文章信息
- 张利, 徐向东, 王丽娟, 卢孟柱
- Zhang Li, Xu Xiangdong, Wang Lijuan, Lu Mengzhu
- 杨树蔗糖转运体基因PagSUT4的鉴定及功能分析
- Identification and Functional Analysis of the Populus Sucrose Transporter Gene PagSUT4
- 林业科学, 2016, 52(8): 21-28
- Scientia Silvae Sinicae, 2016, 52(8): 21-28.
- DOI: 10.11707/j.1001-7488.20160803
-
文章历史
- 收稿日期:2016-03-29
- 修回日期:2016-04-26
-
作者相关文章
糖作为光合作用的主要产物,是植物体代谢过程中最重要的能量来源,同时还能为其他有机物(蛋白质、核酸等)合成提供骨架。此外,糖还是重要的信号分子和渗透调节物。糖的这些重要功能使其在植物的生长发育和对生物及非生物胁迫的响应中发挥着重要作用(Koch, 1996; Lastdrager et al., 2014; Smeekens, 2000)。糖在光合组织(源)中合成,之后在叶片中贮存或转运进入非自养组织(库)进行代谢或贮存。
植物液泡膜上存在的多种糖转运体,在调控糖穿过液泡膜在胞质与液泡之间进行运输的过程中发挥着重要作用(Hedrich et al., 2015)。目前已鉴定出单糖转运家族MSTs(monosaccharide transporters)中的多个成员,在葡萄糖运入或运出液泡的过程中发挥着作用。拟南芥(Arabidopsis thaliana)AtTMT1(tonoplast monosaccharide transporter)和AtVGT1(vacuolar glucose transporter)能促进葡萄糖吸收进入液泡(Aluri et al., 2007; Wormit et al., 2006);AtESL1(early responsive to dehydration6-like1)和AtERDL6(early responsive to dehydration-like6)则在液泡中葡萄糖外运的过程中发挥作用(Poschet et al., 2011; Yamada et al., 2010)。在近期鉴定的拟南芥SWEETs糖转运体家族中,AtSWEET16和AtSWEET17作为液泡膜糖转运体能促进液泡内果糖的外运(Chardon et al., 2013; Guo et al., 2014; Klemens et al., 2013),AtSWEET2则在根部葡萄糖转运进入液泡的过程中发挥作用(Chen et al., 2015)。在植物蔗糖转运体家族SUTs(sucrose transporters)中,拟南芥AtSUC4和水稻(Oryza sativa)OsSUT2均被鉴定为液泡膜定位的蔗糖转运体,能够促进蔗糖转运进入液泡(Eom et al., 2011; Schulz et al., 2011)。
多年生木本植物与1年生草本植物的碳运输和分配机制具有明显区别(Jansson et al., 2007)。在草本植物中,糖在韧皮部的装载主要通过质外体途径(Chen, 2014; Sauer, 2007),而在木本植物中,则主要通过共质体途径(Davidson et al., 2011; Fu et al., 2011; Rennie et al., 2009);草本植物以种子等作为主要的库器官,而木本植物则以木材作为主要的库器官。因此,糖转运体在木本植物木材发育过程中发挥作用的机制可能与草本植物不同,揭示这种机制对研究树木生长发育特别是木材形成具有重要意义。
