文章信息
- 王晓丽, 李海平, 贾程宇, 段立清
- Wang Xiaoli, Li Haiping, Jia Chengyu, Duan Liqing
- 茉莉酸诱导青杨抗性及核型多角体病毒对舞毒蛾幼虫食物利用的影响
- Effects of Induced Resistance of Populus cathayana by Jasmonic Acid and LdNPV on Nutritional Utilization of Lymantria dispar (Lepidoptera: Lymantriidae)
- 林业科学, 2016, 52(10): 96-101
- Scientia Silvae Sinicae, 2016, 52(10): 96-101.
- DOI: 10.11707/j.1001-7488.20161012
-
文章历史
- 收稿日期:2015-10-16
- 修回日期:2016-03-06
-
作者相关文章
2. 内蒙古农业大学农学院 呼和浩特 010019;
3. 兴安盟乌兰浩特市森林病虫防治检疫站 乌兰浩特 137400
2. College of Agriculture, Inner Mongolia Agricultural University Huhhot 010019 ;
3. Prevention and Control of Forest Diseases and Pests of Quarantine Station of Xing'an League Ulanhot Ulanhot 137400
舞毒蛾(Lymantria dispar)是林业上重要的食叶性害虫,寄主植物多,周期性暴发,危害严重(萧刚柔, 1992)。舞毒蛾核型多角体病毒(Lymantria disparnucleopolyhedrovirus, LdNPV)是控制舞毒蛾大暴发的主要病原微生物之一(Elkinton et al., 1990),但其侵染过程较缓慢,野外喷施病毒后幼虫死亡需10~14天,此间幼虫将持续危害寄主植物(Podgwaite et al., 1992)。有些病毒还可刺激害虫取食,如草地贪夜蛾(Spodaptera frugiperda)幼虫受到核型多角体病毒(AcMNPV)感染后,其食量约为未被感染个体的2倍(O’Reily et al., 1992),然而LdNPV对舞毒蛾取食及食物利用的影响尚未见报道。
另外,当遭受植食性昆虫危害时,植物会产生自我保护机制(Howe, 2004),即诱导抗性(Karban et al., 1997):虫害、机械损伤及外源茉莉酸类物质均可诱导植物产生抗性反应(Ingrid et al., 1997),诱导抗性可通过产生不同防御性化学物质引诱害虫天敌或趋避害虫而间接抑制害虫危害(Dicke et al., 2000),也可通过直接影响昆虫的取食、消化、生长发育来阻止害虫发生(Awmack et al., 2002)。前期的研究表明,外源茉莉酸(jasmonic acid,JA)处理可诱导青杨(Populus cathayana)对舞毒蛾产生明显的抗性,使其幼虫发育历期延长、体质量降低(越慧芳等, 2013);进一步的分析表明,JA处理可诱导青杨叶片中单宁酸、绿原酸等含量增加(越慧芳, 2013),而单宁酸、绿原酸、水杨酸可增加舞毒蛾对LdNPV的敏感性(王晓丽等, 2014),但是JA诱导青杨抗性与LdNPV共同作用对舞毒蛾的食物利用、生长发育的影响还不清楚。因此,本文对JA-青杨-舞毒蛾-LdNPV作为研究系统,探讨JA诱导抗性、LdNPV处理及二者联合处理对舞毒蛾幼虫食物利用及生长发育的影响。
1 材料与方法 1.1 试验材料青杨枝条取采自内蒙古农业大学科技园区人工种植的青杨人工林,舞毒蛾卵块采自兴安盟大兴安岭林内。JA购于Sigma公司。
