文章信息
- 万菁娟, 郭剑芬, 纪淑蓉, 任卫岭, 杨玉盛
- Wan Jingjuan, Guo Jianfen, Ji Shurong, Ren Weiling, Yang Yusheng
- 可溶性有机物输入对亚热带森林土壤CO2排放及微生物群落的影响
- Effects of Dissolved Organic Matter Input on Soil CO2 Emission and Microbial Community Composition in a Subtropical Forest
- 林业科学, 2016, 52(2): 106-113
- Scientia Silvae Sinicae, 2016, 52(2): 106-113.
- DOI: 10.11707/j.1001-7488.20160213
-
文章历史
- 收稿日期:2015-01-31
- 修回日期:2015-12-29
-
作者相关文章
可溶性有机物(dissolved organic matter,DOM)是最活跃、最易变的土壤有机质(soil organic matter,SOM)形态,同时也是目前最有争议的土壤碳库之一(Von Lützow et al., 2007)。研究指出,尽管DOM的量很少,但其具有周转速率快、移动能力强等特点,是土壤易变碳库和稳定SOM间的重要中介(Neff et al., 2001; Boddy et al., 2007),在陆地C循环中发挥了重要作用。不同外源DOM的浓度与化学性质均会影响土壤CO2的排放,如Clevel and 等(2010)和Leff等(2012)研究表明,土壤CO2通量随着添加的DOM浓度增加而增加;Wieder等(2008)指出,添加等浓度的不同树种凋落叶DOM到土壤中,土壤CO2累积排放量的差异性很大。但目前关于添加不同外源DOM对土壤微生物群落的影响研究鲜见报道。
已有研究表明,添加外源有机物改变土壤微生物群落结构(Denef et al., 2009; Dungait et al., 2011),主要是通过影响土壤可利用性碳源和营养物质而引起的(Crow et al., 2009; Nemergut et al., 2010; Chen et al., 2012)。Clevel and 等(2007)研究发现,热带雨林土壤中添加凋落物淋溶的DOM后短期内会产生较大的CO2通量,主要是由土壤微生物群落变化引起的(Neff et al., 2001; Clevel and et al., 2004)。土壤中的细菌群落会优先利用外源有机物中易分解的部分(Paterson et al., 2007; Moore-Kucera et al., 2008),尤其是r型细菌会引起假激发效应(Blagodatskaya et al., 2008; Nottingham et al., 2009),而真菌被认为与土壤有机碳矿化速率变化有关(Kuzyakov,2010)。Garcia-Pausas等(2011)添加葡萄糖到土壤中后,增加了土壤真菌和放线菌生物量而增加了土壤有机碳矿化。
前期研究发现,添加外源DOM到土壤中后,培养当天CO2排放速率就达到最大值,但是否添加不同DOM后土壤CO2排放速率均在同一时间达到最大值?CO2排放达到最大值时,土壤微生物群落与CO2排放速率是否具有相关性?这些问题仍不清楚(万菁娟等,2015)。本研究通过室内培养试验探讨添加米槠(Castanopsis carlesii)及杉木(Cunninghamia lanceolata)凋落叶和枯死根DOM对土壤CO2排放(速率和通量)及土壤微生物群落结构的影响,为探讨DOM在森林生态系统碳循环中的作用提供依据。
1 研究区概况研究区位于福建省三明市格氏栲自然保护区(117°28′E,26°11′N),该区东南面和西北面分别与戴云山脉和武夷山脉相连,以低山丘陵为主,平均海拔300 m,平均坡度25°~35°。属中亚热带海洋季风气候,具有冬冷夏热、水热同季、湿润多雨的特点,试验地附近的三明市年均气温20.1 ℃,年降水量1 670 mm,且多集中于3—8月。杉木人工林为在2003年米槠次生林皆伐迹地上营造的纯林,林龄11年。林分表层(0~10 cm)土壤有机碳、全氮和微生物量碳含量分别为17.55 g·kg-1、1.31 g·kg-1和423.5 mg·kg-1;土壤饱和含水量为46.35%,pH为4.56。
2 研究方法 2.1 土壤样品和外源DOM样品取样2014年3月,于杉木人工林的上、中、下坡布设3块20 m × 20 m标准样地,在每块标准样地内用土钻按“S”形取5个点的0~10 cm土层土壤,迅速冷藏并带回实验室用于培养试验。同时选取长势、大小一致的杉木和米槠1年生幼苗进行盆栽种植,2013年5月开始13C标记,7月结束,在人为干旱情况下致死,收获整株树木,分别取叶片、枝干和根,带回实验室烘干保存。
2.2 试验设计与样品测定2014年10月进行样品DOM的浸提。浸提时样品与水的比例为1∶10,即米槠凋落叶、杉木凋落叶、米槠枯死根和杉木枯死根各取30 g,各加入300 mL去离子水,浸泡24 h后,上清液用0.45 μm玻璃纤维过滤器减压过滤,得到DOM浸提液并于4 ℃下保存(表 1)。
