文章信息
- 相昆, 徐颖, 李国田, 王晓芳, 张美勇
- Xiang Kun, Xu Ying, Li Guotian, Wang Xiaofang, Zhang Meiyong
- 外源NO对低温胁迫下核桃幼苗活性氧代谢的影响
- Effects of Exogenous Nitric Oxide on Reactive Oxygen Metabolism of Walnut Seedlings under Low Temperature Stress
- 林业科学, 2016, 52(1): 143-149
- Scientia Silvae Sinicae, 2016, 52(1): 143-149.
- DOI: 10.11707/j.1001-7488.20160117
-
文章历史
- 收稿日期:2014-10-20
- 修回日期:2015-11-01
-
作者相关文章
植物在低温胁迫下生理功能会发生显著变化,如细胞结构(倪郁等,2014)、膜脂过氧化(岳海等,2010; 刘慧英等,2003)、细胞膜透性(刘慧英等,2003; 邓化冰等,2011)、 渗透调节物质(可溶性糖、可溶性蛋白和游离脯氨酸)(邓化冰等,2011; 许楠等,2009)及保护酶活性(SOD、CAT和POD等)(李轶冰等,2009)等的变化。一氧化氮(nitric oxide,NO)是植物体内一种重要的氧化还原信号分子,属于活性氮。近年来,NO与环境胁迫条件下植物抗性的关系受到研究者的广泛关注。研究发现,0.1 mmol ·L-1 SNP处理能显著提高小麦(Triticum aestivum)幼苗的SOD和CAT活性,降低由NaCl处理引起的小麦叶片氧化损伤(Singh et al.,2008); 适宜浓度的外源NO能够通过提高平邑甜茶(Malus hupehensis)体内的SOD,POD,CAT,APX活性和AsA含量,有效缓解20%PEG渗透胁迫对其造成的过氧化伤害(曹慧等,2015); SNP处理可以进一步提高Cu胁迫下番茄(Solanum lycopersicum)体内γ-谷氨酰半胱氨酸合成酶(γ-ECS)、谷胱甘肽合成酶(GS)活性,促进GSH,PCs的合成,增强清除过氧化物的能力,并螯合过多的Cu2+,降低其生物毒性(王建等,2014); 外源NO供体SNP可以通过保护蝴蝶兰(Phalaenopsis spp.)幼苗的细胞膜系统、增加渗透调节物质含量、提高保护酶活性来减轻低温胁迫对蝴蝶兰幼苗的伤害,提高其抗低温胁迫的能力(牟雪姣等,2015); NO可提高黑暗诱导的拟南芥(Arabidopsis thaliana)叶片衰老过程中叶绿体和类囊体膜结构的稳定性,并抑制叶绿素降解(Liu et al., 2013); 低浓度外源NO能够有效缓解镉胁迫对长春花(Catharanthus roseus)产生的毒害作用(刘柿良等,2014)。目前有关外源NO提高植物抗逆性的研究,主要集中在水分胁迫、盐胁迫和金属离子胁迫等方面,研究对象也主要为小麦、玉米(Zea mays)等草本植物,外源NO对低温胁迫下核桃(Juglans regia)幼苗活性氧代谢机制影响的研究鲜见报道。
核桃是喜温树种,为我国重要木本粮油战略树种之一,是丘陵山区农民的重要经济来源。但是在北方,核桃产区易遭受冻害和早春晚霜危害,核桃产量降低,同时也影响核桃幼树期的整形修剪,制约了核桃产业的健康、快速、可持续发展。因此,研究核桃对低温的响应机制以及开展抗寒新品种选育,对于保障核桃安全越冬、预防"倒春寒"都具有重要意义。鉴于此,本研究针对核桃生产实践中存在的低温逆境胁迫问题,在前期研究基础上,以抗寒性不同的'香玲’和'鲁果12号’核桃品种为试材(相昆等,2011),研究外源NO处理对低温胁迫下核桃幼苗活性氧代谢系统的影响,探讨对核桃抗寒性的可能作用机制,以期找到通过施加外源NO提高果树抗逆性的新方法,为外源NO在未来核桃抗逆生产中的广泛应用提供理论基础。
1 材料与方法 1.1 试验材料与处理供试核桃品种为'香玲’和'鲁果12号’,砧木为实生核桃苗。试验在山东农业大学重点实验室进行。2013年3月中旬,将2个核桃品种试材移栽到盆中,每盆栽植1株,正常管理。2014年5月,选取生长一致的2个品种盆栽苗,分成4组,每组5株,移至人工气候室(昼/夜温度为25 ℃)。先预培养15天,然后2组喷蒸馏水、2组喷SNP,再用薄膜覆盖整个植株,保湿1 h,连续喷3天,每天1次。
