文章信息
- 张靖梓, 柏新富, 侯玉平, 董周焱, 卜庆梅
- Zhang Jingzi, Bai Xinfu, Hou Yuping, Dong Zhouyan, Bu Qingmei
- 山东半岛沿海防护林入侵植物美洲商陆及其伴生种生长竞争力的比较
- Comparison on the competitiveness of the invaded pokeweed with its accompanying species in the coastal protection forest of Shandong Peninsula
- 林业科学, 2016, 52(3): 23-29
- Scientia Silvae Sinicae, 2016, 52(3): 23-29.
- DOI: 10.11707/j.1001-7488.20160303
-
文章历史
- 收稿日期:2015-02-09
- 修回日期:2016-01-04
-
作者相关文章
生物入侵可导致原有生物地理分布和生态系统结构与功能的改变、生物多样性的丧失,影响农业生产和人类健康,造成巨大经济损失(Mack et al.,2011; Barney et al.,2013; 闫小玲等,2014)。生境的可入侵性、自然控制机制的缺失及人为干扰等因素及外来生物自身的强生理生态适应性是造成生物成功入侵的重要因素(Pintó-Marijuan et al.,2013; Coccia et al.,2013)。如入侵植物紫茎泽兰(Fupatorium adenophora)具有较高的资源捕获能力(Feng et al.,2009)、对光和养分的获取能力(王俊峰等,2006)等生理生态特性,使其在我国西南地区迅速蔓延,成为危害严重的入侵植物之一。
美洲商陆(Phytolacca americana)又名垂序商陆、十蕊商陆,多为年生草木,高1~2 m,灌木状,最早作为药用植物引入我国,随后在全国大部分地区迅速蔓延,国家林业局2009年发布林业有害生物警示通报(2009第5号)把其列入入侵植物(董周焱等,2014),美洲商陆为多年生草本,与其伴生和在光能、水分、氮素等资源的利用上处于竞争关系的主要是紫穗槐(Amorpha fruticosa)。美洲商陆成为茶园、果园、林地的重要杂草,给入侵地农林业生产及生物多样性带来严重威胁。
国内学者对美洲商陆的入侵性的研究多集中在繁殖、扩散(翟树强等,2010)以及化感作用(闫小红等,2012)等方面,而有关美洲商陆入侵的生理生态机制研究还少见报道。在山东半岛沿海防护林带,美洲商陆主要分布在刺槐(Robinia pseudoacacia)、黑松(Pinus thunbergii)等林下,林缘也有片状分布,该物种已导致沿海防护林下的生物多样性严重降低(付俊鹏等,2012)。本试验通过比较美洲商陆和紫穗槐在不同光能资源环境下光合性能、光能、水分、氮素等资源利用效率以及叶片建成成本等,探索美洲商陆入侵的生理生态机制及在海岸防护林下扩张、泛滥的原因,以便为美洲商陆的风险评价和综合防控提供参考。
1 材料与方法 1.1 研究区概况试验在烟台市北部沿海防护林带进行。烟台属温带季风气候,夏季凉爽,冬季温润; 年平均降水量为651.9 mm,年平均气温11.8 ℃,年平均相对湿度68%,太阳辐射总量年平均值5 224.4 MJ·m-2,年平均风速4~6 m·s-1,平均无霜期210天。试验区近海侧距高潮线约50 m,从高潮线向陆地方向依次为裸露沙滩—海岸草坎—柽柳(Tamarix chinensis)灌丛—黑松和刺槐为主的防护林带。试验区土壤为滨海沙土,基质由疏松的中、粗沙组成,土壤有机质含量为1.01%,速效氮、磷、钾分别为56.8,0.8和52.5 mg·kg-1,pH值为5.8。黑松和刺槐林下密布美洲商陆和点片状紫穗槐与美洲商陆伴生,林缘散布美洲商陆与紫穗槐伴生。林下和林缘有少量的肾叶打碗花(Calystegia soldanella)和白茅(Imperata cylindrica)等分布。
1.2 试验材料以垂直海岸线方向将试验区分为5个区段,每个区段宽约20 m。在每个区段内选择林缘高光照环境条件(简称: 高光照环境)和林下低光照环境条件(仅为林缘光照强度的10%~15%,简称: 低光照环境)下生长的美洲商陆及其伴生的紫穗槐各5株健康植株作为研究对象,于2014年7月上旬选取中部侧枝近顶端的成熟叶进行野外测定和取样。
1.3 试验方法 1.3.1 光合作用指标及光能利用率和水分利用率的测定使用TPS-1便携式光合系统(PP System公司,美国)在08:30—11:30测定记录不同光照强度下净光合速率(Pn)、蒸腾速率(Tr)等指标。光照强度由LED光源控制,在林缘高光照条件设置为1 800,1 600,1 400,1 200,1 000,800,600,400,200,150,100,50和0 μmol·m-2s-1; 林下低光照条件设置为1 000,800,600,400,200,150,100,50和0 μmol·m-2s-1。参照Ye(2007)的直角双曲线修正模型作净光合速率(Pn)-光照强度(PAR)响应曲线,由曲线求出最大净光合速率(Pmax)、光饱和点(Light saturation point,LSP)、光补偿点(Light compensation point,LCP),并利用曲线初始斜率计算表观光量子效率(Apparent quantum yield,AQY); 参照王珊珊等(2011)的方法计算光能利用率[Light use efficiency,LUE(molCO2·mol-1proton)=Pn/PAR]和水分利用率[Water use efficiency,WUE(mmol·mol-1)=Pn/Tr]。
