文章信息
- 余贤美, 侯长明, 王洁, 王海荣, 安淼, 艾呈祥
- Yu Xianmei, Hou Changming, Wang Jie, Wang Hairong, An Miao, Ai Chengxiang
- 山东牛心柿炭疽病菌的分离鉴定及致病性
- Identification of Pathogen Causing Beef Heart Persimmon Anthracnose in Shandong and Its Pathogenicity
- 林业科学, 2015, 51(4): 126-133
- Scientia Silvae Sinicae, 2015, 51(4): 126-133.
- DOI: 10.11707/j.1001-7488.20150416
-
文章历史
- 收稿日期:2013-12-16
- 修回日期:2014-07-02
-
作者相关文章
2. 山东省农业科学院 济南 250100
2. Shandong Academy of Agricultural Sciences Ji'nan 250100
柿(Diospyros kaki)原产于我国秦岭山区,是我国特有的一种果树。 柿子美味多汁,富含胡萝卜素、维生素C、果糖和钙、磷、铁等矿物质,可减轻喉痛、口舌生疮、肺热咳嗽病症,对高血压等有良好的缓解作用,柿子已在中国、日本、韩国、新西兰、意大利、巴基斯坦、法国等诸多国家广泛栽培种植(Kitagawa et al.,1984; Yonemori et al.,2008; Du et al.,2010)。目前柿子已有 2 000多个品种,其中960多个品种为栽培种(Zhang,2008)。牛心柿因其果实形似牛心而得名,其个大味甜、肉细汁多,脱涩后脆酥利口,烘吃汁多甘甜;晒制的牛心柿饼,甜度大、纤维少、质地软、香甜可口,具有较高的鲜食及加工价值。柿树炭疽病是为害柿子的一种重要病害,也是一种毁灭性的病害,该病菌能够侵染嫩梢,叶片(主要为害叶柄和叶脉)和果实,导致嫩梢枯萎、落叶、落果和果实腐烂等,严重的可导致植株死亡,常造成重大经济损失,已成为阻碍柿产业发展的重大病害(刘开启等,1988;Lee et al.,2004;张敬泽等,2005;Zhang,2008)。高温多雨是该病害发生和流行的决定因素,生产上主要通过加强栽培管理措施、提高树势,铲除病毒来源、清除病苗,同时结合药剂保护等措施进行防治(刘开启等,1988)。
炭疽菌是一类重要的植物病原真菌,能够侵染多种植物引起炭疽病,常造成严重的经济损失,但在分类学上很混乱,急需修正(Cannon et al.,2008; Hyde et al.,2009)。形态学分类对于炭疽菌的鉴定尚有不足,分子生物学技术尤其是系统进化树分析已逐渐成为炭疽菌种类界定的有用工具(Shenoy et al.,2007; Than et al.,2008; Cai et al.,2009)。柿树炭疽病菌曾被鉴定为柿盘长孢菌(Gloeosporium kaki Hori)(Hori,1910a;1910b; Ito,1911)、胶孢炭疽菌(Colletotrichum gloeosporioides)(张敬泽等,2005),基于分子生物学和形态学特征,Weir等(2010)将柿树炭疽病菌确定为为柿树炭疽菌(Colletotrichum horii)。
本试验采自感病牛心柿(D.kaki cv.Niuxinshi)果实、叶片和嫩梢的病原物并进行鉴定,分析其致病性,初步了解该病原菌的侵染谱,为柿树病害病原诊断和病害防治提供理论依据和技术支持。
1 材料与方法 1.1 试剂真菌DNA提取试剂盒(E.Z.N.A. Fungal DNA Kit)购自OMEGA公司,TaKaRa TaqTM DNA Polymerase,GoldviewTM Nucleic Acid Stain,TaKaRa Agarose Gel DNA Purification Kit,pGEM-T easy clone vector等试剂购自宝生物工程(大连)有限公司。
1.2 病原物分离和回接试验感病样品采自山东临朐县朱家坡、沂水诸葛镇及山东省果树研究所万吉山试验基地柿子园。采用组织分离法和单孢分离法(方仲达,1998)从感病果实、叶片和嫩梢的病健交界处分离病原物,并按照柯赫氏法则进行回接试验。