在预试验基础上,筛选出SNP 的最适处理浓度为200 μmol ·L-1。试验设4个处理:1)正常生长条件+喷蒸馏水作为对照(Control),昼/夜温度均为25 ℃; 2)低温处理+喷施蒸馏水(Cold),昼/夜温度为8 ℃; 3)正常生长条件+喷施200 μmol ·L-1 SNP(SNP); 4)低温处理+喷施200 μmol ·L-1SNP(Cold+SNP)。连续处理3天后,采取幼苗每枝条的第3片叶片测定各项生理指标,每处理3次重复。
1.2 测定指标及方法质膜相对透性测定参照张宪政等(1994)方法; O2·-产生速率测定采用羟胺氧化法(王爱国等,1990); H2O2,MDA含量测定参照Kramer等(1991)方法; SOD活性测定采用氯化硝基四氮唑蓝(NBT)光化还原法(Beauchamp et al., 1971); CAT活性测定参照Cakmak等(1992)方法; APX和GR酶活性测定参照Pinheiro等(2004)方法; DHAR活性测定参照Nakano等(1981)方法; POD活性测定参照张志良等(2009)方法; AsA含量测定参照Law等(1983)方法; GSH和GSSG含量测定参照Anderson等(1992)方法; 脯氨酸测定采用茚三酮比色法(Bates et al., 1973); 酶提取液中可溶性蛋白含量测定采用(Bradford,1976)。
1.3 数据处理采用Microsoft Excel软件进行数据统计,通过DPS进行差异显著性分析。表中数值为平均值±标准差(mead±SD)。
2 结果与分析 2.1 外源NO对低温胁迫下核桃幼苗叶片叶绿素含量的影响低温胁迫处理降低了核桃幼苗叶片Chla,Chlb,Chl(a+b)和Chla/b含量; SNP处理显著提高了低温胁迫下核桃幼苗叶片Chla,Chlb,Chl(a+b)和Chla/b含量(P<0.05)。由此可见,喷施 SNP处理可减缓低温胁迫下核桃幼苗叶片叶绿素含量的降低,保护光合色素免受胁迫的伤害,有利于维持光合作用,促进幼苗生长;正常生长条件下,SNP处理对叶绿素含量无显著影响(表 1)。
|
|
低温胁迫下2个核桃品种幼苗叶片的相对电导率均上升,与正常生长条件差异显著(P<0.05)。SNP 处理能够缓解低温胁迫对细胞膜的伤害作用。正常生长条件下,SNP处理对质膜相对透性的缓解作用不明显(图 1)。

|
图1 SNP对低温胁迫下核桃幼苗叶片相对电导率的影响 Fig.1 Effect of SNP on relative electrical conductivity in walnut seedlings leaves under low temperature stress |
由表 2可知,正常生长条件下,SNP处理对核桃幼苗叶片的O2·-产生速率、H2O2及MDA含量均无明显影响。低温胁迫处理后,2个核桃品种叶片的O2·-产生速率、H2O2及MDA含量分别较对照提高了102.9%,211.2%,82.6%和68.4%,274.0%,52.1%; SNP处理显著降低了O2·-产生速率和H2O2含量,抑制了膜脂过氧化产物MDA含量的积累,从而缓解了低温胁迫对核桃幼苗叶片细胞膜的伤害。
|
|
低温胁迫下,核桃幼苗叶片SOD和GR活性升高,POD,APX和DHAR活性下降,CAT活性变化在2个品种中表现不一致,'香玲’叶片CAT活性下降,'鲁果12号’叶片CAT活性上升; SNP处理显著提高了SOD,POD,CAT,APX,DHAR和GR活性。 正常生长条件下,SNP处理可显著提高POD,APX,DHAR和GR活性,而对SOD和CAT活性无显著影响(表 3)。
|
|
与对照相比,正常生长条件下,SNP处理显著提高了核桃幼苗叶片AsA含量及AsA/DHA比值,降低了DHA含量。低温胁迫下,AsA含量下降,DHA含量升高,AsA/DHA降低,SNP处理后,2个品种的AsA、DHA含量及AsA/DHA分别提高了58.7%,10.4%,30.8%和52.6%,13.3%,34.6%,达显著水平(图 2)。

|
图2 SNP对低温胁迫下核桃幼苗叶片抗坏血酸循环的影响 Fig.2 Effect of SNP on ascorbic acid cycle in walnut seedlmgs leaves under low temperature stress |
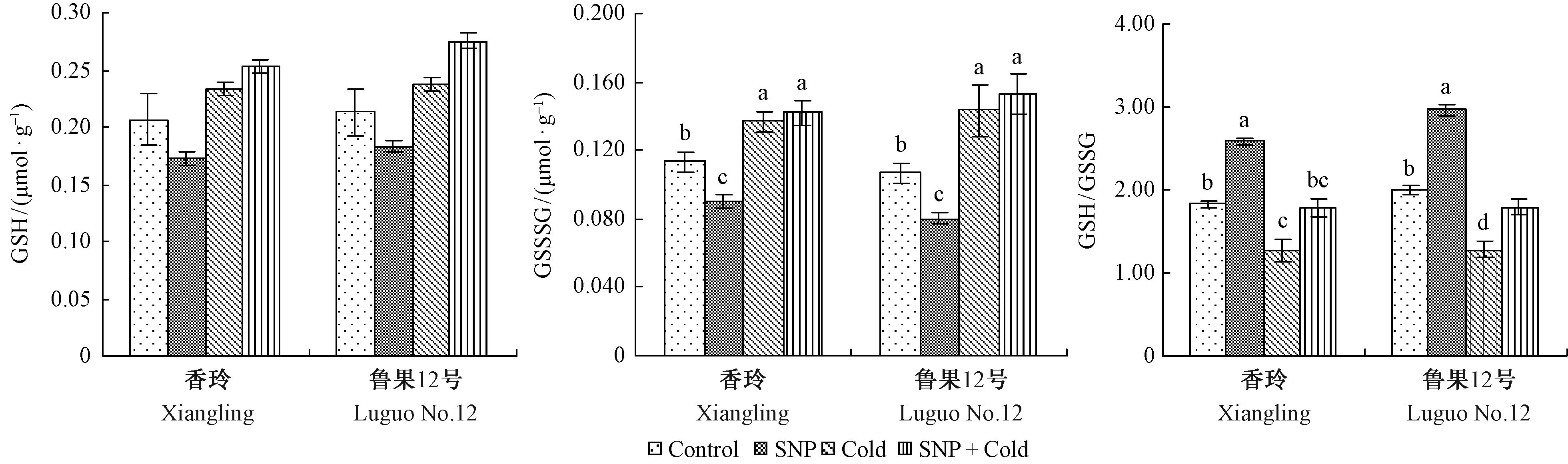