1.3.2 叶片叶绿素含量的测定叶绿素含量用丙酮、乙醇等量混合液提取法(宫兆宁等,2014)提取和测定。每份样品取5个叶片,用内径0.94 cm的打孔器在每个叶片上各打取1个叶圆片(在每个叶片的对称部位另取1个叶圆片烘干称重获得干质量数值),然后用锋利刀片切成细丝状置于试管中,加入丙酮、无水乙醇等量混合液25 mL,密封、闭光提取24 h,在645和663 nm 处测光密度值,根据Arnon公式计算叶绿素含量。根据叶圆片干质量计算单位质量叶绿素含量。
1.3.3 叶绿素荧光参数的测定用便携式植物效率分析仪(Handy-PEA,Hansatech公司,英国)测定叶绿素荧光参数。激发光强为3 000 μmol·m-2s-1,暗适应时间为20 min,记录时间1 s,荧光参数由Handy PEA软件直接从测定结果中导出。每个样本重复测定20个叶片。
1.3.4 光合氮利用效率测定将采集的美洲商陆和紫穗槐的成熟叶片用烘干,称重,测定计算比叶质量(LMA)和比叶面积(SLA)。LMA(g·m-2)=叶片干质量/叶面积,SLA(m2·g-1)=1/LMA。样品烘干、粉碎后用小进样量元素分析仪(Elementar公司,德国)测定叶片全氮含量(以单位质量的氮含量表示,Nmass)。光合氮利用效率(Photosynthetic nitrogen-use efficiency,PNUE)参照苗艳明等(2012)的方法计算,即PNUE(μmolCO2·mol-1s-1)=Pmax/(1/14 Nmass×LMA)。式中,Pmax为最大净光合速率(μmolCO2·m-2s-1),Nmass为叶片含氮量(g·g-1DW),LMA为比叶重(g·m-2),乘以1/14计算氮摩尔分子量。
1.3.5 叶片建成成本的测定与计算灰分含量测定: 利用马福炉干灰化法。精确称取烘干、磨粉后的样品(1.000 0±0.005 0)g,用马福炉在700 ℃下灰化7 h,灰分含量(Ash content,AC,g·g-1)=(灰分质量/样品质量)。
热值测定: 称取烘干粉碎后的样品0.5 g左右压片、再烘干,精确称重(精确至0.000 1g)后用C2000氧弹热量计(德国,IKA公司)测定其热值,即为干质量热值(Gross caloric values,GCV)。去灰分热值(Ash free caloric values,AFCV)=GCV/(1-AC),式中AC为单位质量灰分含量(g·g-1)。测定环境温度在(25±1)℃,每次试验前用苯甲酸标定。
建成成本计算: 叶片单位质量建成成本(mass-based leaf construction cost,CCmass,g glucose·g-1)的计算按照Williams等(1987)的方法: CCmass=[(0.069 68 AFCV- 0.065)(1-AC)+ 7.5(k Nmass /14.006 7)]/EG。式中,AFCV: 去灰分热值(KJ·g-1); AC: 灰分含量(g·g-1); Nmass: 叶氮含量(g·g-1); EG: 生长效率,不同物种的生长效率为0.87(Daehler,2003); k: N的氧化态形式(若为NO3-,k=5; 若为NH4+,k=-3),本试验参照Shen等(2011)的方法,用2种氧化形式计算出的CCmass值的平均数作为结果。叶片单位面积建成成本(area-based leaf construction cost,CCarea,gglucose·m-2)=CCmass/SLA。
1.4 数据分析2个物种的高光照和低光照环境下生长的植株在每个区段内各选5株作为1个样本进行测定和取样(Pn-PAR响应曲线每个区段只选1株测定),除叶绿素荧光参数重复20次外,其他测定指标均重复5次,结果以"平均值±标准差"记。用Origin7.5作图、SPSS17.0 中的"One-Way ANOVA"和"Duncan"进行差异分析。
2 结果与分析 2.1 光合作用相关指标的比较美洲商陆及紫穗槐光合作用相关指标见表 1。除了光饱和点差异不显著(P>0.05)外,不论在林缘高光照环境下还是林下低光照环境下,美洲商陆的表观光量子效率(AQY)、最大净光合速率(Pmax)、叶绿素含量、光系统II最大光化学效率(Fv/Fm)和以吸收光能为基础的光合性能指数(PIABS)均显著高于紫穗槐(P<0.05),而光补偿点和ch1a/b比值则显著低于紫穗槐(P<0.05)。其中,林缘高光照环境下,美洲商陆的AQY,Pmax,Fv/Fm,PIABS和叶绿素含量分别比紫穗槐高10.7%,38.0%,5.0%,24.1%和44.1%,光补偿点和ch1a/b比值则低10.3%和26.6%; 而林下低光照环境下,美洲商陆和紫穗槐各指标的差异率均大于林缘高光照环境下的差异率。另外,对同一物种不同光照条件下的光合性能指标的比较发现,美洲商陆虽然在林下低光照下Pmax比高光照下降低43.0%,但其AQY,PIABS和叶绿素含量却比高光照下增加6.1%,22.8%和32.3%; 而紫穗槐在低光照下Pmax比高光照下降低59.9%,AQY也降低9.5%,仅PIABS和叶绿素含量比高光照下增加3.7%和15.2%。美洲商陆的光合性能和利用弱光的能力显著高于紫穗槐,且在低光照环境下这种优势更为显著。同时,美洲商陆根据光环境的变化来调整自身光合性能的能力也更强。
