文章信息
- 熊福全, 韩雁明, 李改云, 秦特夫, 王思群, 储富祥
- Xiong Fuquan, Han Yanming, Li Gaiyun, Qin Tefu, Wang Siqun, Chu Fuxiang
- 点击化学在木质纤维素化学修饰中的研究现状
- Research Status of Click Chemistry Used for Chemical Modification of Lignocellulose
- 林业科学, 2016, 52(3): 90-96
- Scientia Silvae Sinicae, 2016, 52(3): 90-96.
- DOI: 10.11707/j.1001-7488.20160311
-
文章历史
- 收稿日期:2015-04-20
- 修回日期:2015-05-31
-
作者相关文章
2. 美国田纳西大学再生碳中心 Tennessee 37996-4570
2. Center for Renewable Carbon, University of Tennessee USA Tennessee 37996-4570
随着人们对环境问题的逐步重视,功能生物基材料的发展受到广泛关注,以求其在生物医学、包装和防护涂层等方面都能得到大量应用(Tingaut et al.,2011)。木质纤维基生物质是一种丰富且廉价的可再生资源(Brusa et al.,2014),主要包括纤维素、半纤维素和木质素(Lynd et al.,2002)。纤维素是地球上最丰富的生物聚合物(Mäki-Arvela et al.,2010),由许多β-D-葡萄糖以1-4-β-苷键连接(Zhang et al.,2005),具有密度低、可再生、成本低和机械性能好等优点(Pandey et al.,2005)。半纤维素主要是由木聚糖等组成的一类多糖物质(Brusa et al.,2014)。木质素是一种无规则的高聚物,通过苯基丙烷随机交联而形成三维网状结构,嵌入到到纤维素和半纤维素中(King et al.,2009)。由于木质素结构复杂,通常被认为是废料或低价值的副产品(Casas et al.,2012)。
化学修饰是改善木质纤维素用途的一种重要工具(Hasani et al.,2007),能改善木质纤维素在聚合物基质中的相容性且使其具有特定功能。接枝共聚是化学修饰的一种重要途径,木质纤维素表面具有羟基等活性位点为其接枝修饰提供可能(Brusa et al.,2014; Gao et al.,2014; Yu et al.,2014)。已有许多接枝共聚方法在木质纤维素的化学修饰中被应用,如自由基聚合(Khan,2004)、开环聚合(Zhang et al.,2015)、可逆加成-断裂链转移可控自由基聚合(RAFT)(Liu et al.,2015b; Roy et al.,2005; 2007)、原子转移自由基聚合(ATRP)(Liu et al.,2015a; Meng et al.,2009)和点击化学(Enomoto-Rogers et al.,2012; Liebert et al.,2006; Michinobu et al.,2008; Peng et al.,2012)。
点击化学(click chemistry)是由2001年诺贝尔化学奖获得者Sharpless首次提出的(Kolb et al.,2001),主要通过小单元的拼接,尤其强调在温和的反应条件下高选择性地形成碳-杂原子键(C—X—C)进而合成各种分子。相比传统接枝修饰方法,点击化学具有反应条件温和、环境污染小、副反应少、分离提纯简单、产量和效率高等优点,现已广泛应用在表面修饰(Sedlmeier et al.,2015)、材料科学(Guo et al.,2014)、生物技术(El-Sagheer et al.,2010)等领域。点击化学主要包括铜催化叠氮-炔基环加成反应(CuAAC)、环张力催化叠氮-炔基环加成反应(SPAAC)、巯基-烯/炔点击反应(Thiol-Ene/Yne)和狄尔斯-阿尔德尔环加成反应(Diels-Alder)等类型(图 1),在木质纤维素的化学修饰上已有相关应用。本文分别针对点击化学在纤维素、半纤维素和木质素中的研究进展进行详细分析,以期为点击化学在木质纤维素中的应用提供方法借鉴。
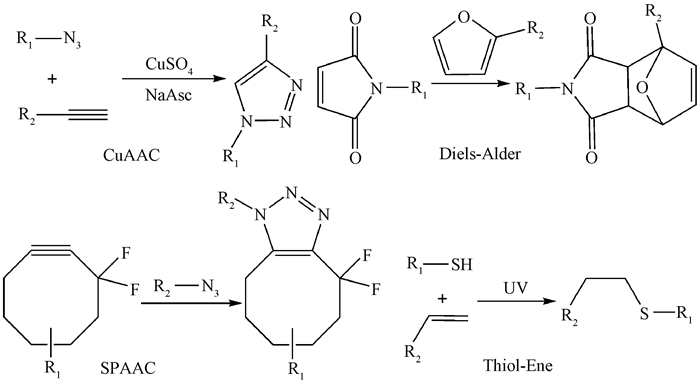 |
图 1 典型点击反应机制(Liebert et al.,2006; Zhao et al.,2010; Navarro et al.,2015)