与对照相比,正常生长条件下,SNP处理的核桃叶片GSH含量及GSH/GSSG显著增加。低温胁迫下,GSH含量下降,GSSG含量升高,GSH/GSSG降低,SNP处理后,2个品种的GSH、GSSG含量及GSH/GSSG分别提高了39.8%,3.6%,40.2%和55.6%,7.0%,39.8%,达显著水平(图 3)。
|
图3 SNP对低温胁迫下核桃叶片谷胱甘肽循环的影响 Fig.3 Effect of SNP on glutathione cycle in walnut leaves under low temperature stress |
低温胁迫下,2个品种核桃幼苗叶片的Pro含量显著高于对照; SNP处理促进了Pro的积累,表明外源NO处理对低温胁迫下核桃幼苗叶片游离Pro积累量的增加具有促进作用;正常生长条件下,喷施SNP对Pro含量无明显影响(图 4)。

|
图4 SNP对低温胁迫下核桃叶片脯氨酸含量的影响 Fig.4 Effect of SNP on contents of Pro in walnut leaves under low temperature stress |
一般认为,冷害胁迫可改变植物叶绿体类囊体膜的膜质组分和膜蛋白,破坏类囊体内膜的超微结构,引起膜质过氧化,进一步影响光能的吸收和传递,引起光合速率下降(许楠等,2009)。本研究发现,低温胁迫降低了核桃幼苗叶片色素含量,但外源NO处理可维持色素含量在较高水平,参与调节植物的光合作用,缓解低温胁迫对核桃幼苗叶片光合作用的破坏。同时,NO是具有强氧化性的活性氮,可作为抗氧化剂直接与O2·-反应,清除活性氧(Laspina et al., 2005)。本研究的低温胁迫下,细胞自身的活性氧清除系统遭受破坏,不能清除过量的活性氧,O2·-和H2O2含量升高,加速了MDA的积累; 外源NO处理降低了核桃幼苗叶片的活性氧水平,减少了膜脂过氧化产物MDA的积累,降低了与膜结合的酶的结构改变,保护膜结构免遭破坏,提高了核桃幼苗抗低温能力,维持了植物细胞内的正常代谢。
SOD,POD,CAT,APX,DHAR和GR 等抗氧化酶构成植物体内主要的活性氧清除系统(杨美森等,2012; 李凯龙等,2013)。本研究的低温胁迫下,SOD和GR活性升高,POD,DHAR和APX活性下降,而'香玲’的CAT活性下降,'鲁果12号’的CAT活性升高。虽然SOD,GR活性有一定的提高,但POD,DHAR和APX活性下降,细胞内活性氧自由基的产生与清除之间失衡,体内H2O2含量和O2·-产生速率显著上升,细胞自身清除活性氧自由基的能力降低,导致ROS积累,活性氧代谢严重失衡,加速了膜脂过氧化作用,致使机体受到伤害。'香玲’CAT活性下降而'鲁果12号’CAT活性升高,可能是因为'鲁果12号’更抗寒的原因,与笔者前期研究结果一致(相昆等,2011)。
大量研究表明,低浓度NO不仅可作为抗氧化剂迅速清除活性氧,而且能诱导抗氧化酶基因的表达,提高酶活性(杨美森等,2012; Uchida et al., 2002)。本研究中,SNP处理显著提高了低温胁迫下核桃幼苗叶片SOD活性,有利于促进O2·-歧化生成H2O2,而H2O2主要通过POD,CAT和APX得以清除。本研究结果亦表明,正常生长条件下,外源NO处理提高了APX和POD活性,抑制了低温胁迫对APX,POD和CAT活性的破坏作用,维持SOD,POD,CAT,APX,DHAR和GR等抗氧化物酶活性在较高水平,减轻了活性氧自由基的破坏作用。 AsA和GSH是植物细胞内主要的抗氧化剂,可直接与活性氧反应,生成氧化态的DHA和GSSG,DHA和GSSG再在DHAR和GR的还原作用下生成AsA和GSH(李秀等,2014)。但是,低温胁迫使核桃幼苗叶片细胞活性氧自由基的产生与清除系统遭受破坏,活性氧积累导致叶绿体膜结构损伤,破坏AsA-GSH循环系统,阻碍AsA和GSH的再生。外源NO处理可维持低温胁迫下核桃幼苗叶片的AsA-GSH循环,提高循环中GR,DHAR酶活性,增强抗氧化物酶活性,促进抗氧化剂AsA和GSH的再生,提高其还原态/氧化态比例,降低O2·-产生速率和H2O2含量,抑制膜脂过氧化产物MDA的积累,缓解低温胁迫对核桃幼苗细胞膜的伤害,从而维护细胞结构的稳定性。
脯氨酸不仅可以作为生物体内有效的渗透调节物质,而且在消除ROS、提高抗氧化能力、稳定大分子结构、降低细胞酸性以及解除氨毒等方面也具有重要作用(Hayat et al.,2012; Szabados et al., 2010)。研究表明,植物抗逆性与Pro含量呈正相关(Hayat et al., 2012)。杨美森等(2012)研究发现,外源NO处理能够提高冷害胁迫下棉花(Gossypium hirsutum)幼苗植株体内Pro含量,保护细胞结构的完整性; 马金虎等(2015)研究发现,外源NO可提高玉米种胚中Pro含量。与其他植物相同,本研究中,外源NO可显著提高低温胁迫下核桃幼苗叶片Pro含量,参与渗透调节,维持细胞正常代谢,使植物具有一定抗寒性。
