<0.01)for CBS115803, followed by CBS100821, and the least(P<0.05)for CNU 120806 and ATCC 74485. Obviously, both the number and proportion of lunate conidia were the highest for CBS 115803 among the four isolates, especially the proportion of lunate conidia on CMA and WA media, on which almost without bacilloid conidia. Although the total conidia number of ATCC 74485 was the significantly highest(P<0.01)among the four isolates, the number and proportion of its lunate conidia were the significantly lowest(P<0.01). In addition, two Asian(ATCC 74485 and CNU 120806)isolates exhibited similar characteristics in both growth and sporulation, which are obviously different with two European (CBS 115803, CBS 100821)isolates(Fig. 2).
2.2 Infectivities of four E. vermicola isolates against PWNCompared to CMA medium, the adhesive rates of four tested isolates were significantly higher(P<0.05)on WA medium at the 12th hour after PWNs inoculation except for CBS 115803, but this difference was disappeared at the 24th hour(Fig. 3A, 3B). Furthermore, PWN mortalities caused by the infection of E. vermicola isolates were significantly higher(P<0.05)on WA medium except for CBS 100821, which did not show significant difference(P>0.05)between CMA and WA media(Fig. 3C, 3D). It seemed that there was a negative correlation between the infectivity of E. vermicola and the nutrition of the culture medium, as the poorer nutrition, the higher infectivity(Fig. 3). Therefore, the poorer nutrition culture medium could be used to improve the infectivity of E. vermicola against PWN.
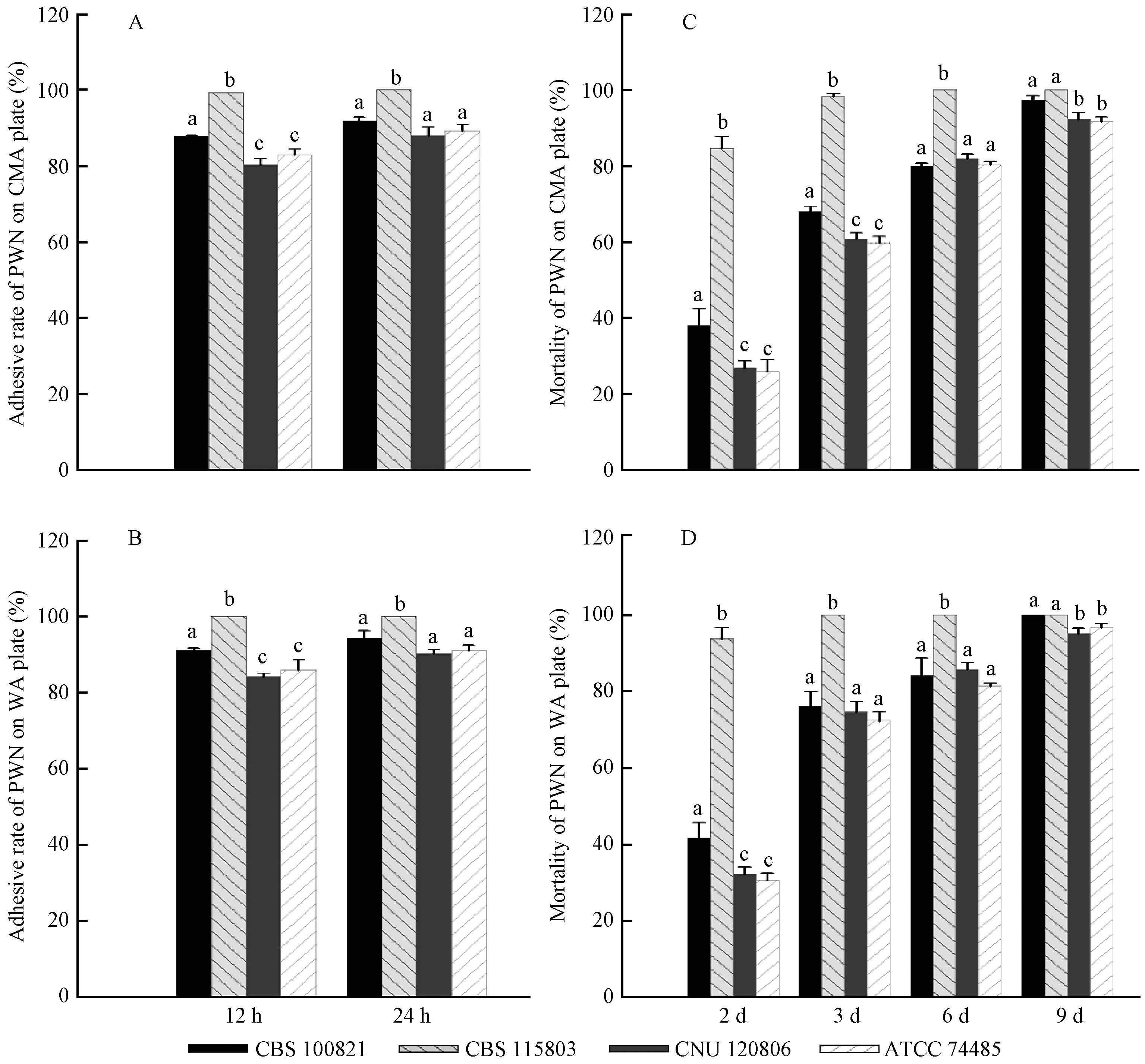
|
Fig.3 Infectivities of four E. vermicola isolates against PWN A, B: Adhesive rates of PWN caused by E. vermicola on CMA and WA media; C, D: PWN mortalities caused by E. vermicola on CMA and WA media. Each bar represents mean±SE. Bars with a different letter above are significantly different(P<0.05). A, B: Adhesive rates of PWN caused by E. vermicola on CMA and WA media; C, D: PWN mortalities caused by E. vermicola on CMA and WA media. Each bar represents mean±SE. Bars with a different letter above are significantly different(P<0.05). |