Fig. 1 Mechanism of typical click reactions
|
铜催化叠氮-炔基环加成反应是一种最普遍的点击反应类型,也是对木质纤维素化学修饰应用最多的一类反应。Liebert等(2006)第一次将点击化学方法应用到纤维素的化学修饰上。首先将磺酸化的微晶纤维素进行叠氮化,然后在二甲基亚砜(DMSO)中将叠氮化的产物分别与丙炔酸甲酯、氨基苯乙炔和乙炔噻吩进行均相点击反应。相比多糖化合物的酯化反应,点击反应使聚合物骨架和官能团之间形成了稳定的键结合。Krouit等(2008)在非均相条件下将聚己内酯大分子链接枝到纤维素表面,首先将十一碳炔酸与微晶纤维素进行酯化反应制备含有炔基的前驱体,分析其取代度大约为0.1,然后对聚己内酯二醇进行叠氮化,最后2种前驱体在非均相混合条件下进行点击反应,通过红外光谱、光电子能谱和元素分析确认点击反应发生。随后,Huang等(2014b)在非均相条件下,通过CuAAC成功将磺基三甲胺乙内酯两性离子基团接枝到纤维素膜表面。
由于铜催化叠氮-炔基环加成反应后催化剂很难移除,因此在一定程度上限定了反应后产品的用途。Koga等(2012)用Cu(I)交换2,2,6,6-四甲基哌啶氧化物自由基(TEMPO)法制备纳米纤维(TOCNs)上的Na,然后通过冻干形成Cu-TOCN气凝胶。将Cu-TOCN气凝胶催化叠氮苄和苯乙炔进行点击反应发现其表现出优异的催化效率,证明Cu(I)高效分散并且充分暴露在TOCNs表面使其能与反应物充分接触。由于Cu-TOCN气凝胶可以回收且TOCNs很容易从天然纤维素中获得,使得Cu-TOCN气凝胶具有潜在应用价值。
相比铜催化叠氮-炔基环加成反应,巯基-烯/炔点击反应不需要有毒的铜作为催化剂,它以光引发自由基反应。Zhao等(2010)第1次将这种点击反应引用到纤维素化学修饰上,成功将芳基、烷基和聚酯基团接枝到纤维素上。随后,Tingaut等(2011)利用巯基-烯点击反应采用3种途径对纤维素膜进行功能化(图 2),即烯基功能化膜与巯基化合物进行点击反应(Route 1)、巯基功能化膜与烯基化合物进行点击反应(Route 2)、巯基化合物和烯基化合物点击反应后再接枝到膜表面(Route 3),发现Route 1和Route 3对纤维素膜功能化效率较Route 2高。
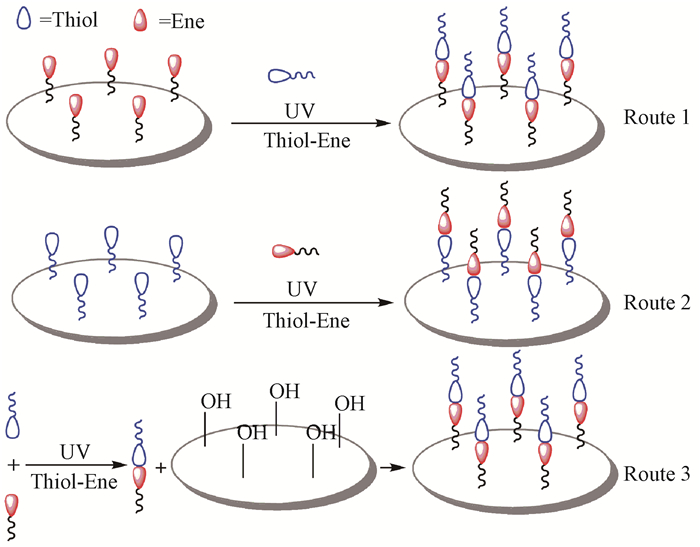 |
图 2 纤维素膜的3种功能化途径(Tingaut et al.,2011)
Fig. 2 Three kinds of functional approach on cellulose film
|
水凝胶是一种亲水性三维网络聚合物(Yu et al.,2008)。Zhang等(2009)首先用叠氮基和炔基分别修饰纤维素和聚N-异丙基丙烯酰胺-co-甲基丙烯酸羟乙酯,然后将2种前驱体通过CuAAC形成一种温敏性水凝胶,通过改变温度确认其具有良好的温度敏感性。随后,Koschella等(2011)同样通过CuAAC制备了一种纤维素基水凝胶材料,首先将磺酸化的纤维素接枝上叠氮基和炔基,然后对接枝后的2种产物进行羧甲基化制备水溶性的纤维素衍生物,最后将2种纤维素衍生物进行点击反应制备水凝胶。制备的水凝胶含有98.4%的水,冻干后形成多孔海绵结构材料。
Filpponen等(2010)通过CuAAC制备了片状纳米纤维素凝胶,首先通过TEMPO氧化方法对纳米纤维素进行氧化,然后通过酰胺键连接形成含有叠氮基和炔基的前驱体,最后将2种前驱体进行点击反应形成一种有规律排列的片状纳米纤维素凝胶。
1.2.2 荧光标记材料荧光材料广泛应用于纳米探针和生物医学等领域。Pahimanolis等(2011)通过CuAAC用氨基乙基联苯功能化制备pH敏感型纳米纤维素时,为确认叠氮化纳米纤维素的存在,将5-甲氨基-N-(2-丙基)-1-萘磺酰胺与叠氮化的纳米纤维素进行点击反应制备了一种强荧光的纳米纤维素。随后,Huang等(2014a)通过巯基-烯点击反应制备功能化的纤维素纳米晶体膜时,使用N-苯基马来酰亚胺对膜表面进行化学修饰,为了确认点击反应的发生,使用荧光染料(N-(1-芘基)马来酰亚胺)替代N-苯基马来酰亚胺与巯基功能化纤维素纳米晶体膜进行点击反应,最终获得了荧光标记的膜材料。