| [1] |
曹慧,王东方,王汉海,等.2015.外源NO对水分胁迫下平邑甜茶幼苗氧化损伤的缓解效应.西北植物学报,35(3):538-562. (Cao H,Wang D F,Wang H H,2015.Alleviated effect of exogenous nitric oxide on oxidative damage in Malus hupehensis(pamp) rehd.seedlings under water stress. Acta Botanica Boreali-Occidentalia Sinica, 35(3):538-562.[in Chinese])(  1) 1)
|
| [2] |
邓化冰,车芳璐,肖应辉.2011.开花期低温胁迫对水稻花粉性状及剑叶理化特性的影响.应用生态学报,22(1):66-72. (Deng H B,Che F L,Xiao Y H. 2011.Effects of low temperature stress during flowering period on pollen characters and flag leaf physiological and biochemical characteristics of rice. Chinese Journal of Applied Ecology, 22(1):66-72.[in Chinese])(  1) 1)
|
| [3] |
李凯龙,王艺潼,韩晓雪,等.2013.低钾胁迫对番茄叶片活性氧及抗氧化酶系的影响.西北植物学报,33(1):66-73. (Li K L,Wang Y T,Han X X, et al.2013.Changes in reactive oxygen species and antioxidative defense mechanism in tomato leaves under low potassium stress. Acta Botanica Boreali-Occidentalia Sinica,33(1):66-73.[in Chinese])(  1) 1)
|
| [4] |
李秀,巩彪,徐坤.2014.外源NO对高温胁迫下姜叶片活性氧代谢的影响.园艺学报,41(92):277-284. (Li X,Gong B,Xu K.2014. Effects of exogenous nitric oxide on reactive oxygen metabolism in ginger leaves under heat stress. Acta Horticulturae Sinica, 41(92):277-284.[in Chinese])(  1) 1)
|
| [5] |
李轶冰,杨顺强,任广鑫,等.2009.低温处理下不同禾本科牧草的生理变化及其抗寒性比较.生态学报, 29(3):1341-1347. (Li Y B,Yang S Q,Ren G X,et al. 2009.Changes analysis in physiological properties of several gramineous grass species and cold-resistance comparison on under cold stress. Acta Ecologica Sinica, 29(3):1341-1347.[in Chinese])(  1) 1)
|
| [6] |
刘慧英,朱祝军,吕国华,等.2003.低温胁迫下西瓜嫁接苗的生理变化与耐冷性关系的研究.中国农业科学,36(11):1325-1329. (Liu H Y,Zhu Z J,Lü G H,et al. 2003.Study on relationship between physiological changes and chilling tolerance in grafted watermelon seedlings under low temperature stress.Scientia Agricultura Sinica, 36(11):1325-1329.[in Chinese])(  1) 1)