12 hours after PWN inoculation, CBS 115803 exhibited the significantly highest(P<0.01)adhesive rate on both CMA and WA media, followed by CBS 100821, while ATCC 74485 and CNU 120806 were the lowest(P<0.05). At the 24th hour, the adhesive rate of CBS 115803 was still significantly higher(P<0.01), but no significant difference(P<0.05)was showed among the other three E. vermicola isolates(Fig. 3A, 3B). At the second, third and sixth day after PWN inoculation, PWN mortalities caused by CBS 115803 were significantly higher(P<0.01)than those caused by the other three isolates on both CMA and WA media(Fig. 3C, 3D). Almost all of the tested(98%)nematodes were colonized and killed by CBS 115803 on the third day, while only 60%-76% nematodes were killed by the other three isolates. Moreover, PWN mortalities caused by CBS 100821 were significantly higher(P<0.05)than those caused by CNU 120806 and ATCC 74485 at the second and third day on CMA medium, as well as at the second day on the WA medium. Until the ninth day, PWN mortalities caused by CBS 115803 and 100821 still were significantly higher(P<0.05)than CNU 120806 and ATCC 74485. Generally, CBS 115803 exhibited the significantly highest(P<0.01)infectivity against PWN, followed by CBS 100821, while CNU 120806 and ATCC 74485 presented the lowest(P<0.05)infectivities. In addition, two Asian isolates, CNU 120806 and ATCC 74485, did not show any significant difference(P>0.05)in their infectivities both on CMA and WA media at the whole test process.
3 DiscussionsFive isolates of E. vermicola have been described so far, and their infectivities against PWN have been investigated separately in previous studies with different methods under different experiment conditions(Du et al., 2014; Liou et al., 1999; Wang et al., 2008; 2009; Wang et al., 2014). It is impossible to compare them and point the best one for the biocontrol of pine wilt disease in the future. In the present study, the sporulation and infectivities of four E. vermicola isolates were systematically investigated with three kinds of most usually used nature culture media. Although the four tested isolates shared the typical traits of E. vermicola in terms of basic morphological characteristics and infection process, diversities were observed on different media in their colonial features, growth speed, sporulation and infectivities (Fig. 1-3). Interestingly, the isolates from the same continent(Asia or Europe)shared similar characteristics in terms of colony morphology, growth, sporulation and infectivity. Wang et al. (2014)also noticed that two Asian isolates(ATCC 74485 and CNU 120806)were closer to each other in molecular characteristics, so did two European isolates(CBS 115803, CBS 100821). It was suggested that the geographical location has an influence on E. vermicola characteristics.