在大多数植物中,糖类长距离运输的主要形式是蔗糖(Kühn et al., 1999; Rennie et al., 2009; Sauer, 2007),SUT在草本植物蔗糖的装载、运输和卸载过程中发挥重要作用(Braun et al., 2009; Slewinski et al., 2010)。杨树SUT家族共包含5个成员,目前已对杂交杨(Populus tremula × P. tremuloides)的PttSUT3和杂交杨(Populus tremula × P. alba)的PtaSUT4进行了研究(Mahboubi et al., 2013; Payyavula et al., 2011)。PttSUT3基因编码细胞膜定位的蔗糖转运体,并在形成层和木质部中表达较高,其RNAi株系的木纤维细胞壁厚度减小且植株高度降低,说明其在树木的次生生长中发挥重要作用(Mahboubi et al., 2013)。PtaSUT4基因编码液泡膜定位的蔗糖转运体,在其RNAi株系的叶片、韧皮部和发育的木质部中均发现了更多蔗糖的积累,说明液泡中蔗糖的外运可能在叶片输出蔗糖的过程中发挥重要作用(Payyavula et al., 2011)。但PtaSUT4对树木木质部发育(木材形成)的影响目前还没有报道。为进一步研究SUT4基因在杨树蔗糖运输和分配中的作用及对木质部发育的影响,本研究对银腺杨(Populus alba×P. glandulosa)PagSUT4基因(GenBank登录号:KX545405)的表达和亚细胞定位进行了分析,创制了PagSUT4的超表达植株,并对其生长和光合指标进行了分析。
1 材料与方法 1.1 植物材料用于瞬时转化的本氏烟草(Nicotiana benthamiana)的培养条件为8 h光照/16 h黑暗,温度为20 ℃。
银腺杨无性系84K,由中国林业科学研究院林木遗传育种国家重点实验室保存并繁殖。用于遗传转化的84K培养条件为16 h光照/8 h黑暗,温度为23~25 ℃。
幼叶、成熟叶、初生茎、次生茎、根取自当年生84K幼株(株高约80 cm),木质部和韧皮部取自84K大树,雄花和雌花取自毛白杨(Populus tomentosa)。各组织分别从3个植株取样。
转基因株系和84K定量分析所用的成熟叶取自温室生长1个月幼苗的顶端第5片叶。各3个独立植株用于转基因株系和84K的定量分析。
1.2 总RNA的提取和实时荧光定量PCR总RNA提取和第1链cDNA的合成分别采用RNeasy Plant Kit (QIAGEN)试剂盒和SuperScript Ⅲ First-Strand Synthesis System(Invitrogen)反转录试剂盒,方法参见试剂盒使用说明。实时荧光定量PCR使用SYBR Premix Ex TaqTM Ⅱ Kit(TaKaRa Dalian, Dalian, China)和LightCycler 480(Roche Applied Science, Penzberg, Upper Bavaria, and Germany)检测系统。基因定量引物(表 1)使用在线引物设计软件Primer3(http://frodo.wi.mit.edu/primer3/input.htm)设计。
|
|
参考毛果杨(Populus trichocarpa)基因组序列设计特异引物(表 1),以银腺杨84K木质部提取的mRNA构建的cDNA为模板,扩增PagSUT4基因的编码区序列,并重组进入pDNOR222.1(Invitrogen)。测序正确后,亚克隆至PMDC32载体,形成35S:PagSUT4载体并通过电转化进入根癌农杆菌(Agrobacterium tumefaciens)GV3101。利用Liu等(2014)描述的根癌农杆菌介导的叶盘法转化84K。选取生长4周的健壮84K无菌苗,取顶端向下第2至4片叶片(叶片颜色均匀、质地较厚)。去除叶片顶端和叶柄,垂直于主叶脉划3~4刀。