1.2 试验方法 1.2.1 青杨扦插、JA溶液配制与喷施将采回的青杨枝条剪成20~23 cm(保证每段有3~4个芽)长,清水浸泡至枝条浸水部分长出根际生根后,扦插于直径10 cm、高25 cm饮料瓶(剪去细颈部),每瓶插穗1枝,置于温室大棚中培养,根据土壤湿度适时浇水,待每个插穗长出3~4个枝条或15~20片叶时进行试验。
将100 mg JA溶解于8 mL丙酮中,再用蒸馏水稀释成浓度为0.1 mmol·L-1的溶液。将青杨苗木分为3组,每组再分为3小组,每1小组30盆,共9小组、270盆苗木。其中1组苗木的3小组分别喷施0.1 mmol·L-1 JA溶液1,5,10天后,接入接毒2龄幼虫(JA+V组)。另2组苗木喷施丙酮+蒸馏水,同样于喷施1,5,10天后接虫,其中1组接入接毒2龄幼虫(V组),另1组接入未接毒幼虫(CK组)。
1.2.2 幼虫孵化及接毒将舞毒蛾卵块去掉表面附毛,用有效氯含量为4.5%~5.5%(W/V)的1% 84消毒液浸泡60 s,再用蒸馏水冲洗3次,置于铺有滤纸的大培养皿内阴干后,转入人工气候培养箱内孵化(T=25 ℃±1 ℃,L:D=16 h:8 h),将初孵幼虫接入装有人工食料的养虫杯中饲养至2龄,选用同一天进入2龄的幼虫备用。
幼虫接毒采用食料法(Duan et al., 2001),将人工食料制成小段(约7.0 mg),移入24孔组织培养皿中,每一小段人工食料上滴1 μL浓度为92 OBs·μL-1的LdNPV病毒液(对照组滴1μL蒸馏水),每孔接入1头2龄备用幼虫,用封口膜密封后盖好盖,放入培养箱内让其取食食料,24 h后将食尽饲料的幼虫转入上述青杨苗木上饲养。
1.2.3 幼虫饲养每盆青杨苗木上接入1~2头幼虫,对其进行编号、称质量。每天观察,发现有幼虫蜕皮至3龄时及时称体质量,并测量其整个2龄阶段的食叶面积,换算出取食量,并收集虫粪进行称质量,记录每头幼虫的2龄发育历期。
根据上述观测结果参照Waldbauer(伊里因斯基, 1957)公式,计算幼虫的相对生长率、食物近似消化率、食物转化率和利用率。
| $ \begin{array}{l} 相对生长率\left( {{\rm{RGR}}} \right) = \\ \frac{{3龄幼虫初始体质量 - 2龄幼虫初始体质量}}{{3龄幼虫初始体质量 + 2龄幼虫初始体质量}} \times 100{\raise0.5ex\hbox{$\scriptstyle 0$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 0$}},\\ 食物近似消化率\left( {{\rm{AD}}} \right) = 1 - \frac{{排粪量}}{{取食量}} \times 100{\raise0.5ex\hbox{$\scriptstyle 0$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 0$}},\\ 食物利用率\left( {{\rm{ECI}}} \right) = \frac{{体质量增加量}}{{取食量}} \times 100{\raise0.5ex\hbox{$\scriptstyle 0$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 0$}},\\ 食物转化率\left( {{\rm{ECD}}} \right) = \frac{{体质量增加量}}{{取食量 - 排粪量}} \times 100{\raise0.5ex\hbox{$\scriptstyle 0$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 0$}}. \end{array} $ |