|
|
美洲商陆及紫穗槐的光能、水分利用率和光合氮利用率如图 1。在林缘高光照环境下,美洲商陆的光能利用率和光合氮利用率分别比紫穗槐高23.5%和34.0%; 水分利用率比紫穗槐低24.2%。在林下低光照环境下,2个物种的3项指标均显著低于高光照下的(P<0.05),但美洲商陆的降幅为16.1%~49.6%,而紫穗槐的降幅为28.5%~69.7%; 且美洲商陆的3项指标分别比紫穗槐高58.3%,43.7%和25.4%。这说明虽然在高光照环境下美洲商陆的水分利用率低于紫穗槐,但其对环境资源的利用效率整体上明显高于紫穗槐,特别是在低光照环境下更具优势。
 |
图 1 美洲商陆及紫穗槐的光能利用率、水分利用率和叶片光合氮利用率 Fig. 1 Light use efficiency (LUE), water use efficiency (WUE) and photosynthetic nitrogen-use efficiency (PNUE) of P. americana and A. fruticosa 不同字母表示在0.05水平上差异显著,下同。Significant differences were denoted with different letters (P<0.05).The same below. |
美洲商陆及紫穗槐的单位质量叶片建成成本(CCmass)和单位面积叶片建成成本(CCarea)如图 2。不论高光照还是低光照环境下,美洲商陆的CCmass和CCarea均显著低于紫穗槐(P<0.05)。这说明美洲商陆能够利用较少的物质和能量消耗来建成植物体,在同样的光能资源环境下具有更强的竞争力。另外,同一物种的CCmass在高光照和低光照环境下变幅较小(美洲商陆和紫穗槐的降幅分别为5.8%和1.7%),而CCarea变幅较大(美洲商陆和紫穗槐的降幅分别为51.2%和51.8%),这表明CCmass主要受物种因素的影响,而CCarea除受物种因素的影响外还受环境因素的较大影响,对环境的响应更为敏感。
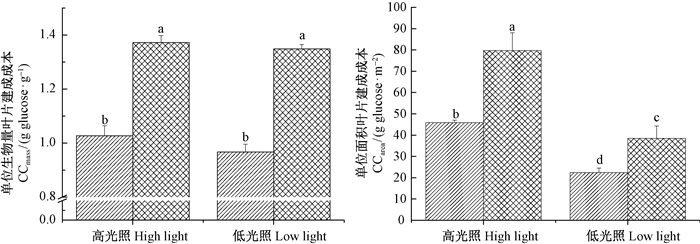 |
图 2 美洲商陆与紫穗槐叶片单位质量建成成本(CCmass)和单位面积建成成本(CCarea)
Fig. 2 Comparisons of the mass-based (CCmass) and area-based (CCarea) leaf construction cost in the invaded P. americana with its accompanying species A. fruticosa
|
外来植物自身的生理生态特性对其种群的入侵、生存和扩展至关重要,有些物种具有比本地种更强的光能利用能力和光合响应机制,从而使它们具有很强的入侵潜力(Heberling et al.,2013)。入侵植物薇甘菊(Mikania micrantha)和五爪金龙(Ipomoea cairica)与本地种相比具有较高的光合能力和水分利用效率等(王宇涛等,2012)。本试验发现,不论在高光照还是低光照环境下,美洲商陆的表观光量子效率、最大光合速率、叶绿素含量、光系统II的最大光化学效率(Fv/Fm)和性能指数(PIABS)均显著高于紫穗槐,而其光补偿点和ch1a/b比值则显著低于紫穗槐,且在林下低光照条件下这种差别更为明显(表 1)。高的叶绿素含量、Fv/Fm,Fv/Fo,PIABS和最大净光合速率表明光合系统对光能的吸收、转递和转化效率高以及对CO2的同化能力较强(刘宝等,2014),高表观光量子效率、低光补偿点和ch1a/b比值则表明植物在弱光条件下能够保持较高的光合能力(黄承建等,2013)。可见,美洲商陆与其伴生种紫穗槐相比具有更高的光能吸收、转化效率和更高的碳素同化能力,其光合生态幅较宽,在强光环境下可以高效地进行光合作用,在弱光环境下具有更强的光合优势。
植物的生长发育涉及对光能、水分、氮素等环境资源的综合利用(Griffin,1994),资源利用效率的高低在一定程度上影响其生存、生长和繁育能力,决定其对环境的适应能力和竞争力(Nagel et al.,2001; Gyenge et al.,2014)。Funk等(2007)的研究表明,在低资源下入侵植物也具有高的瞬时资源利用效率。本试验表明,美洲商陆的光能利用率和光合氮利用效率均显著高于紫穗槐,且在低光照环境下这种差异更为显著; 其水分利用率在高光照环境下低于紫穗槐、在低光照环境下则显著高于后者(图 1)。光能利用效率高的植物一般具有高生产力和快速生长能力(Binkley et al.,2013)、高光合氮利用效率通常也与其高的生长速率相关(Hikosaka,2004),而高的水分利用效率则有利于其在干旱条件下获得高生物产量(Mei et al.,2013)。由此可见,美洲商陆与紫穗槐相比具有更高的生产力和生长速率,且在低光照和干旱并存环境下优势更显著。
