文章信息
- 宋秀华, 李传荣, 许景伟, 胡丁猛, 王超
- Song Xiuhua, Li Chuanrong, Xu Jingwei, Hu Dingmeng, Wang Chao
- 元宝枫、雪松挥发物释放的昼夜节律
- Diurnal Rhythm of Emission of Volatile Compounds Emission from Acer truncatum and Cedrus deodara
- 林业科学, 2015, 51(4): 141-147
- Scientia Silvae Sinicae, 2015, 51(4): 141-147.
- DOI: 10.11707/j.1001-7488.20150418
-
文章历史
- 收稿日期:2014-05-21
- 修回日期:2014-11-07
-
作者相关文章
2. 泰山森林生态站/山东农业大学农业生态与环境重点实验室 泰安 271018;
3. 山东省林业科学研究院 济南 250014
2. Taishan Forest Ecosystem Research Station/Key Laboratory of Agricultural Ecology and Environment, Shandong Agricultural University Tai'an 271018;
3. Shandong Research Institute of Forestry Jinan 250014
绿色植物在吸收CO2释放O2的同时,也释放次生代谢产物,即挥发性有机化合物(volatile organic compounds,VOCs),也称为挥发物(Volatiles)。挥发物可以调节植物的生长、发育及对逆境的适应能力(韩芬等,2008;Kivimäenpää et al.,2013);同时还可以抑制空气微生物生长(孟雪等,2010),还会对人体及其他动物产生心理及生理的影响(Jo et al.,2010;王艳英等,2013)。 挥发物中有许多成分具有较强的生理活性,特别是萜类化合物,其种类繁多,结构复杂,性质各异,生理活性表现多种多样(王峥涛等,2009)。因此,城市植被建设不应只考虑到景观功能,更要考虑到其生态功能,特别能引起人体生理心理变化的植物挥发物的释放情况,通过树种的合理配置,创造一个更有利于人类健康的绿地环境,这已成为科研和生产领域亟待解决的理论和技术问题。
元宝枫(Acer truncatum)为落叶乔木,树冠优美,是华北地区常见的绿化树种,也常作为荒山绿化或风景林的伴生树种。雪松(Cedrus deodara)为常绿乔木,枝条开展,树形优美,是世界著名观赏树种,孤植、列植、群植及与其他树种混置等多种配置方式,应用范围广泛。元宝枫和雪松作为华北地区常见的城市绿化树种,阔叶树和针叶树的典型代表,研究其挥发物的释放,具有一定的典型性。已有学者研究了元宝枫、雪松等常见绿化树种挥发物的释放情况(张风娟等,2007;盖苗苗等,2010),对于树木挥发物释放昼夜节律性的研究,多集中于挥发物释放与昆虫取食行为的关系(王鸿斌等,2005;杨桦等,2011)。因此,本试验采用离体采样固相微萃取(SPME)结合气相色谱质谱联用仪(GC-MS)分析元宝枫、雪松枝叶挥发物的昼夜变化规律并分析对人居环境的影响,旨在预测挥发物的释放量,为人们绿地游憩提供参考,并通过树种的合理组合创造出更有效、更有利于人体健康的植物环境。
1 材料与方法 1.1 试验材料与取样方法山东农业大学校园内生长健康的元宝枫(株高约5.6 m,胸径约15.4 cm)和雪松(株高约11.2 m,胸径约40.5 cm),在7月中旬选取树木中部向阳当年生枝叶,于2:00,5:00,8:00,11:00,14:00,17:00,20:00,23:00共8个时间点采集分析,每次采样3次重复,每重复叶片约20片。
1.2 测定方法挥发物采集与分析方法(SPME-GC-MS):采用固相微萃取法结合日本岛津公司生产的GC-MS-QP 2010 plus气-质联用仪,即SPME-GC-MS法。元宝枫称取新鲜功能叶10 g,剪成 0.5 cm × 0.5 cm碎片,放入100 mL萃取瓶中,铝箔纸封口;雪松称取新鲜枝叶(针叶和嫩枝)10 g,剪成0.5 cm段,放入100 mL萃取瓶中,铝箔纸封口。然后50/30 μm DVB/CAR/PDMS萃取头在 40 ℃的温度中顶空萃取30 min,然后将萃取头插入GC-MS进样口,于250 ℃解吸3 min。色谱条件:柱初温 35 ℃,保持2 min,以6 ℃·min-1上升至 100 ℃,再以8 ℃·min-1上升至140 ℃,随后以12 ℃·min-1上升至250 ℃,保留3 min。质谱条件:EI电离源,电子能量70 eV,离子源温度200 ℃,接口温度230 ℃,全扫描模式,扫描范围45~450 m·z-1。化合物定性与定量分析:经NIST08和NIST08S数据库检索定性,按SI相似度>80%的原则作为鉴定结果,取各化合物的峰面积进行比较分析。
1.3 数据分析采用Microsoft Excel和SPSS软件对数据进行整理、分析和制图,采用SPSS软件对数据进行差异显著性检验和相关分析。