|
| [7] |
刘柿良,潘远智,杨容孑,等.2014.外源一氧化氮对镉胁迫下长春花质膜过氧化、ATPase及矿质营养吸收的影响.植物营养与肥料学报, 20(2):445-458. (Liu S L,Pan Y Z,Yang R J,et al. 2014.Effects of exogenous NO mineral apsorption,lipid peroxidation and ATPase of plasma membrane catharanthus rosues tissues under cadmium stress.Journal of Plant Nutrition and Fertilizer, 20(2):445-458.[in Chinese])(  1) 1)
|
| [8] |
马金虎,邢国芳,杨小环,等.2015.外源EBR和NO信号对低温胁迫下玉米种胚抗氧化系统和低温响应基因表达的影响,应用生态学报,2015, 26(5):1411-1418 (Ma J H, Xing G F,Yang X H,et al.2015.Effects of exogenous EBR and NO signal on antioxidant system and low response gene expression under cold stress on maize embryo.Chinese Journal of Applied Ecology, 26(5):1411-1418.[in Chinese])(  1) 1)
|
| [9] |
牟雪姣,刘理想,孟鹏鹏,等.2015.外源NO缓解蝴蝶兰低温胁迫伤害的生理机制研究.西北植物学报,35(5):978-984. (Mu X J,Liu L X,Meng P P, et al.2015.Physiological mechanism of exogenous nitric oxide on alleviating low temperature stress of Phalaenopsis spp. Acta Botanica Boreali-Occidentalia Sinica, 35(5):978-984.[in Chinese])(  1) 1)
|
| [10] |
倪郁,宋超,王小清. 2014.低温胁迫下拟南芥表皮蜡质的响应机制.中国农业科学,47(2):252-261. (Ni Y, Song C,Wang X Q. 2014.Investigation on response mechanism of epicuticular wax on Arabidopsis thaliana under cold stress. Scientia Agricultura Sinica, 47(2):252-261.[in Chinese])(  1) 1)
|
| [11] |
王爱国,罗广华.1990.植物的超氧物自由基与羟胺反应的定量关系.植物生理学通讯, (6):55-57. (Wang A G,Luo G H.1990.Quanitaitve relaiton between the reaction of hydroxylanmine and superoxide anion radicals in plants.Plant Physiloy Communication, (6):55-57.[in Chinese])(  1) 1)
|
| [12] |
王建,于世欣,张敏,等.2014.外源NO介导Cu胁迫下番茄GSH-PCs合成途径.应用生态学报,25(9):2629-2636. (Wang J,Yu S X,Zhang M,et al.2014. Exogenous NO mediated GSH-PCs synthesis pathway in tomato under copper stress. Chinese Journal of Applied Ecology, 25(9):2629-2636.[in Chinese])(  1) 1)
|
| [13] |
相昆,张美勇,徐颖,等.2011.不同核桃品种耐寒特性综合评价.应用生态学报,22(9):2325-2330. (Xiang K, Zhang M Y,Xu Y,et al. 2011.Cold-tolerance of walnut cultivars:a comprehensive evaluation.Chinese Journal of Applied Ecology, 22(9):2325-2330.[in Chinese])(  2) 2)