|
|
取相当于50 g干土质量的鲜土到500 mL特质瓶中,调节土壤含水量为饱和持水量的40%,放置在25 ℃生化培养箱中先进行15天的预培养,使土壤内部环境趋于稳定。预培养结束后,分别加入米槠及杉木的凋落叶和死根DOM浸提液各4 mL和等量去离子水(4 mL)(对照),调节土壤含水量达到饱和持水量的60%,每处理及对照均3次重复。加入DOM后的第2,5,8,12,24和36 h抽气取样,取样前1 h将瓶盖拧紧,然后将气体注入气相色谱仪(Shimadzu GC-2010,日本)测定土壤CO2排放速率,计算CO2累积排放量。
培养结束后,土壤微生物群落组成采用磷脂脂肪酸 PLFA法测定。即称取8 g干土(冷冻干燥土壤),加入23 mL由磷酸缓冲液、甲醇、氯仿配置而成的提取液,振荡离心,将上层清液转移至分液漏斗中,反复2次,然后将分液漏斗在黑暗环境下静置一夜;收集下层有机相,在氮气下吹干,通过硅胶柱分离出磷脂,加甲醇∶ 甲苯(1∶1,V/V)和0.2 mol·L-1氢氧化钾溶液进行皂化和甲基化形成脂肪酸甲酯。上机测定前,用正十九烷酸甲酯作为内标溶液,将得到的脂肪酸甲酯转移到GC小瓶,通过气相色谱仪(Agilent 6890 N,美国)和MIDI微生物识别系统(MIDI Inc.,Newark,DE)进行鉴定。本研究中,将不同种类PLFA进行归类,i15:0,a15:0,i16:0,i17:0,a17:0表征革兰氏阳性细菌(Denef et al., 2009; Landesman et al., 2010);cy17:0,18:1ω7c,18:1ω5c,cy19:0表征革兰氏阴性细菌(Swallow et al., 2009; Frostegård et al., 2010);18:1ω9c和18:2ω6,9c表征真菌(Swallow et al., 2009);10Me16:0、10Me17:0和10Me18:0表征放线菌;真菌∶细菌为真菌与细菌(包括革兰氏阳性细菌和革兰氏阴性细菌)的磷脂脂肪酸含量之比。
浸提液可溶性有机碳(dissolved organic carbon,DOC)含量采用总有机碳分析仪(SHIMADZU TOC-VCPH/CPN Analyzer)测定; 浸提液可溶性有机氮(dissolved organic nitrogen,DON)含量采用流动注射分析仪(Lachat Qyickchem Automatedion Analyzer)测定。使用紫外可见分光光度计(UV-2450,Shimadzu,Kyoto,Japan)测定浸提液DOM在波长254 nm处的紫外吸收值(UV),在波长250和365 nm处的紫外吸光度比值表示DOM平均分子质量大小。荧光发射光谱用日立-4600 荧光分光光度计测定,激发波长λex为254 nm,狭缝宽10 nm,发射波长λem为300~480 nm,狭缝宽10 nm,扫描速度为2 400 nm·min-1;腐殖化指数(humi fication index,HIX)通过计算发射光谱中Σ435~480与Σ300~345 nm的峰面积比获得(熊丽等,2014)。
2.3 计算方法及数据处理CO2排放速率计算公式为:
F=k×v/m×Δc/Δt×273/(273+T)。
(1)
数据处理在Excel和SPSS 17.0软件中完成,图表采用Origin 8.0 软件制作。采用单因素方差分析(one-way ANOVA)检验添加不同来源DOM对土壤CO2排放的影响,多因素方差分析(multiple comparisons ANOVA)检验树种和凋落物种类对DOM组成和化学性质的影响(P< 0.05),并采用Pearson相关法分析添加外源DOM培养36 h后土壤CO2累积排放量与土壤微生物群落磷脂脂肪酸含量之间的相关性。
3 结果与分析 3.1 不同来源DOM差异多因素方差分析发现,树种和凋落物种类均对DOC浓度具有显著影响(P< 0.01)。如米槠凋落叶浸提得到的DOC浓度最大(3 299 mg·L-1),而杉木枯死根浸提得到的DOC浓度最小(227 mg·L-1),其差异超过了14倍(表 1)。米槠凋落叶DON浓度显著低于米槠枯死根DON浓度(P< 0.05),说明米槠枯死根DOM中含有更多的氮营养物质。杉木凋落叶DOM的HIX,UV均显著低于米槠凋落叶DOM的HIX,UV(P< 0.05),DOM分子质量大小则相反,即阔叶树种凋落叶DOM比针叶树种凋落叶DOM含有更多高分子质量、芳香类的腐殖酸难分解物质。这与相对荧光光谱图的趋势一致(图 1),由针叶树种到阔叶树种最大荧光强度所对应波长向更长的波长转移,表明DOM中共轭体系在增大,分子结构更复杂。另外,凋落叶DOM的疏水性碳(hydrophobic dissolved organic carbon,HDOC)含量显著高于枯死根,而凋落叶DOM的HIX值显著低于枯死根(P< 0.05),不同来源DOM分子质量大小表现为凋落叶高于枯死根,表明凋落叶DOM中含有更多低分子质量、易分解的有机质,而枯死根DOM则以高分子质量的腐殖质为主。
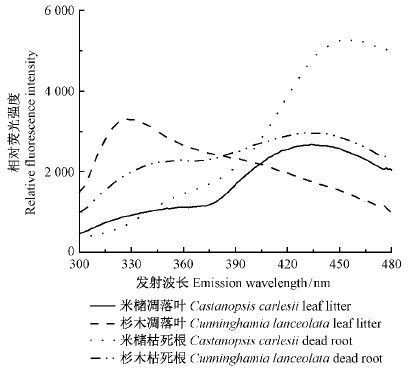
|
图1 不同来源DOM的荧光发射光谱 Fig.1 Fluorescence emission spectra of DOM from different sources |