Navarro等(2015)通过Diels-Alder和Thiol-Ene合成了多色荧光标记的纳米纤维素材料(图 3)。首先将荧光素二乙酸5-马来酰亚胺和N,N’-(4,4’-亚甲基二苯基)双马来酰亚胺与含有呋喃基团的纳米纤维素进行Diels-Alder点击反应,然后将7-巯基-4-甲基香豆素与第一步产物中的N,N’-(4,4’-亚甲基二苯基)双马来酰亚胺进行Thiol-Ene点击反应。紫外-可见分光光度计和荧光光度计证明能成功合成多色纳米纤维素材料,且其在激光共聚焦显微镜下能成像。
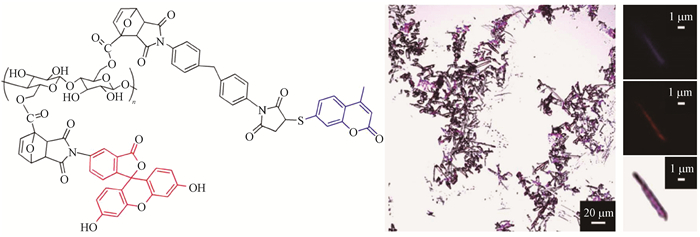 |
图 3 多色荧光标记纳米纤维素的化学结构和激光共聚焦显微镜成像(Navarro et al.,2015)
Fig. 3 Confocal scanning laser microscopy imaging and chemical structure of cellulose nanofibrils with multi-color fluorescent labelling
|
Eyley等(2011)通过CuAAC制备了一种阴离子交换功能的纳米纤维素材料,首先将氯化后的纳米纤维素与NaN3反应,然后将叠氮化产物与离子液体1-甲基-3-丙炔基咪唑溴盐进行点击反应。通过这一方法成功用咪唑嗡盐离子修饰纳米纤维素表面,修饰后纳米纤维素表面的电荷密度为1.17 e nm-2,展现出阴离子交换能力。
Rosilo等(2013)将双键修饰后的纤维素纳米晶须(mCNCs)和聚丁二烯(PBD)与1,9-壬二硫醇在紫外光引发下进行巯基-烯点击反应制备复合膜(图 4),透射电镜观察发现mCNCs硬段与PBD软段交替出现进行自组装。随着mCNCs质量的增加,复合膜由PBD占主导的软材料转为由mCNCs占主导,使其机械性能显著增强,且在mCNCs质量占80%时膜的拉伸应力达到最大值16 MPa;此外,膜对空气湿度的变化不明显。
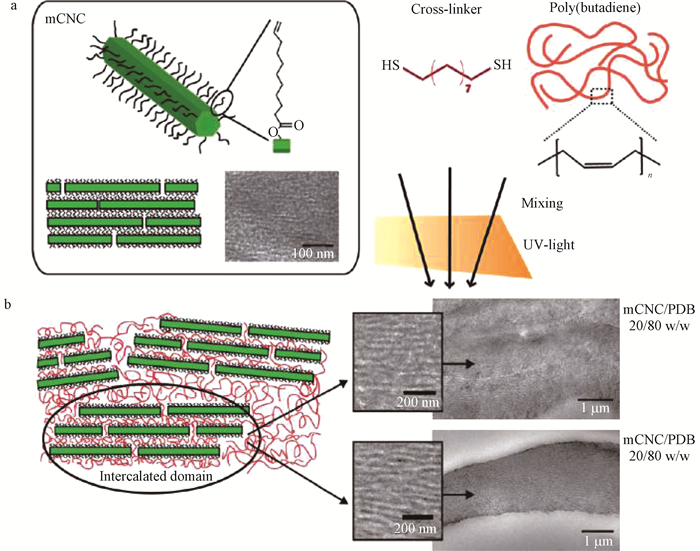 |
图 4 在2种纤维素纳米晶须/聚丁二烯比例下制备复合材料的流程(Rosilo et al.,2013)
Fig. 4 A scheme of the ellulose nanocrystals/poly(butadiene)composites with structures at two length scales
a.纤维素纳米晶须与聚丁二烯复合材料的制备过程Preparation scheme of composites with cellulose nanocrystals and poly(butadiene);b.纤维素纳米晶须与聚丁二烯分别在质量比80/20和20/80时的透射电镜形貌The TEM micrographs are for the compositions cellulose nanocrystals/poly(butadiene)80/20 w/w and 20/80 w/w. |
Agag等(2012)通过点击反应将苯并恶嗪接枝到纤维素表面,使其热分解温度变宽且苯并恶嗪发生交联反应,使其最终分解剩余物由原来的4%增加到40%。Kabiri等(2014)通过点击化学方法将纳米纤维素修饰氧化石墨烯,分析表明氧化石墨烯逐渐被功能化,转化为还原氧化石墨烯,通过热重分析表明氧化石墨烯的功能化程度大约为23%。
2 点击化学在半纤维素和木质素中的研究现状通过化学修饰能使半纤维素功能化,尤其是近些年新材料和功能聚合物的发展,使得半纤维素受到了广泛关注(Heinze et al.,2004)。然而在已有修饰方法中,使用点击化学修饰的报道非常少。Enomoto-Rogers等(2012)将桉树 (Eucalyptus)硫酸盐浆中抽提的木聚糖作为初始材料溶解在DMAc/LiCl中,用6-溴己酰氯和二甲基氨基嘧啶对其酯化,然后将酯化后的木聚糖溶解在DMF中,用NaN3处理,从而将其末端的官能团由溴转化为叠氮基。此外,用炔丙基醇作引发剂,辛酸锡作催化剂,将3种不同分子质量的聚乳酸端基分别接上炔丙基。最后将末端基为叠氮基的木聚糖,末端基为炔丙基的聚乳酸和五甲基二乙烯基三胺按特定比例溶解在DMF中,用溴化亚铜做催化剂在氮气氛围下进行点击反应。DSC测量显示共聚物的玻璃化转变温度较叠氮化木聚糖低,主要是由于共聚物中接入的聚乳酸扮演塑化剂的作用。TGA分析表明共聚物的分解温度较聚合前2种前驱体高,且共聚物的解聚温度随木聚糖主链上接枝的聚乳酸支链的减少而增加。