外来植物成功入侵还与其能量利用策略以及较低的叶片建成成本有关(屠臣阳等,2013)。叶片建成成本,即生产单位叶片所需要消耗的葡萄糖当量,包括建造叶片的所有有机物质所需碳骨架、还原剂和ATP消耗的葡萄糖总量(Williams et al.,1987)。叶片建成成本是评价和预测植物入侵潜力的普遍适用的指标之一(Nagel et al.,2001),较低的建成成本反映了植物较高的能量利用效率,通常与其较高的生长速率和竞争力相联系(宋莉英等,2009; Van Kleunen et al.,2010)。有研究发现,入侵植物的叶片建成成本低于本地植物(Baruch et al.,1999; 屠臣阳等,2013)。本试验中,美洲商陆叶片单位质量和单位面积建成成本均显著低于其伴生种紫穗槐(图 2),表明美洲商陆比紫穗槐具有更高效的物质和能量利用效率,它的叶片结构构建对物质和能量的需求较低,能够将更多的物质和能量投资到其他竞争策略中,如: 增加种子产量和生物量、提高相对生长速率等,这也是其成功入侵的重要原因之一。
3.2 结论入侵植物美洲商陆与其伴生种相比具有更高的光合能力、更高效的资源利用效率和更低的构建自体的成本(物质和能量)消耗,这些特性导致其具有高生长速率和强竞争力。同时,美洲商陆还具有花期长、结实量大、种子寿命长、能够克隆繁殖、具有较强的化感作用和超强的扩散能力等特点(翟树强等,2010; 闫小红等,2012; 周兵等,2013)。这些繁殖和生理生态特性为其成功入侵奠定了基础,特别是在林下低光照环境下,美洲商陆强竞争力更为凸现。这就预示着美洲商陆有可能在我国进一步扩张蔓延,从而对农林产业造成严重威胁。在各种防护林和各类自然保护区的林缘和林下美洲商陆可能成为相关生态系统演替和生物多样性维持的重要威胁因素。因此,美洲商陆的危害评价和防控应引起人们的密切关注和高度重视。
| [1] |
董周焱,柏新富,张靖梓,等. 2014.入侵植物美洲商陆对光环境的适应性.生态学杂志, 33(2):316-320. (Dong Z Y, Bai X F, Zhang J Z, et al. 2014. Adaptability of an invasive plant Phytolacca americana to varied light environment. Chinese Journal of Ecology, 33(2):316-320[in Chinese]).(  1) 1)
|
| [2] |
付俊鹏,李传荣,许景伟,等. 2012.沙质海岸防护林入侵植物垂序商陆的防治.应用生态学报, 23(4):991-997. (Fu J P, Li C R, Xu J W, et al. 2012. Prevention and control of invaded plant Phytolacca americana in sandy coastal shelter forests. Chinese Journal of Applied Ecology, 23(4):991-997[in Chinese]).(  1) 1)
|
| [3] |
宫兆宁,赵雅莉,赵文吉,等. 2014.基于光谱指数的植物叶片叶绿素含量的估算模型.生态学报, 34(20):5736-5745. (Gong Z N, Zhao Y L, Zhao W J, et al. 2014. Estimation model for plant leaf chlorophyll content based on the spectral index content.Acta Ecologica Sinica, 34(20):5736-5745[in Chinese]).(  1) 1)
|
| [4] |
黄承建,赵思毅,王龙昌,等. 2013.马铃薯/玉米套作对马铃薯品种光合特性及产量的影响.作物学报, 39(2):330-342. (Huang C J, Zhao S Y, Wang L C, et al. 2013. Effect of potato/maize intercropping on photosynthetic characteristics and yield in two potato varieties. Acta Agronomica Sinica, 39(2):330-342[in Chinese]).(  1) 1)
|
| [5] |