添加米槠凋落叶DOM、杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM到土壤中后,土壤CO2排放速率均显著高于对照(P< 0.05)(图 2),其中添加米槠和杉木枯死根DOM的土壤CO2排放速率在第2 h达到最大值,分别是对照的7.3和8.3倍;添加米槠和杉木凋落叶DOM的土壤CO2排放速率在第12 h达到最大值,分别是对照的20.6和13.2倍。添加凋落叶DOM的土壤CO2排放速率一直高于添加枯死根DOM的土壤CO2排放速率。
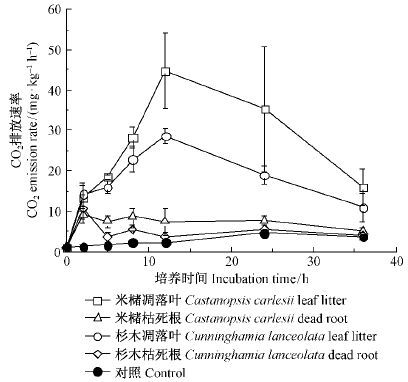
|
图2 添加不同来源DOM土壤的CO2排放速率 Fig.2 CO2 emission rate after DOM addition from different sources |
在培养36 h时,添加不同树种和不同种类凋落物DOM均对土壤CO2累积排放量有显著影响(P< 0.05)(图 3)。添加米槠凋落叶DOM、杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM的土壤CO2累积排放量均明显大于对照。添加凋落叶DOM的土壤CO2累积排放量显著高于添加相同树种枯死根DOM的土壤CO2累积排放量,如添加米槠凋落叶DOM的土壤CO2累积排放量是添加米槠枯死根的4倍,添加杉木凋落叶DOM的土壤CO2累积排放量是添加杉木枯死根的3.7倍。添加米槠凋落叶DOM的土壤CO2累积排放量显著高于添加杉木凋落叶的53.9%,添加米槠枯死根DOM土壤CO2累积排放量显著高于添加杉木枯死根的43.5%。在培养36 h时,添加不同来源DOM的土壤CO2累积排放量的大小表现为: 添加米槠凋落叶DOM >添加杉木凋落叶DOM >添加米槠枯死根DOM >添加杉木枯死根DOM。
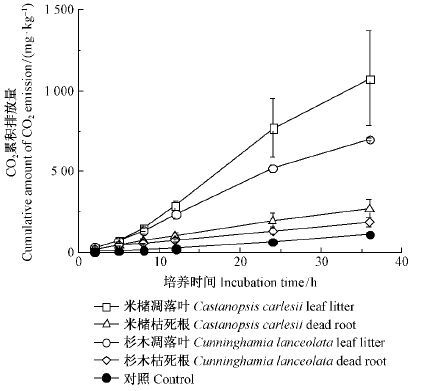
|
图3 添加不同来源DOM的土壤CO2累积排放量 Fig.3 Cumulative CO2 emission after DOM addition from different sources |
添加米槠凋落叶DOM、杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM的土壤微生物磷脂脂肪酸(phospholipid fatty acid,PLFA)种类没有显著差异,但对照(添加去离子水)土壤的PLFA种类少了10Me 18:0。添加不同来源DOM到土壤中,含量较高的3种PLFA种类依次是16:0、cy19:0 w8c和i15:0。归类结果分析(表 2)表明,添加杉木凋落叶和枯死根DOM的土壤中G+、G-、放线菌和真菌的PLFAs含量显著大于对照(P< 0.05)。类似地,添加米槠凋落叶和枯死根DOM的土壤中G+、G-、放线菌和真菌的PLFAs含量亦大于对照,但差异不显著(P>0.05)。从添加不同树种凋落叶DOM来看,添加杉木凋落叶DOM的土壤中G+、G-、放线菌和真菌PLFAs含量及真菌∶细菌均显著大于添加米槠凋落叶DOM(P< 0.05),但添加杉木凋落叶DOM的土壤G+∶G-显著低于添加米槠凋落叶DOM(P< 0.05)。比较添加杉木和米槠枯死根DOM的土壤微生物PLFAs含量可知,添加杉木枯死根DOM的土壤中G+、G-和真菌PLFAs含量显著高于添加米槠枯死根DOM(P< 0.05),而放线菌PLFAs含量、G+∶G-、真菌∶细菌均没有显著差异。此外,添加杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM的土壤中G+∶G-低于培养前,而添加米槠凋落叶DOM和对照(添加去离子水)高于培养前;添加米槠凋落叶DOM的真菌∶细菌低于培养前,而其他3种处理均高于培养前,可见添加不同来源DOM对土壤微生物群落生物量的影响亦不一致。通过Pearson相关分析发现,培养36 h时添加不同来源DOM的土壤G+、G-、放线菌和真菌PLFAs含量与土壤CO2排放量之间没有显著的相关性(r=0.089,P=0.752)。
|
|
本研究中,添加不同凋落物DOM的土壤CO2最大排放速率出现的时间不一致,这可能与外源添加DOM中DOC量的大小有关(表 1),因为DOC是土壤微生物生长和代谢过程的重要能量来源(Leff et al., 2012)。外源添加米槠和杉木枯死根DOC量分别是1.5和0.9 mg,而外源添加米槠和杉木凋落叶DOC量分别是13.2和5.8 mg。在培养过程中,添加米槠和杉木凋落叶DOM的土壤CO2排放速率一直大于添加米槠和杉木枯死根DOM的土壤CO2排放速率,因为外源添加的凋落叶DOM相比枯死根DOM中含有更多可利用有机碳且分子结构更简单,因而更容易被微生物所利用(万菁娟等,2015)。此外,培养期间对照土壤CO2排放速率一直高于培养前,这可能是由于土壤水分从饱和含水量的40%调整到60%,促进了土壤有机碳的矿化(Housman et al., 2006;Potts et al., 2006)。