木糖在生物细胞中是一种特殊的多糖,仅在蛋白聚糖中呈现。蛋白聚糖主要由核蛋白和糖胺聚糖(GAG)侧链组成。GAG侧链在众多生物功能管理中扮演重要角色,含有疏水性糖苷配基基团的木糖苷能引导GAG链的合成。Brusa等(2014)通过两步法合成苯三唑连接的木糖苷和木二糖苷。首先在水相中将山毛榉 (Fagus)的木聚糖用木聚糖酶催化合成含有炔丙基的木糖苷和木二糖苷,然后将其与各种含有叠氮基的脂肪族和芳香族化合物通过CuAAC反应形成苯三唑连接高产率的邻位木糖苷和木二糖苷。
化学修饰被认为是木质素作为聚合物和化学合成起始材料的最好方法(Laurichesse et al.,2014)。Michinobu等(2008)用木质素的衍生物2-吡喃酮- 4,6-羧基酸作原料,通过酯化接上炔基,然后与1,2-二(2-叠氮乙氧基)-乙烷进行点击反应。由于缺少配位体,聚合速率非常慢,甚至在15 h后通过GPC观察任然是小分子片段,且通过红外观测发现聚合物中任有叠氮基残留。聚合时间从27 h增加到70 h,发现小分子片段完全转化为高分子的聚合物。在聚合时间为70 h时,聚合物的最高分子量已经高于106。
Chung等(2013)将聚苯乙烯端基用叠氮基功能化,木质素羟基用炔基功能化,然后用铜催化二者进行点击反应,1H NMR表明聚苯乙烯端基完全功能化且反应后木质素和聚苯乙烯片段没有残余;此外,GPC测量表明点击反应在5 h反应完全。这种木质素基共聚物为其在加工过程中作为一种热塑性聚合物展示了较高的灵活性。Van deáWouwer等(2014)在用生物正交点击化学描叙活体植物细胞壁的木质化过程中(图 5),将炔基或叠氮基标记的愈创木基合成木质素与相应标记的染料进行点击反应时发现,铜催化叠氮-炔基环加成反应比环张力促进的叠氮-炔环加成反应有效,但环张力促进的叠氮-炔环加成反应能避免使用有毒的铜作为催化剂。
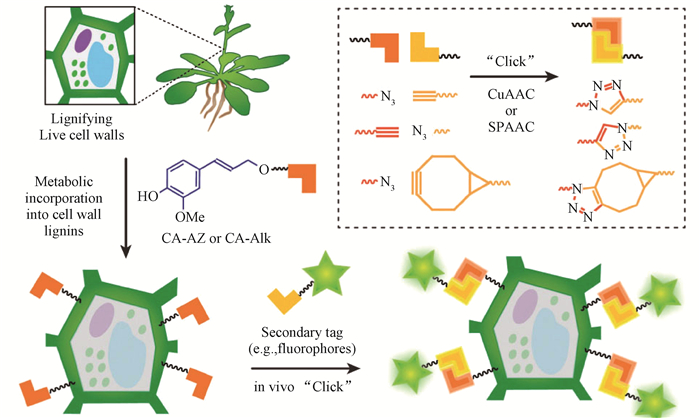 |
图 5 植物细胞壁木质素的点击化学标记(Van deáWouwer et al.,2014)
Fig. 5 Click chemistry labeling of plant cell wall lignin
|
点击化学具有高度选择性和广泛适用性,能广泛应用于聚合物的功能化、大分子构建、材料的设计和合成;然而,点击化学在木质纤维素上的应用还没有深入探索,扩大点击化学在木质纤维素上的应用范围仍然需要进一步探索:
1)由于点击反应的条件比较温和,很多反应都能在室温下进行,非常有利于在生物医用材料领域的研究,并且纤维素和半纤维素等多糖又具有良好的生物相容性,为纤维素和半纤维素在生物领域的应用创造了有利条件。此外,点击化学具有较高的接枝效率,这为木质素的化学修饰提供了有利条件。
2)由于铜催化的毒性作用,无铜催化的点击反应可能会作为一个重要发展方向,像环张力促进的叠氮-炔环加成反应、狄尔斯-阿尔德尔环加成反应、热促进的叠氮-炔环加成反应和巯基-烯/炔点击反应等。无铜点击反应避免了有毒铜催化剂的使用,这将有效拓展化学修饰后产品的用途。
| [1] |
Agag T, Vietmeier K, Chernykh A, et al. 2012. Side-chain type benzoxazine-functional cellulose via click chemistry. Journal of Applied Polymer Science,125(2):1346-1351.( 1) 1)
|
| [2] |
Brusa C, Ochs M, Rémond C, et al. 2014. Chemoenzymatic synthesis of "click" xylosides and xylobiosides from lignocellulosic biomass. RSC Advances,4(18):9330-9338.( 4) 4)
|
| [3] |
Casas A, Alonso M V, Oliet M, et al. 2012. FTIR analysis of lignin regenerated from Pinus radiata and Eucalyptus globulus woods dissolved in imidazolium-based ionic liquids. Journal of Chemical Technology and Biotechnology,87(4):472-480.( 1) 1)
|
| [4] |
Chung H, Al-Khouja A, Washburn N R. 2013. Lignin-Based Graft Copolymers via ATRP and Click Chemistry. Washington, DC:American Chemical Society.( 1) 1)