2 结果与分析 2.1 元宝枫挥发物昼夜节律释放检测到的元宝枫挥发物中主要是酯、醇、醛和萜烯类化合物,表 1所列化合物共21种,占所测化合物相对含量的80.0%以上,有些物质只检测到一次且含量较低,在表中未列出(如丁酸-2-戊烯酯、丁酸己酯、β-罗勒烯等)。酯类物质有5种,醇、醛类物质有5种,萜烯类物质共11种,分别占总种类的23.8%,23.8%和52.4%;相对含量较高的物质主要有乙酸叶醇酯、乙酸己酯、 3-己烯醇、3-己烯醛和β-石竹烯,占总量的70.0%以上。其中,3-己烯醛、3-己烯醇和乙酸叶醇酯,具有青叶香味,乙酸己酯具有水果香味,这些主要成分使元宝枫叶片呈现青叶香气,可使人产生愉悦意识(Jo et al.,2010)。
|
|
挥发物的昼夜释放节律可分为3类,C6醇、醛类的释放高峰在17:00和2:00—5:00,低谷在14:00和20:00左右;C8酯类化合物释放高峰在14:00,低谷在5:00;C10单萜类化合物释放量白天高于夜晚,在23:00—2:00间释放量最低,多数C15倍半萜类化合物释放呈现“2峰2谷”型,高峰在17:00和5:00左右,低谷出现在8:00和23:00左右(表 1)。
元宝枫总挥发物峰面积呈现“2峰2谷”型变化,从早8:00开始逐渐降至11:00,随后升高至17:00达最高峰,晚23:00降至最低,凌晨2:00出现次高峰。主要挥发物乙酸叶醇酯、β-石竹烯、乙酸己酯的峰面积变化与此相似,3-己烯醛、3-己烯醇变化规律与此相反(图 1)。同时对此5种主要挥发物峰面积值进行Pearson相关分析(表 2),3-己烯醛与3-己烯醇呈极显著正相关,与乙酸叶醇酯呈极显著负相关;3-己烯醇与乙酸叶醇酯呈极显著负相关;其他挥发物之间无显著相关性,这与图 1中的变化趋势一致。
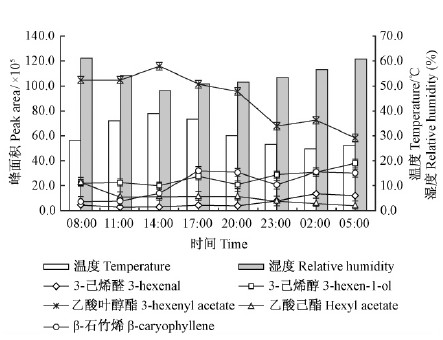 |
图 1 元宝枫主要挥发物峰面积昼夜变化及温湿度变化 Fig. 1 Diurnal variation of the main compounds peak area from A. truncatum and the variations of temperature and relative humidity |
|
|
检测到的雪松挥发物主要是萜烯类化合物,表 3所列化合物共26种,占所测化合物相对含量的87.0%以上。其中,酯类物质有3种,醇类物质有1种,萜烯类物质共22种,单萜8种,倍半萜14种,萜烯类物质的相对含量达84.0%以上。这些萜烯类物质对人体均有益,其中α-蒎烯、β-蒎烯、β-月桂烯具有杀菌、抑菌、镇咳、祛痰、抗炎作用,柠檬烯可治疗胆结石,同时这些物质可令人精神放松,利于人们的身心健康(孙启祥等,2004),这些化合物使雪松枝叶呈现树脂香。
|
|
所测挥发物的昼夜释放规律可分为3类:大多数单萜物质,如α-蒎烯、β-蒎烯、β-月桂烯、D-柠檬烯等释放高峰在14:00左右,低谷出现在23:00—次日2:00;大多数倍半萜类物质,如长叶烯、β-石竹烯、α-石竹烯等,呈现“2峰2谷”型变化,释放高峰在17:00和2:00,在23:00和5:00达最低;酯类物质如乙酸叶醇酯、乙酸己酯、乙酸龙脑酯等白天释放量高于夜晚,且在2:00—5:00达最低(表 3)。
雪松总挥发物峰面积日变化规律呈现“2峰2谷”型,从早8:00开始逐渐升高至14:00达高峰,晚23:00降至最低,次日凌晨2:00出现次高峰,5:00达谷底,与元宝枫总挥发物的变化规律相似。其中主要单萜类物质的峰面积在8:00—14:00较高,在23:00—次日2:00达到谷底;主要倍半萜类物质释放高峰在17:00和2:00,23:00和5:00则达最低。总之,雪松枝叶挥发物的释放高峰在14:00—17:00,低谷在23:00和5:00(图 2)。同时对6种主要挥发物峰面积值进行Pearson相关分析(表 4),α-蒎烯与β-蒎烯极显著相关,与D-柠檬烯显著相关;β-蒎烯与β-月桂烯极显著相关,与D-柠檬烯显著相关;β-月桂烯与D-柠檬烯极显著相关,这4种单萜物质相关性较高。β-石竹烯与吉马烯D极显著相关,与单萜物质无显著相关。
 |
图 2 雪松主要挥发物峰面积昼夜变化及温湿度变化 Fig. 2 Diurnal variation of the main compounds peak area from C. deodara and the variations of temperature and relative humidity |
|
|
环境因子如温度、湿度等影响着植物的代谢活动,进而对枝叶挥发物的释放产生间接的影响。本试验的结果表明(图 1,2):在不考虑其他影响因子的条件下,在一定范围内随温度升高、湿度减小,挥发物的释放量增加,这与已有研究结论较一致(李娟等,2011;Ziru et al.,2011)。
图 1,2表明,温度升高,湿度降低时段,挥发物总峰面积较高。8:00开始,温度增高,光照增强,植物生理活动逐渐增加,光合合成物质增多,伴随的次生代谢物质释放量逐渐增大,在17:00达高峰。随后光照减弱,光合作用逐渐停止,湿度逐渐增大,部分气孔关闭,挥发物释放量减少,光合产物在植株体内仍进行着代谢活动(相比白天要弱),次生代谢产物在体内逐渐累积,挥发物达到一定浓度时,气孔内外的压强差使气孔张开,储藏的挥发物释放,在2:00左右出现小高峰。从表 5可知,挥发物总峰面积与温度呈正相关,与湿度呈负相关,这与图 1,2的变化趋势是一致的。