4.2 添加不同来源DOM的土壤微生物群落差异许多研究表明,树种会影响土壤微生物群落组成,在不同树种下会形成独特的微生物群落(Lejon et al., 2005)。本研究供试土壤来自于杉木人工林,由于针叶凋落物中总酚、木质素、脂溶性物质多等因素(程东升,1993),使得土壤中K策略者真菌含量较大,而r策略者细菌含量较少(Fontaine et al., 2003)。添加不同来源DOM的土壤微生物群落
PLFAs含量均高于对照,其原因是添加可溶性有机C源后,土壤中r策略者的能量限制得到缓解,能迅速增殖而促进胞外酶增加,因而也有利于增加K策略者(Kuzyakov et al., 2000; Paterson,2009)。外源碳添加会改变土壤微生物群落组成(Feng et al., 2009; Dungait et al., 2011; Wang et al., 2013)。Garcia-Pausas等(2011)研究发现,添加葡萄糖到土壤中后增加了土壤真菌与放线菌PLFAs含量。Wang等(2013)直接添加凋落物到土壤中后,增加了土壤微生物活性,降低了细菌∶真菌的值。本研究发现,添加杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM的土壤真菌∶细菌高于培养前,但添加米槠凋落叶DOM的土壤真菌∶细菌低于培养前,表明添加米槠凋落叶DOM的土壤细菌生物量增加得更多。添加杉木凋落叶DOM、米槠枯死根DOM和杉木枯死根DOM后土壤的G+∶G-均低于培养前,表明3种来源DOM添加促进了革兰氏阴性细菌群落的繁殖(Kuzyakov et al., 2000; Garcia-Pausas et al., 2011),而添加米槠凋落叶DOM后土壤G+∶G-高于培养前,这可能是因为不同微生物群落对碳源的利用具有选择性,其中革兰氏阴性细菌会优先利用外源添加有机物,而革兰氏阳性细菌会优先利用原有土壤有机物(Kramer et al., 2006; Tavi et al., 2013)。
4.3 土壤CO2排放与微生物群落相关性分析在培养36 h时,添加不同来源DOM的土壤中G+、G-、放线菌和真菌PLFAs含量与土壤CO2累积排放量的相关性很低,这可能与供试土壤有关,因为外源添加杉木凋落叶和枯死根DOM在杉木人工林土壤中会有一定的主场优势(Ayres et al., 2009),也可能由于土壤CO2排放是一个渐进的过程,培养过程中存在微生物的更替,不同生存策略的微生物对CO2排放的贡献不一致(Gärdenäs et al., 2011)。另外,本研究培养时间短暂,需较长时间培养以进行验证。
5 结论36 h的短期培养试验发现,添加米槠和杉木枯死根DOM的土壤CO2排放速率在第2 h达到最大值,而添加米槠和杉木凋落叶DOM的土壤CO2排放速率最大值出现在第12 h,土壤CO2排放速率最大值出现时间不同可能与外源添加DOM的性质差异有关。培养36 h时,添加不同来源DOM的土壤G+、G-、放线菌和真菌PLFAs含量均高于培养前,添加杉木凋落叶和枯死根DOM的土壤G+、G-和真菌PLFAs含量均显著高于添加米槠凋落叶和枯死根DOM,可见外源添加DOM影响了土壤微生物群落,但DOM对土壤微生物的影响机制还有待进一步研究。
| [1] |
程东升. 1993.森林微生物生态学. 哈尔滨:东北林业大学出版社, 81-84. (Cheng D S.1993.Forest microbial Ecology. Harbin:Northeast Forestry University Press, 81-84.[in Chinese]) |
| [2] |
万菁娟,郭剑芬,纪淑蓉,等. 2015.杉木和米槠凋落叶DOM对土壤碳矿化的影响. 生态学报, 35(24):8148-8154. (Wan J J, Guo J F, Ji S R, et al. 2015.Effects of dissolved organic matter from Cunninghamia lanceolata and Castanopsis carlesii leaf litter on soil C mineralization. Acta Ecologica Sinica, 35(24):8148-8154.[in Chinese])(  2) 2)
|
| [3] |
熊丽, 杨玉盛, 王巧珍. 等. 2014.可溶性有机碳在米槠天然林土壤中的淋溶特征. 亚热带资源与环境学报, 9(1):46-52. (Xiong L, Yang Y S, Wang Q Z, et al. 2014.Movement of dissolved organic carbon in natural forest soil of Castanopsis carlesii. Journal of Subtropical Resources and Environment, 9(1):46-52.[in Chinese])(  1) 1)
|
| [4] |
Ayres E, Steltzer H, Simmons B L, et al. 2009.Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. Journal of Ecology, 97(5):901-912.( 1) 1)