采用SPSS 17.0的方差分析(ANOVA)和EXCEL软件对获得的试验数据进行统计分析及作图,并用Duncan氏新复极差法对平均值进行多重比较(P<0.05),检验不同处理之间、不同时间之间试验数据的差异显著性。
2 结果与分析 2.1 对幼虫发育历期及体质量的影响JA+V组舞毒蛾2龄幼虫的发育历期比V组及CK组显著延长(P<0.05)(表 1),取食诱导1,5,10天青杨叶片组的分别比CK组延长了1.0,1.2,1.7天(P<0.05),诱导天数间无显著差异;与V组的差异只在诱导10天时显著,前者比后者延长了1.0天(P<0.05)。V组的幼虫发育历期比CK组显著延长,但10天组发育历期的延迟与CK组的无差异显著。
|
|
JA+V组舞毒蛾2龄幼虫的体质量显著低于V组及CK组(P<0.05)(表 1),且3个处理组之间存在显著差异,体质量从重到轻为CK组>V组>JA+V组,JA+V组体质量在1,5,10天分别比CK组的体质量下降27.8%,37.9%,34.3%(P<0.05);比V组降低11.8%,18.2%和14.4%(P<0.05);V组比CK组降低18.2%,24.1%,23.2%。JA诱导时间长短对舞毒蛾的体质量影响差异不显著。
2.2 幼虫取食量及相对生长率JA+V组、V组及CK组舞毒蛾2龄幼虫取食量变化最大组出现在诱导第5天组,JA+V组取食量显著低于V组及CK组(表 2),取食量比V组和CK组分别降低24.2%和21.6%,而诱导1天和10天组的取食量无显著差异。JA对青杨诱导时间长短对舞毒蛾幼虫取食量影响差异不显著。
|
|
JA+V组、V组及CK组舞毒蛾2龄幼虫的相对生长率有显著影响(表 2)。在诱导1,5,10天JA+V组的幼虫相对生长率比CK组分别显著降低46.3%,58.1%和58.8%(P<0.05),比V组分别降低20.7%,28.5%,26.6%(P<0.05);V组比CK组分别降低32.3%,41.4%和43.8%(P<0.05);JA+V组较V组相对生长率降低,1,5天差异显著,但10天时未达到统计检验的显著性。JA诱导青杨时间对幼虫相对生长率影响差异不显著。
2.3 幼虫食物消化率、转化率和利用率JA+V组和V组处理对舞毒蛾2龄幼虫食物消化率、利用率和转化率都有明显影响,其中JA+V组的影响最显著,在诱导1,5,10天时JA+V组处理的消化率比其对应的CK组分别降低5.2%,8.4%和8.2%(P<0.05);利用率分别降低45.1%,53.0%和65.4%(P<0.05),转化率分别降低40.4%,49.0%和63.2%(P<0.05)。幼虫食物消化率、利用率和转化率的降低程度有随JA诱导时间的延长而越加明显的趋势,且在诱导10天JA+V组与V组之间均存在显著差异(图 1,图 2,图 3);JA+V组及V组降低了舞毒蛾幼虫对食物营养的利用率。
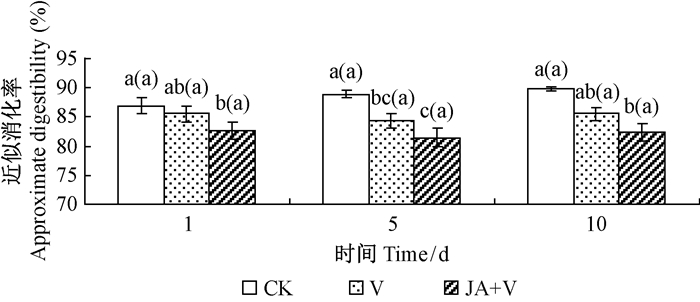
|
图 1 JA诱导青杨抗性及LdMNPV对舞毒蛾幼虫食物近似消化率的影响 Fig.1 Effects of induc resistance ofP. cathayana by JA and LdNPV on food approximate digestibility of gypsy moth larvae |

|
图 2 JA诱导青杨抗性及LdMNPV对舞毒蛾幼虫食物利用率的影响 Fig.2 Effects of induced resistance ofP. cathayana by jasmonic acid and LdNPV on efficiency of convertion of ingested food of gypsy moth larvae |