挑含载体的农杆菌单克隆于含抗生素[34 mg·L-1利福平(rifampicin)、10 mg·L-1庆大霉素(gentamycin)和50 mg·L-1卡那霉素(kanamycin)]的YEP液体培养基,培养至菌液OD600=0.6~0.8。叶片在菌液中浸泡10 min,用滤纸洗净表面菌液后接入芽分化培养基(1/2 MS基本培养基加0.5 mg·L-1 6-BA和0.05 mg·L-1的NAA)并在23~25 ℃培养室暗培养3~4天。暗培养后的叶片转移至添加了200 mg·L-1特美汀(Timentin)和3 mg·L-1潮霉素(hygromycin)B的芽分化培养基上进行筛选,每2周更换1次新的培养基。当不定芽高度达到1 cm左右时,将芽转入添加200 mg·L-1特美汀和3 mg·L-1潮霉素B的生根培养基(1/2 MS基本培养基加0.05 mg·L-1 IBA和0.02 mg·L-1的NAA)上诱导生根。
1.4 PagSUT4的亚细胞定位分析通过入门载体,将PagSUT4基因的编码区序列亚克隆至pEarleyGate104(ABRC stock DB3-686)载体,形成35 S:YFP-PagSUT4载体。利用Zheng等(2005)描述的方法进行烟草的瞬时转化。烟草叶片在转化后3天进行观察。烟草叶片YFP荧光信号的观察和成像采用蔡司LSM510共聚焦显微镜(Carl Zeiss, Oberkochen, Germany)。
1.5 杨树光合测定采用Li-6400便携式光合作用测定系统,对在温室生长2个月的84K和转基因株系第4片叶的光合作用进行测定,测量时间为9:00—12:00。设定光合有效辐射(photosynthetic active radiation、PAR)为2 000 μmol·m-2s-1作为测定光强,在平衡90~120 s读数稳定后测定光合,并由仪器自动记录净光合速率Pn(net photosynthetic rate)、胞间CO2浓度Ci(intercellular CO2concentration)、气孔导度Gs(stomatal conductance)、蒸腾速率Tr(transpiration rate)、PAR等数值。水分利用效率WUE(water use efficiency)=Pn/Tr,光能利用效率LUE(light use efficiency)=Pn/PAR。每个株系测定4株,每株重复测定4次。
1.6 杨树的生长和组织切片分析杨树在温室培养2个月后,对其地径和株高进行测量。从第7节间中部取1 mm厚度的材料放入2.5%的戊二醛固定液中固定。固定的材料经过酒精梯度脱水后用Spurr树脂包埋。用徕卡Leica EM UC7超薄切片机(Leica 283 Microsystems GmbH Wetzlar, Germany)按厚度为3 μm的标准进行切片。使用连接AxioCam MRC5相机的蔡司Zeiss Axio Imager A1(Carl Zeiss)进行拍照。
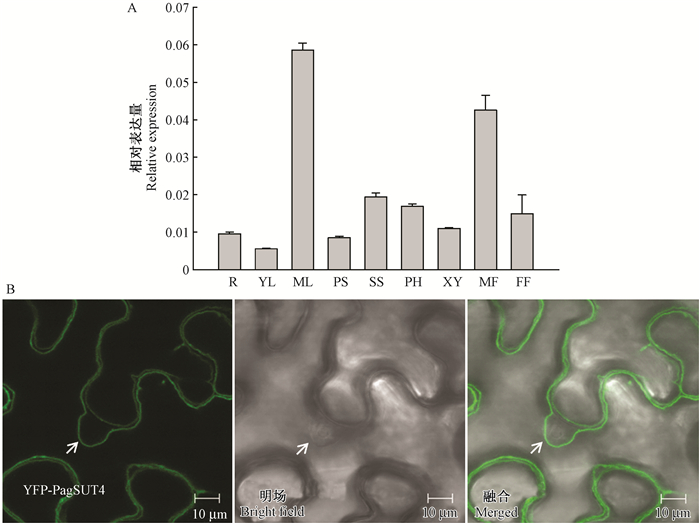
2 结果与分析 2.1 PagSUT4的组织表达和亚细胞定位为研究PagSUT4的组织表达特异性,采用实时荧光定量PCR,分别分析银腺杨84K根、幼叶、成熟叶、初生茎、次生茎、雄花和雌花以及木质部、韧皮部组织中PagSUT4的表达(图 1A)。结果显示,PagSUT4在所有被检测的器官和组织中均有表达,并且在成熟叶、雄花、次生茎和韧皮部的表达较高。通过构建35 S:YFP-PagSUT4载体瞬时转化本氏烟草并对叶片中YFP信号进行检测,发现YFP荧光信号分布于位于细胞核附近的液泡膜,这说明PagSUT4不是在质膜、核膜定位,而是定位于液泡膜(图 1B)。
|