|
| [5] |
Blagodatskaya E, Kuzyakov Y. 2008.Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure:critical review. Biology and Fertility of Soils, 45(2):115-131.( 1) 1)
|
| [6] |
Boddy E, Hill P W. 2007.Fast turnover of low molecular weight components of the dissolved organic carbon pool of temperate grassland field soils. Soil Biology and Biochemistry, 39(4):827-835.( 1) 1)
|
| [7] |
Chen Q H, Feng Y, Zhang Y P, et al. 2012.Short-term responses of nitrogen mineralization and microbial community to moisture regimes in greehouse vegetable soils. Pedosphere, 22 (2):263-272.( 1) 1)
|
| [8] |
Cleveland C C, Neff J C, Townsend A R, et al. 2004.Composition, dynamics, and fate of leached dissolved organic matter in terrestrial ecosystems:results from a decomposition experiment. Ecosystems, 7(3):175-285.( 1) 1)
|
| [9] |
Cleveland C C, Nemergut D R, Schmidt S K, et al. 2007.Increases in soil respiration following labile carbon additions linked to rapid shifts in soil microbial community composition. Biogeochemistry, 82(3):229-240.( 1) 1)
|
| [10] |
Cleveland C C, Wieder W R, Reed S C, et al. 2010.Experimental drought in a tropical rain forest increases soil carbon dioxide losses to the atmosphere. Ecology, 91(8):2313-2323.( 1) 1)
|
| [11] |
Crow S E, Lajtha K, Filley T R, et al. 2009.Sources of plant-derived carbon and stability of organic matter in soil:implications for global change. Global Change Biology, 15(8):2003-2019.( 1) 1)
|
| [12] |
Denef K, Roobroeck D, Manimel Wadu M C, et al. 2009.Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biology and Biochemistry, 41(1):144-153.( 2) 2)
|
| [13] |
Dungait J A J, Kemmitt S J, Michallon L, et al. 2011.Variable responses of the soil microbial biomass to trace concentrations of 13C-labelled glucose, using 13C-PLFA analysis. European Journal of Soil Science, 62(1):117-126.( 2) 2)
|
| [14] |
Feng W T, Zou X M, Schaefer D. 2009.Above- and belowground carbon inputs affect seasonal variations of soil microbial biomass in a subtropical monsoon forest of southwest China. Soil Biology and Biochemistry, 41(5):978-983.( 1) 1)
|
| [15] |
Fontaine S, Mariotti A, Abbadie L. 2003.The priming effect of organic matter:a question of microbial competition? Soil Biology and Biochemistry, 35(6):837-843.( 1) 1)