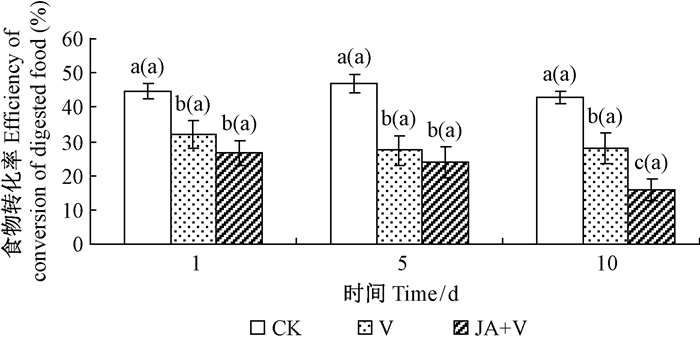
|
图 3 JA诱导青杨抗性及LdMNPV对舞毒蛾幼虫食物转化率的影响 Fig.3 Effects of induced resistance ofP. cathayanaby JA and LdNPV on efficiency of conversion of digested food of gypsy moth larvae |
前期研究表明,JA诱导使青杨次生物质及酶活性改变,降低了青杨的营养成分,增加了木质素、绿原酸、黄酮及单宁等抗性化合物的含量,还能够激发青杨保护性酶活性(王予彤等, 2015; An et al., 2006; 越慧芳等, 2013),从而降低舞毒蛾幼虫对食物营养的利用,导致其体质量下降,发育历期延迟。自然界中昆虫个体发育历期的延长,意味着其暴露在不利环境的机会就大大增加,不但能增加病原微生物的侵染机会,也间接增加其被天敌捕食或寄生的可能性。而寄主植物的质量影响幼虫的生长发育及其生理状况(秦秋菊等, 2005; Pechan et al., 2000),反过来又影响其对病原物的敏感性(Shikano et al., 2009),已有研究证实,当核型多角体病毒粒子侵染昆虫寄主中肠细胞时,中肠细胞环境对多角体的溶解性及病毒粒子的侵染活性有影响(Keating et al., 1988),白杨(P. tremuloides)中水解单宁酸含量的变化影响LdNPV对舞毒蛾的致病性(Keating et al., 1990),说明JA诱导青杨次生物质的改变对舞毒蛾中肠环境的影响及与病原物致病性之间存在一定的关系。病毒的致病力与寄主植物间的关系是由两者的性质和数量特性决定的,尤其是植物的酚酸物质(Keating et al., 1988),其含量的变化对病毒的致病力有影响(Keating et al., 1990),Hoover等(1998)利用棉花(Gossypium hirsutum)和番茄(Lycopersicon esculentum)植物对夜蛾试验证实,不同酚类物质含量不同会使昆虫对病毒做出不同反应,也有研究用酚苷物质和人工饲料对舞毒蛾进行毒性试验,结果表明酚苷和单宁含量会影响苏云金杆菌(Bacillus thuringensis)对舞毒蛾的致病性(Arteel et al., 1992)。因此,研究JA诱导抗性及其与害虫对病原微生物敏感性的关系可能是揭示舞毒蛾周期性发生与核型多角体病毒病暴发关系的关键。
4 结论感染LdNPV的舞毒蛾幼虫生长发育及食物利用受到其取食寄主植物诱导抗性的影响,其发育历期、体质量、相对生长量及食物利用指标均比单独病毒感染组及对照组显著降低,且取食量只有JA诱导5天组与对照差异显著,而仅病毒感染的舞毒蛾幼虫取食量与对照差异不显著,JA诱导抗性增加了LdNPV对舞毒蛾幼虫生长发育及食物利用的负面影响。感染病毒的舞毒蛾幼虫对诱导5天组青杨叶片取食量最低,可能与叶片酚类等次生物质含量较高有关,次生物质含量改变了寄主植物的可食性。
食物消化率、转化率和利用率可反映昆虫对植物的利用情况、嗜食程度及其生长优劣(钦俊德, 1987),感染病毒的幼虫通过取食JA诱导的青杨叶片,其食物利用的3个指标均显著低于对照组,同时低于单独病毒组,说明JA诱导抗性和LdNPV对舞毒蛾幼虫生长和代谢的负面影响有明显的协同增强效应。另一方面也说明JA作为诱导因子可诱导青杨产生积极的防御反应,这种防御反应也影响了LdNPV。
JA诱导青杨对感染LdNPV的舞毒蛾幼虫生长发育及食物利用影响在笔者设置的诱导时间(1,5,10天)间显著差异,是否与时间间隔较短有关还有待进一步研究。
| [] |
钦俊德. 1987. 昆虫与植物关系--论昆虫与植物的相互作用及其演化. 北京: 科学出版社 : 133 -149.