图 1 PagSUT4基因在银腺杨不同组织中的表达及PagSUT4的亚细胞定位分析 Fig.1 The expression of PagSUT4 in different tissues and subcellular localization of PagSUT4 R:根; YL:幼叶; ML:成熟叶; PS:初生茎; SS:次生茎; PH:韧皮部; XY:木质部; MF:雄花; FF:雌花。PagSUT4的表达水平用PtACTIN基因进行归一化处理。箭头所指位置表示YFP-PagSUT4标记的液泡膜位于细胞核内侧。 R: Root; YL: Young leaf; ML: Mature leaf; PS: Primary stem; SS: Secondary stem; PH: Phloem; XY: Xylem; MF: Male flower; FF: Female flower. The expression levels of the PagSUT4 gene were normalized to that of PtACTIN. The arrows point to the YFP-PagSUT4-labeled vacuole membranes lining the inside of the nucleus. |
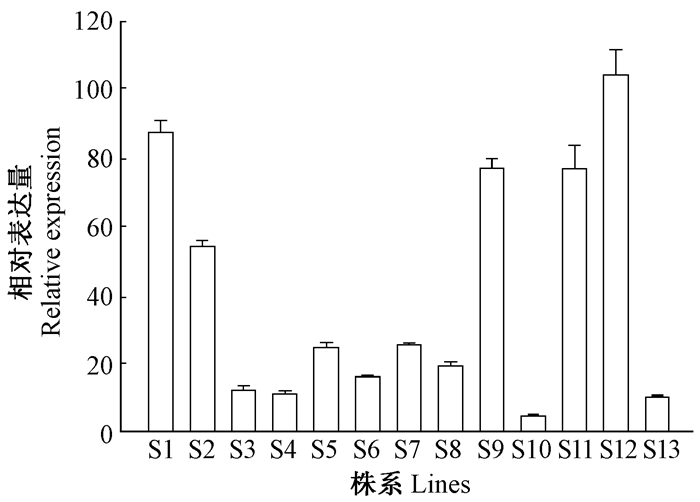
通过农杆菌介导的叶盘法将构建的35 S:PagSUT4载体转化银腺杨84K,并对转基因植株经PCR检测。所获得的13个转基因株系,依次命名为S1-S13。通过实时荧光定量PCR检测了84K和转基因株系PagSUT4的表达,发现13个转基因株系成熟叶片中的PagSUT4表达量是对照的5~105倍(图 2)。从中选取表达量较高的株系S1和S12,进行生长性状和光合指标的测定,并对其茎进行解剖学分析。
|
图 2 银腺杨转基因株系中PagSUT4基因的表达分析 Fig.2 The PagSUT4 expression analysis of the transgenic poplar 非转基因84K中PagSUT4基因的表达水平设定为1,转基因杨树中PagSUT4基因的表达量为与84K的比值。 The expression levels of the PagSUT4 in 84K were set to 1, and the levels in transgenic poplar were given as the relative ratio to this. |
为研究超表达PagSUT4对杨树光合作用的影响,测定了PagSUT4超表达株系和非转基因84K的光合指标(表 2)。2个超表达株系S1和S12的气孔导度与对照相比分别高出14%,9%,水分利用效率分别比对照高出11%,15%。S1的蒸腾速率显著高出对照12%,S12高出7%。S1和S12的胞间CO2浓度则低于对照,分别低3%和5%。超表达株系的净光合速率分别比对照高出24%和21%,光能利用率则分别比对照高出24%和22%。
|
|
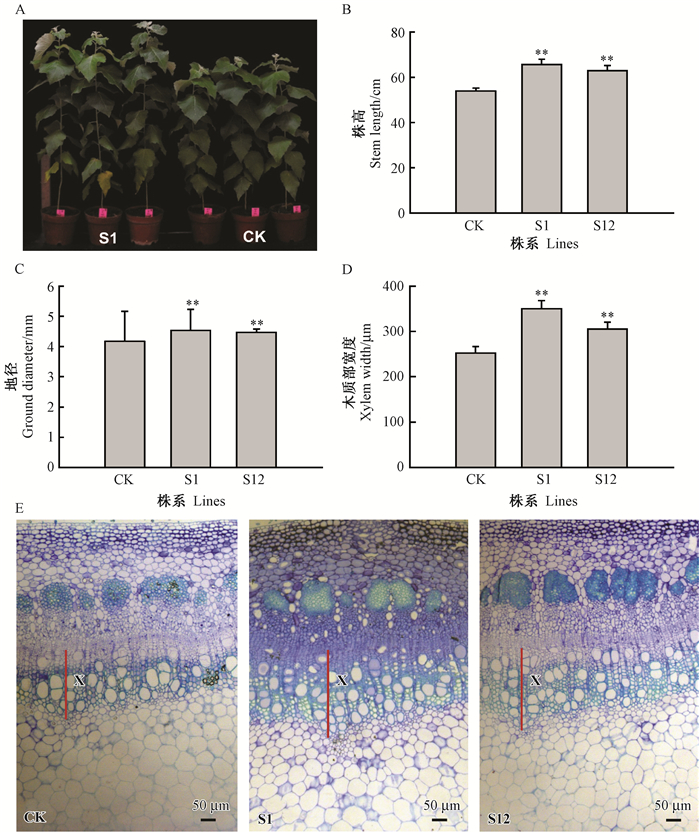
为揭示PagSUT4在杨树生长和木质部发育中的作用,测量了生长2个月的PagSUT4超表达株系和非转基因84K的株高和地径,并分别对其第7节间进组织解剖分析。与非转基因84K相比,PagSUT4超表达植株的株高分别增加了22%和17%(图 3A,B),地径分别增加了9%和7%(图 3C)。
|