|
| [14] |
许楠,孙广玉.2009.低温锻炼后桑树幼苗光合作用和抗氧化酶对冷胁迫的响应.应用生态学报, 20(4):761-766. (Xu N, Sun G Y.2009.Responses of mulberry seedlings photosynthesis and antioxidant enzymes to chilling stress after low-temperature acclimation.Chinese Journal of Applied Ecology, 20(4):761-766.[in Chinese])(  2) 2)
|
| [15] |
岳海,李国华,李国伟,等.2010.澳洲坚果不同品种耐寒特性的研究.园艺学报,37(1):31-38. (Yue H,Li G H,Li G W,et al.2010.Studieson cold resistance of different macadamia cultivars.Acta Horticulturae Sinica, 37(1):31-38.[in Chinese])(  1) 1)
|
| [16] |
杨美森,王雅芳,干秀霞,等.2012.外源一氧化氮对冷害胁迫下棉花幼苗生长、抗氧化系统和光合特性的影响.中国农业科学,45(15):3058-3067. (Yang M S,Wang Y F,Gan X X,et al.2012.Effects of exogenous nitric oxide on growth, antioxidant system and photosynthetic characteristics in seedling of cotton cultivar under chilling injury stress.Scientia Agricultura Sinica, 45(15):3058-3067.[in Chinese])(  3) 3)
|
| [17] |
张宪政,陈凤玉,王荣富.1994.植物生理学实验技术.沈阳:辽宁科学技术出版社. (Zhang X Z,Chen F Y,Wang R F. 1994.Guide of experimental technology of physiology.Shenyang:Liaoning Science and Technology Press.[in Chinese])(  1) 1)
|
| [18] |
张志良,瞿伟菁,李小方.2009.植物生理学实验指导.北京:高等教育出版社. (Zhang Z L, Qu W J,Li X F. 2009.Laboratory guide of plant physiology.Beijing:Higher Education Press.[in Chinese](  1) 1)
|
| [19] |
Anderson J V,Chevone B I,Hess J L.1992.Seasonal variation in the antioxidant system of eastern white pine needles.Plant Physiology,98(2):501-508.( 1) 1)
|
| [20] |
Bates L,Waldren R,Teare I.1973.Rapid determination of free proline for water-stress studies.Plant and Soil,39(1):205-207.( 1) 1)
|
| [21] |
Beauchamp C, Fridovich I.1971.Superoxide dismutase:improved assays and an assay applicable to acrylamide gels.Analytical Biochemistry,44(1):276-287.( 1) 1)
|
| [22] |
Bradford M.1976.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding.Analytical Biochemistry,72(1/2):248-254.( 1) 1)
|
| [23] |