刘宝,陈存及,林达定,等. 2014. 21个闽楠种源叶片光合色素含量及叶绿素荧光参数分析.江西农业大学学报, 36(1):115-121. (Liu B, Chen C J, Lin D D, et al. 2014. Analyses of photosynthetic pigment content and chlorophyll fluorescence parameter in leaves of 21 provenances of Phoebe bournei. Acta Agriculturae Universitatis Jiangxiensis, 36(1):115-121[in Chinese]).(  1) 1)
|
| [6] |
苗艳明,吕金枝,毕润成,等. 2012.翅果油树叶性特征的动态变化.植物学报, 47(3):257-263. (Miao Y M, Lu J Z, Bi R C, et al. 2012. Dynamic changes of traits of leaves from Elaeagnus mollis. Chinese Bulletin of Botany, 47(3):257-263[in Chinese]).(  1) 1)
|
| [7] |
宋莉英,彭长连,彭少麟. 2009.华南地区3种入侵植物与本地植物叶片建成成本的比较.生物多样性, 17(4):378-384. (Song L Y, Peng C L, Peng S L. 2009. Comparison of leaf construction costs between three invasive species and three native species in South China. Biodiversity Science, 17(4):378-384[in Chinese]).(  1) 1)
|
| [8] |
王俊峰,冯玉龙. 2006.人工群落中苗期紫茎泽兰的化感作用和对光环境的适应.生态学报, 26(6):1809-1817. (Wang J F, Feng Y L. 2006. Allelopathy and light acclimation characteristic for Ageratina adenophora seedlings grown in man-made communities. Acta Ecologica Sinica, 26(6):1809-1817[in Chinese]).(  1) 1)
|
| [9] |
王珊珊,陈曦,王权,等. 2011.新疆古尔班通古特沙漠南缘多枝柽柳光合作用及水分利用的生态适应性.生态学报, 31(11):3082-3089. (Wang S S, Chen X, Wang Q, et al. Ecologicaladaptability of photosynthesis and water use for Tamarix ramosissima in the southern periphery of Gurbantunggut Desert, Xinjiang. Acta Ecologica Sinica, 31(11):3082-3089[in Chinese]).(  1) 1)
|
| [10] |
王宇涛,麦菁,李韶山,等. 2012.华南地区严重危害入侵植物薇甘菊和五爪金龙入侵机制研究.华南师范大学学报:自然科学版, 44(4):1-5. (Wang Y T, Mai J, Li S S, et al. 2012. Invasion mechanisms of the exotic and noxious invasive plants Mikania micrantha and Ipomoea cairica in South China. Journal of South China Normal University:Natural Science Edition, 44(4):1-5[in Chinese]).(  1) 1)
|
| [11] |
屠臣阳,皇甫超河,姜娜,等. 2013.入侵植物黄顶菊与5种共生植物叶片建成成本的比较.生态学杂志, 32(11):2985-2991. (Tu C Y, Huangpu C H, Jiang N, et al. 2013. Comparison of leaf construction cost between invasive plant Flaveria bidentis and its five co-occuring plants. Chinese Journal of Ecology, 32(11):2985-2991[in Chinese]).(  2) 2)
|
| [12] |
闫小玲,刘全儒,寿海洋,等. 2014.中国外来入侵植物的等级划分与地理分布格局分析.生物多样性, 22(5):667-676. (Yan X L, Liu Q R, Shou H Y, et al. 2014. The categorization and analysis on the geographic distribution patterns of Chinese alien invasive plants. Biodiversity Science, 22(5):667-676[in Chinese]).(  1) 1)
|
| [13] |