|
| [16] |
Frostegård Å, Tunlid A, Bååth E. 2011.Use and misuse of PLFA measurements in soils. Soil Biology and Biochemistry, 43(8):1621-1625.( 1) 1)
|
| [17] |
Garcia-Pausas J, Paterson E. 2011.Microbial community abundance and structure are determinants of soil organic matter mineralisation in the presence of labile carbon. Soil Biology and Biochemistry, 43(8):1705-1713.( 3) 3)
|
| [18] |
Gärdenäs A I, Ågren G I, Bird J A, et al. 2011.Knowledge gaps in soil carbon and nitrogen interactions -from molecular to global scale. Soil Biology and Biochemistry, 43 (4):702-717.( 1) 1)
|
| [19] |
Housman D C, Powers H H, Collins A D, et al. 2006.Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. Journal of Arid Environments, 66(4):620-634.( 1) 1)
|
| [20] |
Kramer C, Gleixner G. 2006.Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biology and Biochemistry, 38(11):3267-3278.( 1) 1)
|
| [21] |
Kuzyakov Y. 2010. Priming effects:interactions between living and dead organic matter. Soil Biology and Biochemistry, 42(9):1363-1371.( 1) 1)
|
| [22] |
Kuzyakov Y, Friedel J K, Stahr K. 2000.Review of mechanisms and quantification of priming effects. Soil Biology and Biochemistry, 32(11):1485-1498.( 2) 2)
|
| [23] |
Landesman W J, Dighton J. 2010.Response of soil microbial communities and the production of plant-available nitrogen to a two-year rainfall manipulation in the New Jersey Pinelands. Soil Biology and Biochemistry, 42(10):1751-1758.( 1) 1)
|
| [24] |
Leff J W, Nemergut D R, Grandy A S, et al. 2012.The effects of soil bacterial community structure on decomposition in a tropical rain forest. Ecosystems, 15(2):284-298.( 2) 2)
|
| [25] |
Lejon D P, Chaussod R, Ranger J, et al. 2005.Microbial community structure and density under different tree species in an acid forest soil (Morvan, France). Microbial Ecology, 50(4):614-625.( 1) 1)
|
| [26] |
Moore-Kucera J, Dick R P. 2008.Application of 13C-labeled litter and root materials for in situ decomposition studies using phospholipid fatty acids. Soil Biology and Biochemistry, 40(10):2485-2493.( 1) 1)
|
| [27] |
Neff J C, Asner G P. 2001.Dissolved organic carbon in terrestrial ecosystems:synthesis and a model. Ecosystems, 4(1):29-48.( 2) 2)