|
| [5] |
El-Sagheer A H, Brown T. 2010. Click chemistry with DNA. Chemical Society Reviews,39(4):1388-1405.( 1) 1)
|
| [6] |
Enomoto-Rogers Y, Iwata T. 2012. Synthesis of xylan-graft-poly (L-lactide) copolymers via click chemistry and their thermal properties. Carbohydrate Polymers,87(3):1933-1940.( 2) 2)
|
| [7] |
Eyley S, Thielemans W. 2011. Imidazolium grafted cellulose nanocrystals for ion exchange applications. Chemical Communications,47(14):4177-4179.( 1) 1)
|
| [8] |
Filpponen I, Argyropoulos D S. 2010. Regular linking of cellulose nanocrystals via click chemistry:synthesis and formation of cellulose nanoplatelet gels. Biomacromolecules,11(4):1060-1066.( 1) 1)
|
| [9] |
Gao G, Karaaslan M A, Kadla J F, et al. 2014. Enzymatic synthesis of ionic responsive lignin nanofibres through surface poly (N-isopropylacrylamide) immobilization. Green Chemistry,16(8):3890-3898.( 1) 1)
|
| [10] |
Guo J, Xie Z, Tran R T, et al. 2014. Click chemistry plays a dual role in biodegradable polymer design. Advanced Materials,26(12):1906-1911.( 1) 1)
|
| [11] |
Hasani M M, Westman G. 2007. New coupling reagents for homogeneous esterification of cellulose. Cellulose,14(4):347-356.( 1) 1)
|
| [12] |
Heinze T, Koschella A, Ebringerova A. 2004. Chemical functionalization of xylan:a short review. Washington, DC:American Chemical Society.( 1) 1)
|
| [13] |
Huang J L, Li C J, Gray D G. 2014a. Functionalization of cellulose nanocrystal films via "thiol-ene" click reaction. RSC Advances,4(14):6965-6969.( 1) 1)
|
| [14] |
Huang W, Huang J, Xu C, et al. 2014b. Surface functionalization of cellulose membrane via heterogeneous "click" grafting of zwitterionic sulfobetaine. Polymer Bulletin,71(10):2559-2569.( 1) 1)
|
| [15] |
Kabiri R, Namazi H. 2014. Surface grafting of reduced graphene oxide using nanocrystalline cellulose via click reaction. Journal of nanoparticle research,16(7):1-13.( 1) 1)
|
| [16] |