图 3 PagSUT4转基因杨树表型分析 Fig.3 Phenotypes of PagSUT4 transgenic poplars A.在温室生长2个月的PagSUT4超表达株系和非转基因84K杨(CK);E.第7节间横切面,红线代表木质部(X)分布。数值表示平均值±标准差。超表达株系与对照的t检验P值用星号表示:*,P < 0.05;**,P < 0.01。 A. Phenotypes of two-month-old non-and transgenic plants grown in the greenhouse; E. Cross-sections from the seventh internode. Red lines represent the xylem (X) distribution. Values shown are means±SD. P values of student's t test for overexpression lines and controls are denoted with asterisks: *, P < 0.05; **, P < 0.01. |
对转基因株系和非转基因84K的第7节间茎的横切面进行解剖学分析,结果显示木质部细胞的形态学特征没有明显差异;但通过对木质部宽度进行统计学分析发现,转基因株系木质部宽度明显大于非转基因84K,S1和S12比对照各高出32%和21%(图 3D,E)。
3 讨论植物SUTs家族成员按照其进化关系可以分为4个进化组(Ⅰ到Ⅳ),杨树PtSUT1和PtSUT3属于Ⅱ组成员,PtSUT5和PtSUT6属于Ⅲ组成员,PtSUT4为Ⅳ组成员(Payyavula et al., 2011)。在1年生双子叶植物成熟叶片中,Ⅱ组成员的表达较高,Ⅳ组成员只有微弱表达且主要在幼叶中表达(Chincinska et al., 2008; Endler et al., 2006; Srivastava et al., 2008)。通过实时荧光定量PCR对银腺杨无性系84K PagSUT4的组织表达模式进行分析,发现其在成熟叶片、次生茎和雄花中具有较高表达量,这与之前对杨树SUT4表达分析的报道相似(Payyavula et al., 2011)。作为Ⅳ组成员,杨树PagSUT4虽与草本植物一样定位于液泡膜上并参与蔗糖的外运,但在木本植物中的表达模式发生了改变,预示PagSUT4在木本植物的蔗糖运输中发挥的作用与草本植物不同,并可能除了参与成熟叶片中蔗糖的外运外,还参与了更多的运输过程,例如蔗糖在茎维管组织中的运输。这表明其可能在木本植物碳水化合物的源、库分配过程中发挥更重要的作用。
在植物贮藏组织(库)和具有光合活性的叶肉细胞(源)中,中央大液泡能占到细胞体积的90%左右,在糖的长期和暂时贮存中发挥重要作用(Etxeberria et al., 2012; Martinoia et al., 2007; Winter et al., 1993)。由于液泡可贮存大量的糖,因此能在植物饥饿时提供碳源或为其他渗透调节物质的合成提供原料(Echeverria et al., 1988)。目前的研究表明,糖对光合作用具有反馈调节作用,库组织或器官糖的利用效率和源组织或器官糖的转运效率可能都会通过基因的反馈网络影响光合作用,因此液泡中糖的贮藏与外运,可能在光合作用的调节中也发挥重要作用(Ainsworth et al., 2011; Rolland et al., 2006; Stitt et al., 2010)。对PagSUT4超表达株系和非转基因84K的光合作用分析发现,超表达PagSUT4基因能显著提高杨树的光合速率和光能利用率。这说明超表达PagSUT4可能促进了叶片中糖的外运,降低了叶片中糖的浓度,进而通过信号的反馈调节促进光合作用。光合作用增强,使汇组织能更高效地进行碳的固定,为植物其他非自养组织提供更多碳源。草本植物营养生长期较短,其光合作用产物转运主要是用于生殖生长,而木本植物具有较长的童期,其更多是要把碳水化合物运输到茎部,形成木材来固定碳水化合物。木材由维管形成层经过细胞分裂、细胞扩张、次生细胞壁的沉积和成熟(细胞死亡)4个主要发育过程产生(Mellerowicz et al., 2001)。在细胞的分裂、扩张和次生细胞壁聚合物的形成过程中,需要不断地从光合组织获得碳源。因此,更高效的糖的合成和运输能够促进树木的次生生长。