|
| [28] |
Nemergut D R, Cleveland C C, Wieder W R, et al. 2010.Plot-scale manipulations of organic matter inputs to soils correlate with shifts in microbial community composition in a lowland tropical rain forest. Soil Biology and Biochemistry, 42(12):2153-2160.( 1) 1)
|
| [29] |
Nottingham A T, Griffiths H, Chamberlain P M, et al. 2009.Soil priming by sugar and leaf-litter substrates:a link to microbial groups. Applied Soil Ecology, 42(3):183-190.( 1) 1)
|
| [30] |
Paterson E. 2009.Comments on the regulatory gate hypothesis and implications for C-cycling in soil. Soil Biology and Biochemistry, 41(6):1352-1354.( 1) 1)
|
| [31] |
Paterson E, Gebbing T, Abel C, et al. 2007.Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytologist, 173(3):600-610.( 1) 1)
|
| [32] |
Potts D L, Huxman T E, Cable J M, et al. 2006.Antecedent moisture and seasonal precipitation influence the response of canopy-scale carbon and water exchange to rainfall pulses in a semi-arid grassland. New Phytologist, 170(4):849-860.( 1) 1)
|
| [33] |
Swallow M, Quideau S, MacKenzie M, et al. 2009.Microbial community structure and function:the effect of silvicultural burning and topographic variability in northern Alberta. Soil Biology and Biochemistry, 41(4):770-777.( 1) 1)
|
| [34] |
Tavi N M, Martikainen P J, Lokko K, et al. 2013.Linking microbial community structure and allocation of plant-derived carbon in an organic agricultural soil using 13CO2 pulse-chase labelling combined with 13C-PLFA profiling. Soil Biology and Biochemistry, 58(3):207-215.( 1) 1)
|
| [35] |
Von Lützow M, K gel-Knabner I, Ekschmitt K, et al. 2007.SOM fractionation methods:relevance to functional pools and to stabilization mechanisms. Soil Biology and Biochemistry, 39(9):2183-2207.( 1) 1)
|
| [36] |
Wang Q, He T, Wang S, et al. 2013.Carbon input manipulation affects soil respiration and microbial community composition in a subtropical coniferous forest. Agricultural and Forest Meteorology, 178-179:152-160.( 1) 1)
|
| [37] |
Wieder WR, Cleveland C, Townsend A R. 2008.Tropical tree species composition affects the oxidation of dissolved organic matter from litter. Biogeochemistry, 88(2):127-138.( 1) 1)
|
 2016, Vol. 52
2016, Vol. 52