Khan F. 2004. Photoinduced graft-copolymer synthesis and characterization of methacrylic acid onto natural biodegradable lignocellulose fiber. Biomacromolecules,5(3):1078-1088.( 1) 1)
|
| [17] |
King A, Kilpeläinen I, Heikkinen S, et al. 2009. Hydrophobic interactions determining functionalized lignocellulose solubility in dialkylimidazolium chlorides, as probed by 31P NMR. Biomacromolecules,10(2):458-463.( 1) 1)
|
| [18] |
Koga H, Azetsu A, Tokunaga E, et al. 2012. Topological loading of Cu (Ⅰ) catalysts onto crystalline cellulose nanofibrils for the Huisgen click reaction. Journal of Materials Chemistry,22(12):5538-5542.( 1) 1)
|
| [19] |
Kolb H C, Finn M, Sharpless K B. 2001. Click chemistry:diverse chemical function from a few good reactions. Angewandte Chemie International Edition,40(11):2004-2021.( 1) 1)
|
| [20] |
Koschella A, Hartlieb M, Heinze T. 2011. A "click-chemistry" approach to cellulose-based hydrogels. Carbohydrate Polymers,86(1):154-161.( 1) 1)
|
| [21] |
Krouit M, Bras J, Belgacem M N. 2008. Cellulose surface grafting with polycaprolactone by heterogeneous click-chemistry. European Polymer Journal,44(12):4074-4081.( 1) 1)
|
| [22] |
Laurichesse S, Avérous L. 2014. Chemical modification of lignins:Towards biobased polymers. Progress in Polymer Science,39(7):1266-1290.( 1) 1)
|
| [23] |
Liebert T, Hänsch C, Heinze T. 2006. Click chemistry with polysaccharides. Macromolecular rapid communications,27(3):208-213.( 3) 3)
|
| [24] |
Liu X, Yin H, Zhang Z, et al. 2015a. Functionalization of lignin through ATRP grafting of poly (2-dimethylaminoethyl methacrylate) for gene delivery. Colloids and Surfaces B:Biointerfaces,125:230-237.( 1) 1)
|
| [25] |
Liu Y, Jin X, Zhang X, et al. 2015b. Self-assembly and chiroptical property of poly (N-acryloyl-l-amino acid) grafted celluloses synthesized by RAFT polymerization. Carbohydrate Polymers,117:312-318.( 1) 1)
|
| [26] |