与温湿度的相关系数 Tab.5 Correlation coefficients between the total
volatile compounds and temperature and relative humidity
|
|
挥发物的昼夜释放节律,不同树种表现出不同的规律,特别是阔叶树和针叶树表现出不同的规律,通常认为在正午和午夜有2个释放高峰(李金龙等,1994)。Li等(2003)研究复叶槭(Anoplophora glabripennis)7,8月的释放规律时发现,高峰分别在14:00和10:00左右,且挥发物种类和相对含量明显不同。胡永建等(2007)研究马尾松(Pinus massoniana)萜烯类化合物释放高峰多在10:30,低峰期在13:30和22:30,湿地松的释放高峰在12:00—15:00之间;李娟等(2011)研究侧柏(Biota orientalis)春季挥发物释放规律呈现“3峰2谷”型,即5:00,13:00,19:00呈现高峰,23:00— 1:00和17:00呈现低谷。在许多植物中,单萜合成依赖于温度和光照,如意大利松(Pinus pinea)光下释放的挥发物远大于暗处,Standt等(1997)认为单萜释放的最初来源是植物体内重新合成而非树脂道中储存的。但同时正午强光照导致气孔部分关闭,也会影响挥发物的释放。本研究中,元宝枫和雪松枝叶挥发物的释放高峰集中在 8:00,17:00和2:00,具体挥发物的释放规律不同,但主要成分释放情况与此相同;挥发物的释放受环境条件的影响,在温度升高、湿度减小的时段,挥发物的释放量增加。
植物挥发物之间还存有一定的相关性,这与挥发物产生的前体物质及合成酶有关,如Julian等(2003)发现矮牵牛(Petunia hybrid line W115)晚间PAL和SAM表达增多,促使合成苯环烃类物质增多,花的香气更浓。Ben等(2010)发现蚕豆(Vicia faba)的主要挥发物不仅呈现昼夜节律变化,而且3-己烯醇与乙酸叶醇酯、甲基庚烯酮与乙酸叶醇酯显著相关。元宝枫醛、醇、酯类挥发物主要通过脂肪酸合成途径产生,在代谢过程中受植物体内代谢酶的影响,醛在醇脱氢酶ADH作用下生成醇,然后醇在酰基转移酶AAT作用下生成酯,3-己烯醛和3-己烯醇作为乙酸叶醇酯的前体物质,白天光合作用旺盛期合成乙酸叶醇酯量高,晚上合成量降低,造成3-己烯醛和3-己烯醇累积,呈现与乙酸叶醇酯负相关变化。雪松针叶挥发物主要通过类异戊二烯合成途径产生,萜类合成酶(TPS)分别催化前体底物香叶酯二磷酸(GPP)、法呢基焦磷酸(FPP)、牻牛儿基焦磷酸(GGPP),形成单萜、倍半萜和二萜(龚治等,2010),但是同样底物的酶在不同植物体内的产物却不尽相同。本试验中,雪松枝叶挥发物中α-蒎烯、β-蒎烯、β-月桂烯、D-柠檬烯等这4种单萜物质相关性较高,而β-石竹烯与吉马烯D则极显著相关,这与单萜和倍半萜具体合成途径和酶有关。
元宝枫、雪松作为阔叶树和针叶树的典型代表,其挥发物主要成分不同且呈现不同的昼夜释放规律,但主要是对人体有益的成分。元宝枫释放的主要是酯、醇、醛和萜烯类化合物,其中C8酯类化合物释放高峰在14:00,低谷在5:00;雪松释放的主要是萜烯类物质,其中单萜类物质释放高峰在14:00左右,低谷在23:00—2:00,倍半萜类物质释放高峰在17:00和2:00,低谷在23:00和5:00。树木释放到空气的挥发物多为微量或痕量物质,浓度到达怎样程度才能对人体产生影响还需要做进一步研究。同时各类挥发物由于产生和释放机理不同呈现的变化规律各不相同,呈现这些规律的原因也有待于进一步研究。
| [1] |
盖苗苗, 周春玲, 曲宁, 等. 2010. 雪松的挥发性物质成分及抑菌效益研究. 中国农学通报, 26(7): 311-313. (Gai M M, Zhou C L, Qu N, et al. 2010. The study of volatile substances from Cedar and its antibacterial benefit. Chinese Agricultural Science Bulletin, 26(7): 311-313[in Chinese]).(  1) 1)
|
| [2] |
龚治, 李典谟, 张真. 2010. 针叶树萜类合成酶研究进展. 林业科学, 46(1): 123-130. (Gong Z, Li D M, Zhang Z. 2010. Research progress of terpene synthases in conifers. Scientia Silvae Sinicae, 46(1): 123-130[in Chinese]).(  1) 1)
|
| [3] |