1.3 病原菌形态特征观察单孢分离后接种于PDA培养基上于25 ℃、光周期12 h的条件下进行培养。培养2周后观察记录菌落形态,从培养7~10天的平板中挑取分生孢子进行镜检并记录孢子的形状、大小。
1.4 rDNA-ITS序列分析和系统进化树分析 1.4.1 DNA提取和PCR扩增采用真菌DNA提取试剂盒从纯培养物菌丝中提取总DNA,操作严格按照说明书进行。提取的DNA溶液保存于-20 ℃备用。
以真菌转录间隔区通用引物ITS6(Gardes et al.,1996)和ITS4(White et al.,1990)为引物,以菌丝总DNA为模板,对rDNA-ITS区进行扩增。PCR扩增反应在ABI VeritiTM 96孔热循环仪[Applied Biosystems(USA),Co.,Ltd]上进行。引物ITS6(5′-GAA GGT GAA GTC GTA ACA AGG-3′)和ITS4(5′-TCC TCC GCT TAT TGA TAT GC-3′)由北京赛百盛基因技术有限公司合成,配制成100 μmol·L -1的母液备用。
25 μL反应体系中含有1 μL DNA模板,0.2 μL(5 U·μL-1)Taq 酶,2.5 μL 10 × PCR buffer,2 μL dNTPs(各2.5 mmol·L-1),1 μL ITS6(10 μmol·L-1),1 μL ITS4(10 μmol·L-1)和17.3 μL灭菌水。PCR反应条件如下:94 ℃ 5 min;94 ℃ 1 min,50 ℃ 40 s,72 ℃ 1 min,30循环;72 ℃延伸10 min。
取2 μL PCR产物经1.2%琼脂糖凝胶电泳检测,回收PCR产物,将产物与pGEM-T easy clone vector连接,并转化大肠埃希菌(Escherichia coli)DH5α感受态细胞,挑取阳性克隆,委托宝生物工程(大连)有限公司进行测序。
1.4.2 rDNA-ITS序列分析和系统进化树构建测序所得序列通过Blastn数据库进行同源性搜索,采用DNAssist2.2软件进行序列比对,利用Mega4.0软件构建系统进化树,并对其基因序列的区域定位进行分析。
1.5 致病性分析通过离体接种试验测定病原菌对不同品种柿树以及桃(Prunus persica ‘Chanhong’)、苹果(Malus domestica ‘Royal Gala’)、梨(Pyrus communis ‘Yellow Gold’)的致病性。将牛心柿分离物和次郎甜柿分离物分别接种在PDA平板上,一部分于25 ℃培养,长满平板后,打成直径0.5 cm的菌饼备用,另一部分于25 ℃黑暗培养,产孢后用无菌水将孢子洗脱,配制成105个·mL-1的孢子悬浮液。选择新鲜、健康的柿、桃、苹果、梨的叶片和果实,用1.5%次氯酸钠溶液浸泡消毒3~5 min,无菌水冲洗3次,置于超净工作台上吹干。用菌饼对处理后的叶片和果实进行伤口和非伤口接种,同时用20 μL孢子悬浮液进行喷雾接种。每处理设10个叶片或果实,以喷20 μL无菌水接种为对照,重复3次。置于垫有无菌吸水纸的盒子里保湿,于25 ℃黑暗培养,观察发病情况,接种第7天测定病斑面积(S),分别记作-,+++++(S>4 cm2)、++++(3 cm2<S≤4 cm2)、+++(2 cm2<S≤3 cm2)、++(1 cm2<S≤2 cm2)和+(0<S≤1 cm2),不发病记为-(邓维萍等,2013)。从发病部位重新分离病原菌,观察菌落形态及分生孢子形态特征,与原接种菌株进行比较。
2 结果与分析 2.1 病害症状自然条件下,柿树炭疽菌能够侵染嫩梢,叶柄、叶脉和果实,在牛心柿嫩梢、叶片和果实上均发现典型的炭疽病病斑。嫩梢上的病斑往往绕茎1周,病斑暗灰色或深褐色,在病健交界处有明显的分界线。在病斑中心往往能产生橙色的分生孢子堆。在适宜的条件下,相邻病斑连成一片,使病斑扩大直至整个嫩梢(图 1A)。
 |