闫小红,张蓓玲,周兵,等. 2012.外来入侵植物美洲商陆提取物的化感活性.生态与农村环境学报, 28(2):139-145. (Yan X H, Zhang B L, Zhou B, et al. 2012. Allelopathic activity of the extract from Phytolacca americana-an invasive alien plant. Journal of Ecology and Rural Environment, 28(2):139-145[in Chinese]).(  2) 2)
|
| [14] |
翟树强,李传荣,许景伟,等. 2010.灵山湾国家森林公园刺槐林下垂序商陆种子雨时空动态.植物生态学报, 34(10):1236-1242. (Zhai S Q, Li C R, Xu J W, et al. 2010. Spatial and temporal dynamics of Phytolacca americana seed rain under Robinia pseudoacacia forest in Lingshan Bay National Forest Park, Shandong, China. Chinese Journal of Plant Ecology, 34(10):1236-1242[in Chinese]).(  2) 2)
|
| [15] |
周兵,张蓓玲,闫小红,等. 2013.外来入侵植物美洲商陆的繁殖生物学特性及其与入侵性的关系.生态环境学报, 22(4):567-574. (Zhou B, Zhang B L, Yan X H, et al. 2013. Traits of reproductive biology associated with invasiveness in alien invasive plant Phytolacca americana. Ecology and Environmental Sciences, 22(4):567-574[in Chinese]).(  1) 1)
|
| [16] |
Barney J N, Tekiela D R, Dollete E S J, et al. 2013. What is the "real" impact of invasive plant species? Frontiers in Ecology and the Environment, 11(6):322-329( 1) 1)
|
| [17] |
Baruch Z, Goldstein G. 1999. Leaf construction cost, nutrient concentration, and net CO2 assimilation of native and invasive species in Hawaii. Oecologia, 121(2):183-192.( 1) 1)
|
| [18] |
Binkley D, Laclau J P, Sterba H. 2013. Why one tree grows faster than another:patterns of light use and light use efficiency at the scale of individual trees and stands. Forest ecology and management, 288(1):1-4.( 1) 1)
|
| [19] |
Coccia C, Calosi P, Boyero L, et al. 2013. Does ecophysiology determine invasion success? A comparison between the invasive boatman Trichocorixa verticalis verticalis and the native Sigara lateralis (Hemiptera, Corixidae) in South-West Spain. PLOS One, 8(5):1-10( 1) 1)
|
| [20] |
Daehler C C. 2003. Performance comparisons of co-occurring native and alien invasive plants:implications for conservation and restoration. Annual Review of Ecology, Evolution, and Systematics, 34(1), 183-211.( 1) 1)
|
| [21] |
Feng Y L, Lei Y B, Wang R F, et al. 2009. Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proceedings of the National Academy of Sciences of the United States of America, 106(6):1853-1856.( 1) 1)