According to the results, all of the E. vermicola isolates had a quicker growth speed, developed more and thicker hyphae, produced more total conidia and lunate conidia on nutrition-rich medium than on nutrition-weak and -poor media(Fig. 1-2). Except for CNU 120806, however, there was a negative correlation between the nutrition of culture medium and the proportion of lunate infective conidia. On nutrition-rich PDA medium, ATCC 74485 produced numerous conidia, but the proportion of lunate conidia was very low(5.9%). This result was consistent with the report of Liou et al. (1999)that ATCC 74485 mainly produced cylindrical or bacilloid, non-adhesive conidia on enriched culture medium. Kubátová et al., (2000)found CBS 115803 produced both types of conidia on nutrition medium. In this study, CBS 115803 also produced two types of conidia on PDA medium, but the proportion of lunate conidia(70.5%)was higher than that of bacilloid one. As to CNU 120806, both the number and proportion of lunate conidia were higher on PDA medium than CMA and WA media. The same result has been revealed by Wang et al. (2008)that more lunate infective conidia were produced by CNU 120806 on nutrition-richer culture medium. In addition, there was a negative correlation between the infectivity of E. vermicola and nutrition of culture medium. Under unfavorable conditions, nemato-phagous fungi usually produce more infective conidia and show higher infection ability against nematodes for nutrition(Cooke, 1963; Meyer et al., 2005; Rubner, 1996). This is responsible for the higher infectivity of E. vermicola isolates in nutrition-poor WA medium. Accordingly, these results suggested the carbon nutrition of culture medium had different influences on the growth, sporulation and infectivity of different E. vermicola isolates. The selection of culture medium should depend on the specific isolate and the real objective. In general, PDA is the best culture medium for the growth and sporulation of E. vermicola, but WA is the best one for producing high proportion of lunate infective conidia and high infectivity against PWN.
Since only lunate conidia can infect PWN, the infectivity of E. vermicola is directly related to the number and proportion of lunate conidia, as the more and higher proportion of lunate conidia are produced, the higher the infectivity is. In this study, the lunate conidia number of CBS 100821 was little higher(P>0.05)than CBS 115803 on CMA and WA media, but CBS 115803 showed the higher proportion of lunate conidia and exhibited higher adhesive rate as well as mortality than CBS 100821. According to the report of Du et al. (2014), the shed lunate conidia of CBS 115803 also exhibited higher infection effectiveness than CUN 120806 and CBS 10082 under the same conidia concentration. The results suggested that the number and proportion of lunate conidia may be not the only factors influencing the infectivities of E. vermicola isolates against PWN. In the future, it is necessary to investigate the infectivity of lunate conidia produced by different E. vermicola isolates. Moreover, the influencing factors, such as the adhesive ability, germination and growth speed of lunate conidia, should also be studied. Among the tested four isolates, CBS 115803 exhibited the significantly best PWN control effect. Although ATCC 74485 and CNU 120806 showed more rapid growth speed and better saprophytic ability than CBS 115803 and CBS 100821 did, but their infectivities against PWN were lower. This result was consistent with the conclusion drawn by Cooke(1963)that rapid growth rate and good saprophytic ability are accompanied by lower predacious efficiency, which reflects the dependence of nematophagous fungi on nematodes for nutrition.
4 ConclusionsAlthough E. vermicola CBS 115803 showed slower growth speed, it produced the biggest amount of infective conidia and exhibited the significantly highest(P<0.01)infectivity against PWN than the other three isolates did. Therefore, CBS 115803 can be selected and developed into the biocontrol agent against pine wilt disease in the future. This experiment will provide more knowledge and information for the control of disastrous pine wilt disease.
| [1] |
Cooke R C.1963. Ecological characteristics of nematode-trapping hyphomycetes. I.Preliminary studies.Annals of Applied Biology , 52 : 431–437.