Lynd L R, Weimer P J, Van Zyl W H, et al. 2002. Microbial cellulose utilization:fundamentals and biotechnology. Microbiology and molecular biology reviews,66(3):506-577.( 1) 1)
|
| [27] |
Mäki-Arvela P, Anugwom I, Virtanen P, et al. 2010. Dissolution of lignocellulosic materials and its constituents using ionic liquids-A review. Industrial Crops and Products,32(3):175-201.( 1) 1)
|
| [28] |
Meng T, Gao X, Zhang J, et al. 2009. Graft copolymers prepared by atom transfer radical polymerization (ATRP) from cellulose. Polymer,50(2):447-454.( 1) 1)
|
| [29] |
Michinobu T, Inazawa Y, Hiraki K, et al. 2008. A novel biomass-based polymer prepared from lignin-derived stable metabolic intermediate by copper (Ⅰ)-catalyzed azide-alkyne Click reaction. Chemistry Letters,37(2):154-155.( 2) 2)
|
| [30] |
Navarro J R, Conzatti G, Yu Y, et al. 2015. Multi-color fluorescent labelling of cellulose nanofibrils by click-chemistry. Biomacromolecules,16(4):1293-1300.( 3) 3)
|
| [31] |
Pahimanolis N, Hippi U, Johansson L S, et al. 2011. Surface functionalization of nanofibrillated cellulose using click-chemistry approach in aqueous media. Cellulose,18(5):1201-1212.( 1) 1)
|
| [32] |
Pandey J K, Kumar A P, Misra M, et al. 2005. Recent advances in biodegradable nanocomposites. Journal of Nanoscience and Nanotechnology,5(4):497-526.( 1) 1)
|
| [33] |
Peng P, Cao X, Peng F, et al. 2012. Binding cellulose and chitosan via click chemistry:synthesis, characterization, and formation of some hollow tubes. Journal of Polymer Science Part A:Polymer Chemistry,50(24):5201-5210.( 1) 1)
|
| [34] |
Rosilo H, Kontturi E, Seitsonen J, et al. 2013. Transition to reinforced state by percolating domains of intercalated brush-modified cellulose nanocrystals and poly (butadiene) in cross-linked composites based on thiol-ene click chemistry. Biomacromolecules,14(5):1547-1554.( 2) 2)
|
| [35] |
Roy D, Guthrie J T, Perrier S. 2005. Graft polymerization:grafting poly (styrene) from cellulose via reversible addition-fragmentation chain transfer (RAFT) polymerization. Macromolecules,38(25):10363-10372.( 1) 1)