PagSUT4在茎中的表达较高,说明其可能参与糖在茎中运输和卸载的过程,并可能在茎的生长发育中发挥重要作用。在之前的研究(Payyavula et al., 2011)中发现,PtaSUT4的RNAi株系中叶片和茎中糖的积累增加,说明PtaSUT4可能参与了叶片蔗糖的外运和茎中蔗糖的运输和卸载等过程;但是,PtaSUT4是否参与了杨树次生生长,对杨树生物量的积累产生何种影响,这些问题单纯利用降低PtaSUT4表达的转基因植株的分析难以回答。本研究中,对PagSUT4超表达株系生长指标的测定发现,转基因株系光合作用增强,并导致转基因植株的株高、地径均高于对照,这说明超表达PagSUT4可能促进了杨树中糖的运输效率,从而促进源组织光合效率的提高、促进库组织的发育。超表达转基因植株的径向生长主要依赖木质部的增厚,也就是强化了木材形成过程。这说明木材作为树木的主要库组织,需要从韧皮部不断获得碳源,这个过程是糖由韧皮部卸载,供给到木质部。而进入发育中的木质部还需要经过多个过程,已有的研究表明,PttSUT3在该过程中发挥着重要作用(Mahboubi et al., 2013)。本研究对PagSUT4超表达株系的解剖学分析发现,转基因株系木质部的发育明显比对照要快,这进一步证明PagSUT4可能在韧皮部糖的运输和卸载中发挥重要作用,能够调控木本植物碳的流向。
4 结论杨树SUT4基因编码定位于液泡膜的蔗糖转运蛋白,主要在成熟叶、次生茎和韧皮部及花中表达,可能参与了叶片中蔗糖的外运和茎中蔗糖的卸载过程。超表达PagSUT4基因可能促进了蔗糖的运输和分配,并进一步促进了光合作用。蔗糖运输效率的提高和光合作用的增强促进了杨树木质部的发育和生长量的积累。通过对PagSUT4超表达株系的研究,揭示了PagSUT4在木本植物碳运输与分配中的作用,为更加深入理解PagSUT4的功能提供了重要线索。同时也说明该基因可以作为靶标,用于林木速生或材性改良的分子育种。
| [] |
Ainsworth E A, Bush D R.2011. Carbohydrate export from the leaf:a highly regulated process and target to enhance photosynthesis and productivity. Plant Physiology , 155 (1) : 64–69.
DOI:10.1104/pp.110.167684 ( 0) 0)
|
| [] |
Aluri S, Büttner M.2007. Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proceedings of the National Academy of Sciences , 104 (7) : 2537–2542.
DOI:10.1073/pnas.0610278104 ( 0) 0)
|
| [] |
Braun D M, Slewinski T L.2009. Genetic control of carbon partitioning in grasses:roles of sucrose transporters and tie-dyed loci in phloem loading. Plant Physiology , 149 (1) : 71–81.
DOI:10.1104/pp.108.129049 ( 0) 0)
|
| [] |
Chardon F, Bedu M, Calenge F, et al.2013. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Current Biology , 23 (8) : 697–702.