韩芬, 王辉, 边银霞, 等. 2008. 华北落叶松枝叶挥发性物质的化学成分及其化感作用. 应用生态学报, 19(11): 2327-2332. (Han F, Wang H, Bian Y X, et al. 2008. Chemical components and their allelopathic effects of the volatiles from Larix principis-rupprechtii leaves and branches. Chinese Journal of Applied Ecology, 19(11): 2327-2332[in Chinese]).(  1) 1)
|
| [4] |
胡永建, 任琴, 金幼菊, 等. 2007. 马尾松(Pinus massoniana)、湿地松(Pinus elliottii)挥发性化学物质的昼夜节律释放. 生态学报, 27(2): 565-570. (Hu Y J, Ren Q, Jin Y J, et al. 2007. Diurnal cycle of emission of volatile compounds from Pinus massoniana and Pinus elliottii. Acta Ecologica Sinica, 27(2): 565-570.[in Chinese])(  1) 1)
|
| [5] |
李金龙, 白郁华, 胡建信, 等. 1994. 油松排放萜烯类化合物浓度的日变化及排放速率的研究. 中国环境科学, 14(3): 165-169. (Li J L, Bai Y H, Hu J X, et al. 1994. Diurnal variation in the concentration of terpenes and its emission rate measurements from oil pine. China Environmental Science, 14(3): 165-169[in Chinese]).(  1) 1)
|
| [6] |
李娟, 王成, 彭镇华, 等. 2011. 侧柏春季挥发物浓度日变化规律及其影响因子研究. 林业科学研究, 24(1):82-90. (Li J, Wang C, Peng Z H, et al. 2011. The diurnal variation and influence factors of VOC of Platycladus orientalis in spring. Forest Research, 24(1):82-90[in Chinese]).(  2) 2)
|
| [7] |
孟雪, 王志英, 吕慧. 2010. 绿萝和常春藤主要挥发性成分及其对5种真菌的抑制活性. 园艺学报, 37(6): 971-976. (Meng X, Wang Z Y, Lv H. 2010. The volatile constituents analysis of Scindapsus aureum and Hedera nepalensis var. sinensis and their inhibition against five fungi. Acta Horticulturae Sinica, 37(6): 971-976[in Chinese]).(  1) 1)
|
| [8] |
孙启祥, 彭镇华, 张齐生. 2004. 自然状态下杉木木材挥发物成分及其对人体身心健康的影响. 安徽农业大学学报, 31(2): 158-163. (Sun Q X, Peng Z H, Zhang Q S. 2004. Volatiles of wood of Chinese fir in nature and its effect on human health. Journal of Anhui Agricultural University, 31(2): 158-163[in Chinese]).(  1) 1)
|
| [9] |
王鸿斌, 张真, 孔祥波, 等. 2005. 油松萜烯类挥发物释放规律与红脂大小蠹危害的关系. 北京林业大学学报, 27(2): 75-80. (Wang H B, Zhang Z, Kong X B, et al. 2005. Relationship between release regularity of volatiles from Pinus tabulaeformis and the damage by Dendroctonus valens. Journal of Beijing Forestry University, 27(2): 75-80[in Chinese]).(  1) 1)
|
| [10] |
王艳英, 王成, 郄光发, 等. 2013.4个针叶树种枝叶气味对小白鼠自发行为影响的比较分析, 林业科学, 49(5): 188-193. (Wang Y Y, Wang C, Qie G F, et al. 2013. Comparative analysis on effects of VOCs from branches and leaves of four conifer species on locomotor activity of mice. Scientia Silvae Sinicae, 49(5): 188-193[in Chinese]).(  1) 1)