( Qin J D. 1987. The relationships between insects and plants-on the interactions between insects and plants and their evolution. Beijing: Science Press : 133 -149. [in Chinese] ) |
| [] |
秦秋菊, 高希武. 2005. 昆虫取食诱导的植物防御反应. 昆虫学报 , 48 (1) : 125–134.
( Qin Q J, Gao X W.2005. Plant defense responses induced by insect herbivory. Acta Entomologica Sinica , 48 (1) : 125–134. [in Chinese] ) |
| [] |
王晓丽, 王予彤, 段立清, 等. 2014. 四种植物酚类物质对舞毒蛾生长发育及繁殖的影响. 昆虫学报 , 57 (7) : 831–836.
( Wang X L, Wang Y T, Duan L Q, et al.2014. Effects of four plant phenolics on the development and fecundity of the gypsy moth, Lymantria dispar (Lepidoptera:Lymantriidae). Acta Entomologica Sinica , 57 (7) : 831–836. [in Chinese] ) |
| [] |
王予彤, 越慧芳, 王晓丽, 等. 2015. 外源茉莉酸诱导青杨生化抗性及其对舞毒蛾幼虫食物利用的影响. 昆虫学报 , 58 (6) : 673–679.
( Wang Y T, Yue H F, Wang X L, et al.2015. Biochemical response of green poplar induced by exogenous jasmonic acid and its effects on food utilization of larval Lymantria dispar (Lepidoptera:Lymantriidae). Acta Entomologica Sinica , 58 (6) : 673–679. [in Chinese] ) |
| [] |
伊里因斯基. 1957.森林食叶害虫的观察及其大量发生的预测.殷殿忠译.北京:中国林业出版社. ( Ilyinsky A N. 1957. Observation on the forest defoliators and prediction of their massive occurrence. Translated by Yin Dianzhong. Beijing:China Forestry Publishing House.[in Chinese][in Chinese]) |
| [] |
萧刚柔. 1992. 中国森林昆虫. 北京: 中国林业出版社 : 1086 -1087.
( Xiao G R. 1992. Forest insects of China. Beijing: China Forestry Publishing House : 1086 -1087. [in Chinese] ) |
| [] |
越慧芳, 段立清, 李海平, 等. 2013. 外源茉莉酸诱导的青杨叶片保护性酶活性变化及其对舞毒蛾幼虫生长发育的影响. 昆虫学报 , 56 (3) : 270–275.