|
| [22] |
Funk J L, Vitousek P M. 2007. Resource-use efficiency and plant invasion in low-resource systems. Nature, 446(7139):1079-1081.( 1) 1)
|
| [23] |
Griffin K. 1994. Calorimetric estimates of construction cost and their use in ecological studies. Functional ecology, 8(5):551-562.( 1) 1)
|
| [24] |
Gyenge J, Fernández M E. 2014. Patterns of resource use efficiency in relation to intra-specific competition, size of the trees and resource availability in ponderosa pine. Forest Ecology and Management, 312(1):231-238.( 1) 1)
|
| [25] |
Heberling J M, Fridley J D. 2013. Resource-use strategies of native and invasive plants in Eastern North American forests. New Phytologist, 200(2):523-533.( 1) 1)
|
| [26] |
Hikosaka K. 2004. Interspecific difference in the photosynthesis-nitrogen relationship:patterns, physiological causes, and ecological importance. Journal of Plant Research, 117(6):481-494.( 1) 1)
|
| [27] |
Mack R N, Smith M C. 2011. Invasive plants as catalysts for the spread of human parasites. NeoBiota, 9:13-29.( 1) 1)
|
| [28] |
Mei X R, Zhong X L, Liu X Y. 2013. Improving water use efficiency of crops by exploring variety differences. Acta Agronomica Sinica, 39(5):761-766.( 1) 1)
|
| [29] |
Nagel J M,Griffin K L.2001. Construction cost and invasive potential:Comparing Lythrum salcaria (Lythraceae) with co-occurring native species along pond banks. American Journal of Botany, 88(12):2252-2258.( 2) 2)
|
| [30] |
Pintó-Marijuan M, Munné-Bosch S. 2013. Ecophysiology of invasive plants:osmotic adjustment and antioxidants. Trends in Plant Science, 18(12):660-666( 1) 1)
|
| [31] |
Shen X Y, Peng S L, Chen B M, et al. 2011. Do higher resource capture ability and utilization efficiency facilitate the successful invasion of native plants? Biological Invasions, 13(4):869-881.( 1) 1)
|
| [32] |
Williams K, Percival F, Merino J, et al. 1987. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant, Cell & Environment, 10(9):725-734.( 2) 2)
|
| [33] |
Van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters, 13(2):235-245.( 1) 1)
|
| [34] |
Ye Z P. 2007. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica, 45(4):637-640.( 1) 1)
|
 2016, Vol. 52
2016, Vol. 52