DOI:10.1016/j.cub.2013.03.021 ( 0) 0)
|
| [] |
Chen H Y, Huh J H, Yu Y C, et al.2015. The Arabidopsis vacuolar sugar transporter SWEET2 limits carbon sequestration from roots and restricts Pythium infection. Plant Journal , 83 (6) : 1046–1058.
DOI:10.1111/tpj.2015.83.issue-6 ( 0) 0)
|
| [] |
Chen L Q.2014. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytologist , 201 (4) : 1150–1155.
DOI:10.1111/nph.12445 ( 0) 0)
|
| [] |
Chincinska I A, Liesche J, Krügel U, et al.2008. Sucrose transporter StSUT4 from potato affects flowering, tuberization, and shade avoidance response. Plant Physiology , 146 (2) : 515–528.
( 0) 0)
|
| [] |
Davidson A, Keller F, Turgeon R.2011. Phloem loading, plant growth form, and climate. Protoplasma , 248 (1) : 153–163.
DOI:10.1007/s00709-010-0240-7 ( 0) 0)
|
| [] |
Echeverria E, Valich J.1988. Carbohydrate and enzyme distribution in protoplasts from Valencia orange juice sacs. Phytochemistry , 27 (1) : 73–76.
DOI:10.1016/0031-9422(88)80593-4 ( 0) 0)
|
| [] |
Endler A, Meyer S, Schelbert S, et al.2006. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiology , 141 (1) : 196–207.
DOI:10.1104/pp.106.079533 ( 0) 0)
|
| [] |
Eom J S, Cho J I, Reinders A, et al.2011. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiology , 157 (1) : 109–119.
DOI:10.1104/pp.111.176982 ( 0) 0)
|
| [] |
Etxeberria E, Pozueta-Romero J, Gonzalez P.2012. In and out of the plant storage vacuole. Plant Science , 190 : 52–61.
DOI:10.1016/j.plantsci.2012.03.010 ( 0) 0)
|
| [] |
Fu Q, Cheng L, Guo Y, et al.2011. Phloem loading strategies and water relations in trees and herbaceous plants. Plant Physiology , 157 (3) : 1518–1527.
DOI:10.1104/pp.111.184820 ( 0) 0)
|
| [] |
Guo W J, Nagy R, Chen H Y, et al.2014. SWEET17, a facilitative transporter, mediates fructose transport across the tonoplast of Arabidopsis roots and leaves. Plant Physiology , 164 (2) : 777–789.
DOI:10.1104/pp.113.232751 ( 0) 0)
|
| [] |
Hedrich R, Sauer N, Neuhaus H E.2015. Sugar transport across the plant vacuolar membrane:nature and regulation of carrier proteins. Current Opinion in Plant Biology , 25 : 63–70.
DOI:10.1016/j.pbi.2015.04.008 ( 0) 0)
|
| [] |
Jansson S, Douglas C J.2007. Populus:a model system for plant biology. Annual Review of Plant Biology , 58 : 435–458.
DOI:10.1146/annurev.arplant.58.032806.103956 ( 0) 0)
|
| [] |
Kühn C, Barker L, Bürkle L, et al.1999. Update on sucrose transport in higher plants. Journal of Experimental Botany , 50 : 935–953.
DOI:10.1093/jxb/50.Special_Issue.935 ( 0) 0)
|
| [] |
Klemens P A, Patzke K, Deitmer J, et al.2013. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiology , 163 (3) : 1338–1352.
DOI:10.1104/pp.113.224972 ( 0) 0)
|
| [] |
Koch K.1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Biology , 47 (1) : 509–540.
DOI:10.1146/annurev.arplant.47.1.509 ( 0) 0)
|
| [] |
Lastdrager J, Hanson J, Smeekens S.2014. Sugar signals and the control of plant growth and development. Journal of Experimental Botany , 65 (3) : 799–807.
DOI:10.1093/jxb/ert474 ( 0) 0)
|
| [] |
Liu B, Zhang J, Wang L, et al.2014. A survey of Populus PIN-FORMED family genes reveals their diversified expression patterns. Journal of Experimental Botany , 65 (9) : 2437–2448.