|
| [36] |
Roy D, Knapp J S, Guthrie J T, et al. 2007. Antibacterial cellulose fiber via RAFT surface graft polymerization. Biomacromolecules,9(1):91-99.( 1) 1)
|
| [37] |
Sedlmeier A, Gorris H H. 2015. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chemical Society Reviews,44(6):1526-1560.( 1) 1)
|
| [38] |
Tingaut P, Hauert R, Zimmermann T. 2011. Highly efficient and straightforward functionalization of cellulose films with thiol-ene click chemistry. Journal of Materials Chemistry,21(40):16066-16076.( 3) 3)
|
| [39] |
Van deáWouwer D. 2014. A click chemistry strategy for visualization of plant cell wall lignification. Chemical Communications,50(82):12262-12265.( 2) 2)
|
| [40] |
Yu H Y, Qin Z Y. 2014. Surface grafting of cellulose nanocrystals with poly (3-hydroxybutyrate-co-3-hydroxyvalerate). Carbohydrate Polymers,101:471-478.( 1) 1)
|
| [41] |
Yu L, Ding J. 2008. Injectable hydrogels as unique biomedical materials. Chemical Society Reviews,37(8):1473-1481.( 1) 1)
|
| [42] |
Zhang H, Wu J, Zhang J, et al. 2005. 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid:a new and powerful nonderivatizing solvent for cellulose. Macromolecules,38(20):8272-8277.( 1) 1)
|
| [43] |
Zhang J, Xu X D, Wu D Q, et al. 2009. Synthesis of thermosensitive P (NIPAAm-co-HEMA)/cellulose hydrogels via "click" chemistry. Carbohydrate Polymers,77(3):583-589.( 1) 1)
|
| [44] |
Zhang X, Chen M, Liu C, et al. 2015. Homogeneous ring opening graft polymerization of ε-caprolactone onto xylan in dual polar aprotic solvents. Carbohydrate Polymers,117:701-709.( 1) 1)
|
| [45] |
Zhao G L, Hafrén J, Deiana L, et al. 2010. Heterogeneous "Organoclick" derivatization of polysaccharides:photochemical thiol-ene click modification of solid cellulose. Macromolecular Rapid Communications,31(8):740-744.( 2) 2)
|
 2016, Vol. 52
2016, Vol. 52