( Yue H F, Duan L Q, Li H P, et al.2013. Changes in activities of protective enzymes in green poplar induced by exogenous jasmonic acid and the effects on larval development of the gypsy moth, Lymantria dispar (Lepidoptera:Lymantriidae). Acta Entomologica Sinica , 56 (3) : 270–275. [in Chinese] ) |
| [] |
越慧芳. 2013.外源茉莉酸对青杨的诱导防御反应.呼和浩特:内蒙古农业大学硕士学位论文. ( Yue H F. 2013. Induced defense responses of green poplar (Populus cathayana Rehd) by exogenous jasmonic acid. Huhhot:MS thesis of Inner Mongolia Agricultural University.[in Chinese][in Chinese]) |
| [] | An Y, Shen Y B, Wu L J, et al.2006. A change of phenolic acids content in poplar leaves induced by methyl salicylate and methyl jasmonate. Journal of Forestry Research , 17 (2) : 107–110. DOI:10.1007/s11676-006-0025-1 |
| [] | Arteel G E, Lindroth R L.1992. Effects of aspen phenolic on gypsy moth (Lepidoptera:Lymantriidae) susceptibility to Bacillus thuringiensis. Great Lakes Entomol , 25 : 239–244. |
| [] | Awmack C, Leather S R.2002. Host plant quality and fecundity in herbivorous insects. Annual Review of Entomology , 47 : 817–844. DOI:10.1146/annurev.ento.47.091201.145300 |
| [] | Dicke M, Loon J J.2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomologia Experimentalis et Applicata , 97 : 237–249. DOI:10.1046/j.1570-7458.2000.00736.x |
| [] | Duan L, Otvos I S.2001. Influence of larval age and virus concentration on mortality and sublethal effects of a nucleopolyhedrovirus on the western Spruce Budworm (Lepidoptera:Tortricidae). Environmental Entomology , 30 (1) : 136–146. DOI:10.1603/0046-225X-30.1.136 |
| [] | Elkinton J S, Liebhold A M.1990. Population dynamics of gypsy moth in North America. Annual Review of Entomology , 35 : 571–596. DOI:10.1146/annurev.en.35.010190.003035 |
| [] | Hoover K, Stout M J, Alaniz S A, et al.1998. Influence of induced plant defenses in cotton and tomato on the efficacy of baculoviruses on noctuid larvae. Chemoecology , 24 : 253–271. |
| [] | Howe G A.2004. Jasmonates as signals in the wound response. Journal of Plant Growth Regulation , 23 (3) : 223–237. DOI:10.1007/s00344-004-0030-6 |
| [] | Ingrid M, Dale M N, Erik V N.1997. Gypsy moth (Lymantira dispar) larval development and survival to pupation on diet plus extractables from green ash foliage. Entomologia Experimentalis et Applicata , 84 : 247–254. DOI:10.1046/j.1570-7458.1997.00222.x |
| [] | Karban R, Baldwin I T. 1997. Induced responses to herbivory. London: University of Chicago Press Ltd. : 187 -191. |
| [] | Keating S T, Yendol W G, Schultz J C.1988. Relationship between susceptibility of gypsy moth larvae (Lepidoptera:Lymantriidae) to a baculovirus and host plant foliage constituents. Environmental Entomology , 17 : 952–958. DOI:10.1093/ee/17.6.952 |
| [] | Keating S T, Hunter M D, Schultz J C.1990. Leaf phenolic inhibition of gypsy moth nuclear polyhedrosis virus. Journal of Chemical Ecology , 16 : 1445–1457. DOI:10.1007/BF01014080 |
| [] | Maffei M E, Mithofer A, Boland W.2007. Insects feeding on plants:rapid signals and responses preceding the induction of phytochemical release. Phytochemistry , 68 : 2946–2959. DOI:10.1016/j.phytochem.2007.07.016 |
| [] | O'Reily D R, Brrown M R, Miller L K.1992. Alteration of ecysterpoid metabolism due to baculovirus infection of the fall armyworm Spodaptera frugiperda:host ecdysteroids are conjugated with galactose. Insect Biochemistry and Molecular Biology , 22 (4) : 313–320. DOI:10.1016/0965-1748(92)90069-Q |
| [] | Pechan T, Ye J, Chang Y M, et al.2000. A unique 33-kD cysteine proteinase accumulates in response to larval feeding inmaize genotypes resistant to fall armyworm and other Leidoptera. Plant Cell , 12 (7) : 1031–1040. DOI:10.1105/tpc.12.7.1031 |
| [] | Podgwaite J D, Reardon R C, Walton G S, et al.1992. Effects of aerially applied Gypchek on gypsy moth (Lepidoptera:Lymantriidae) populations in Maryland woodlots. Journal of Economical Entomology , 85 : 1136–1139. DOI:10.1093/jee/85.4.1136 |
| [] | Shikano I, Ericsson J D, Cory J S, et al.2009. Indirect plant-mediated effects on insect immunity and disease resistance in a tritrophic system. Basic and Applied Ecology , 11 (1) : 15–22. |
 2016, Vol. 52
2016, Vol. 52