Cakmak I,Marschner H.1992.Magnesium deficiency and high light intensity enhance activities of superoxide dismutase,ascorbate peroxidase,and glutathione reductase in bean leaves.Plant Physiology,98(4):1222-1227.( 1) 1)
|
| [24] |
Hayat S,Hayat Q,Alyemeni M N,et al.2012.Role of proline under changing environments:a review.Plant Signal Behav,7 (11):1-11.( 1) 1)
|
| [25] |
Kramer G, Norman H, Krizek D, et al.1991.Influence of UV-B radiation on polyamines lipid peroxidation and membranelipids in cucumber. Phytochemistry, 30(7):2101-2108.( 1) 1)
|
| [26] |
Laspina N V,Groppa M D,Tomaro M L,et al.2005.Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sciences,169(2):323-330.( 1) 1)
|
| [27] |
Law M Y,Charles S A,Halliwell B.1983.Glutathione and ascorbic acid in spinach(Spinacia oleracea)chloroplasts. The effect of hydrogen peronide and of paraguat.Biochemical Journal,210(2):899-903.( 1) 1)
|
| [28] |
Liu F,Guo F Q. 2013.Nitric oxide deficiency accelerates chlorophyll breakdown and stability loss of thylakoid membranes during dark-induced leaf senescence in Arabidopsis. PLoS One, 8(2):1-12.( 1) 1)
|
| [29] |
Nakano Y,Asada K.1981.Hydragen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. J Plant Cell Physiol,22(5):867-880.( 1) 1)
|
| [30] |
Pinheiro H A, DaMatta F M, Chaves A R M, et al. 2004.Drought tolerance in relation to protection against oxidative stress in clones of Coffea canephora subjected to long-term drought. Plant Science,167(6):1307-1314.( 1) 1)
|
| [31] |
Singh H P,Batish D R,Kaur G,et al.2008.Nitric oxide(as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots.Environmental and Experimental Botany,63(1/3):158-167.( 1) 1)
|
| [32] |
Szabados L, Savoure A.2010.Proline:a multifunctional amino acid.Trends Plant Sci,15(2):89-97.( 1) 1)
|
| [33] |
Uchida A,Jagendorf A T,Hibino T.2002.Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Science,163(3):515-523.( 1) 1)
|
 2016, Vol. 52
2016, Vol. 52