|
| [11] |
王峥涛, 梁光义. 2009. 中药化学. 上海: 上海科学技术出版社, 200-222. (Wang Z T, Liang G Y. 2009. Chemistry of Chinese material medica. Shanghai: Shanghai Science and Technology Publishing House, 200-222.[in Chinese])(  1) 1)
|
| [12] |
杨桦, 杨伟, 杨茂发, 等. 2011. 法国冬青和光皮桦挥发物日节律及云斑天牛的触角电位反应. 应用生态学报, 22(2): 357 -363. (Yang H, Yang W, Yang M F, et al. 2011. Diurnal rhythm of Viburnum awabuki and Betula luminifera volatiles and electroantennogram response of Batocera horsfieldi. Chinese Journal of Applied Ecology, 22(2): 357 -363[in Chinese]).(  1) 1)
|
| [13] |
张风娟, 李继泉, 徐兴友, 等. 2007. 皂荚和五角枫挥发性物质组成及其对空气微生物的抑制作用. 园艺学报, 34(4): 973-978. (Zhang F J, Li J Q, Xu X Y, et al. 2007. The volatiles of two greening tree species and the antimicrobial activity. Acta Horticulturae Sinica, 34(4): 973-978[in Chinese]).(  1) 1)
|
| [14] |
Ben W, Salvador G, Toby B, et al. 2010. Between plant and diurnal variation in quantities and ratios of volatile compounds emitted by Vicia faba plants. Phytochemistry, 71(1): 81-89.( 1) 1)
|
| [15] |
Jo H J, Fujii E, Cho T D. 2010. An experimental study of physiological and psychological effects of pine scent. Journal of the Korea Institute of Landscape Architecture, 38(4): 1-10.( 2) 2)
|
| [16] |
Julian C V, Ric de vos C H, Harrie A V, et al. 2003. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry, 62: 997-1008.( 1) 1)
|
| [17] |
Kivimäenpää M, Riikonen J, Ahonen V, et al. 2013. Sensitivity of Norway spruce physiology and terpenoid emission dynamics to elevated ozone and elevated temperature under open-field exposure. Environmental and Experimental Botany, 90: 32-42.( 1) 1)
|
| [18] |
Li J G, Jin Y J, Luo Y Q, et al. 2003. Leaf volatiles from host tree Acer negundo: diurnal rhythm and behavior responses of Anoplophora glabripennis to volatiles in field. Acta Botanica Sinica, 45: 177-182.( 1) 1)
|
| [19] |
Staudt M, Bertin N, Hansen U, et al. 1997. Seasonal and diurnal patterns of monoterpene emissions from Pinus pinea (L.) under field conditions. Atmos Environ, 31(S): 145-156.( 1) 1)
|
| [20] |
Ziru L, Ellen A R, Thomos D S. 2011. Effect of temperature on postillumination isoprene emission in oak and poplar. Plant Physiology, 155: 1037-1046.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