DOI:10.1111/aab.1963.52.issue-3 ( 0) 0)
|
| [2] |
Chu W H, Dou Q, Chu H L, et al.2015. Research advance on Esteya vermicola,a high potential biocontrol agent of pine wilt disease. Mycological Progress , 14 (12) : 1–9.
( 0) 0)
|
| [3] |
Du T, Zhang Y A, Wang Y Z, et al.2014. Infectivity test on Esteya vermicola conidia against pinewood nematode. Forest Research , 27 (2) : 174–187.
( 0) 0)
|
| [4] | Fang Z M,Wang C Y,Wang Z,et al.2010.A biocontrol agent of pinewood nematode and application method.South Korea Patent:10-1033270. |
| [5] |
KubátováA, NovotnýD, Práil K, et al.2000. The nematophagous hyphomycete Esteya vermicola found in the Czech Republic. Czech Mycology , 52 (3) : 227–235.
( 0) 0)
|
| [6] |
Liou J Y, Shih J Y, Tzean S S.1999. Esteya,a new nematophagous genus from Taiwan,attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycological Research , 103 (2) : 242–248.
DOI:10.1017/S0953756298006984 ( 0) 0)
|
| [7] |
Meyer S L F, Carta L K, Rehner S A.2005. Morphological variability and molecular phylogeny of the nematophagous fungus Monacrosporium drechsleri. Mycologia , 97 (2) : 405–415.
DOI:10.3852/mycologia.97.2.405 ( 0) 0)
|
| [8] | Mota M,Vieira P.2008.Pine wilt disease:a worldwide threat to forest ecosystems.Springer,Berlin. |
| [9] |
Rubner A.1996. Revision of predacious hyphomycetes in the Dactylella-Monacrosporium complex. Studies in Mycology , 39 : 1–134.
( 0) 0)
|
| [10] | Tzean S S,Liou J Y,Shih J Y.2001.Nematophagous fungus Esteya vermicola.United States Patent:006168947B1. |
| [11] |
Wang C Y, Fang Z M, Sun B S, et al.2008. High infectivity of an endoparasitic fungus strain,Esteya vermicola,against nematodes. Journal of Microbiology , 46 (4) : 380–389.
DOI:10.1007/s12275-007-0122-7 ( 0) 0)
|
| [12] |
Wang C Y, Fang Z M, Wang Z, et al.2009. High infection activities of two Esteya vermicola isolates against pinewood nematode. African Journal of Microbiology Research , 3 (10) : 581–584.
( 0) 0)
|
| [13] |
Wang C Y, Fang Z M, Wang Z, et al.2011. Biological control of the pinewood nematode Bursaphelenchus xylophilus by application of the endoparasitic fungus Esteya vermicola. Biocontrol , 56 (1) : 91–100.
DOI:10.1007/s10526-010-9302-1 ( 0) 0)
|
| [14] |
Wang C Y, Wang Z, Fang Z M, et al.2010. Attraction of pinewood nematode to endoparasitic nematophagous fungus Esteya vermicola. Current Microbiology , 60 (5) : 387–392.
DOI:10.1007/s00284-009-9556-y ( 0) 0)
|
| [15] |
Wang T T, Wang X, Wang J C, et al.2014. Infection characteristics of nematophagous fungus Esteya vermicola to plant parasitic nematodes. Journal of Plant Protection , 4 (5) : 540–546.
( 0) 0)
|
| [16] |
Xiang M C, Yang X H, Wang Z X, et al.2007. Variability of morphology,parasitism,and nucleotide sequences among isolates and species of nematophagous Hirsutella. Biological Control , 41 (1) : 110–119.
DOI:10.1016/j.biocontrol.2006.12.016 ( 0) 0)
|
 2016, Vol. 52
2016, Vol. 52