DOI:10.1093/jxb/eru129 ( 0) 0)
|
| [] |
Mahboubi A, Ratke C, Gorzsas A, et al.2013. Aspen SUCROSE TRANSPORTER3 allocates carbon into wood fibers. Plant Physiology , 163 (4) : 1729–1740.
DOI:10.1104/pp.113.227603 ( 0) 0)
|
| [] |
Martinoia E, Maeshima M, Neuhaus H E.2007. Vacuolar transporters and their essential role in plant metabolism. Journal of Experimental Botany , 58 (1) : 83–102.
( 0) 0)
|
| [] |
Mellerowicz E J, Baucher M, Sundberg B, et al.2001. Unravelling cell wall formation in the woody dicot stem. Plant Molecular Biology , 47 (1/2) : 239–274.
DOI:10.1023/A:1010699919325 ( 0) 0)
|
| [] |
Payyavula R S, Tay K H C, Tsai C J, et al.2011. The sucrose transporter family in Populus:the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. The Plant Journal , 65 (5) : 757–770.
DOI:10.1111/tpj.2011.65.issue-5 ( 0) 0)
|
| [] |
Poschet G, Hannich B, Raab S, et al.2011. A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds. Plant Physiology , 157 (4) : 1664–1676.
DOI:10.1104/pp.111.186825 ( 0) 0)
|
| [] |
Rennie E A, Turgeon R.2009. A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA , 106 (33) : 14162–14167.
DOI:10.1073/pnas.0902279106 ( 0) 0)
|
| [] |
Rolland F, Baena-Gonzalez E, Sheen J.2006. Sugar sensing and signaling in plants:conserved and novel mechanisms. Annual Review of Plant Biology , 57 : 675–709.
DOI:10.1146/annurev.arplant.57.032905.105441 ( 0) 0)
|
| [] |
Sauer N.2007. Molecular physiology of higher plant sucrose transporters. FEBS Letters , 581 (12) : 2309–2317.
DOI:10.1016/j.febslet.2007.03.048 ( 0) 0)
|
| [] |
Schulz A, Beyhl D, Marten I, et al.2011. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. The Plant Journal , 68 (1) : 129–136.
DOI:10.1111/j.1365-313X.2011.04672.x ( 0) 0)
|
| [] |
Slewinski T L, Braun D M.2010. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Science , 178 (4) : 341–349.
DOI:10.1016/j.plantsci.2010.01.010 ( 0) 0)
|
| [] |
Smeekens S.2000. Sugar-induced signal transduction in plants. Annual Review of Plant Biology , 51 (1) : 49–81.
DOI:10.1146/annurev.arplant.51.1.49 ( 0) 0)
|
| [] |
Srivastava A C, Ganesan S, Ismail I O, et al.2008. Functional characterization of the Arabidopsis AtSUC2 sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiology , 148 (1) : 200–211.
DOI:10.1104/pp.108.124776 ( 0) 0)
|
| [] |
Stitt M, Lunn J, Usadel B.2010. Arabidopsis and primary photosynthetic metabolism-more than the icing on the cake. The Plant Journal , 61 (6) : 1067–1091.
DOI:10.1111/tpj.2010.61.issue-6 ( 0) 0)
|
| [] |
Winter H, Robinson D G, Heldt H W.1993. Subcellular volumes and metabolite concentrations in barley leaves. Planta , 191 (2) : 180–190.
( 0) 0)
|
| [] |
Wormit A, Trentmann O, Feifer I, et al.2006. Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. The Plant Cell , 18 (12) : 3476–3490.
DOI:10.1105/tpc.106.047290 ( 0) 0)
|
| [] |
Yamada K, Osakabe Y, Mizoi J, et al.2010. Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. Journal of Biological Chemistry , 285 (2) : 1138–1146.
DOI:10.1074/jbc.M109.054288 ( 0) 0)
|
| [] |
Zheng H, Camacho L, Wee E, et al.2005. A Rab-E GTPase mutant acts downstream of the Rab-D subclass in biosynthetic membrane traffic to the plasma membrane in tobacco leaf epidermis. The Plant Cell , 17 (7) : 2020–2036.
DOI:10.1105/tpc.105.031112 ( 0) 0)
|
 2016, Vol. 52
2016, Vol. 52