图 1 牛心柿炭疽病症状 Fig. 1 The symptoms of anthracnose disease of beef heart persimmon A:嫩梢 受害症状Symptoms on twig;B:叶片 受害症状Symptoms on leaf;C:果实 受害症状Symptoms on fruit. |
当叶片受侵染时,发病初期叶柄和叶脉上出现圆形小病斑,随着病情的发展形成较大的紫色或深褐色的圆形或不规则圆形凹陷病斑,直径8~10 mm,但叶柄和叶脉上的病斑不会连成一片(图 1B)。
果实在发病初期出现针头大紫色至深紫色的圆形或椭圆形斑点,直径3~8 mm。随着病情发展,果实上的病斑进一步扩大,直径可超过20 mm。病斑中心最后变成灰白色,边缘黑色或黑紫色,在病斑中心出现大量的橙色分生孢子堆,发展到后期病斑开裂(图 1C)。
2.2 形态学特征从感病样品中获得9个病原物纯培养物,它们的菌落特征完全一致。通过柯赫氏法则验证,重新分离获得了与原分离物一致的分离物。
在PDA培养基平板上,菌落呈绒毛絮状,灰色到灰黑色,边缘规则,在同心轮纹上通常可见大量橙色的分生孢子团(图 2A),背面灰黑色到深褐色,随菌龄增长菌落颜色变深,并产生同心轮纹(图 2B)。25 ℃条件下,菌落生长速率为每天12.7 mm±0.6 mm。分生孢子形成在橙色的分生孢子堆中。分生孢子柱形或长椭圆形,表面光滑,两端钝圆,无色,单孢,大小为 [17.1-(20.3)-22.5]μm× [4.6-(5.5)-5.9]μm(n=30)(图 2C)。
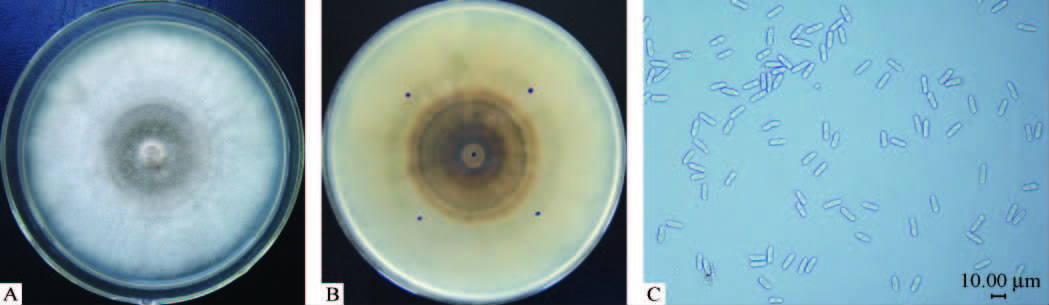 |
图 2 山东牛心柿分离物形态特征
Fig. 2 Morphological characteristics of beef heat persimmon isolate
A:PDA平板上的菌落形态;B:PDA平板反面的菌落形态;C:分生孢子。 A: View of colony; B: Reverse view of colony; C: Conidia. |
上述病原菌在自然寄主和培养条件下的形态特征与张敬泽等(2005)、Xie等(2010)描述的柿树炭疽菌的形态基本一致。据此,将样品病害确定为炭疽病,病原确定为炭疽菌(Colletotrichum sp.)。
2.3 rDNA-ITS基因序列特征经PCR扩增,从9个病原物纯培养物中均获得了598 bp的rDNA-ITS基因片段,而且9个片段的核苷酸序列完全一致。Blastn搜索显示,该片段与炭疽菌的同源性高达98%以上。采用DNAssist2.2软件进行序列比对,结果显示,该片段与次郎甜杮分离物(JQ957543)(余贤美等,2014)、浙江无核柿(D.kaki cv. Wuheshi)分离物(AY787483)同源性为100%,与柿炭疽菌新西兰分离物(GQ329690)同源性为99.8%(图 3)。
 |
图 3 山东牛心柿分离物与次郎甜杮、浙江无核柿和新西兰分离物的rDNA-ITS核甘酸序列比对 Fig. 3 The nucleotide alignment of rDNA-ITS genes of beef heart persimmon anthracnose pathogen with that of anthracnose pathogen on Jiro persimmon, D. kaki cv. Wuheshi in Zhejiang and New Zealand isolate JQ957543:次郎甜杮Jiro persimmon; AY787483:浙江无核杮D.kaki cv.Wuheshi in Zhejiang; GQ329690:新西兰分离物New zealand isolate;Niuxinshi:牛心杮Beef heart persimmon. |
对rDNA-ITS基因序列的区域定位进行分析,结果显示,牛心柿分离物和次郎甜杮分离物(JQ957543)(余贤美等,2014)一样,它们的rDNA-ITS基因片段中,1~54 bp为18S rRNA,55~226 bp为ITS1区,227~383 bp为5.8S rRNA,384~540 bp为 ITS2区,541~598为28S rRNA;而浙江无核柿分离物(AY787483和AY791890)的rDNA-ITS基因片段中,1~51 bp为18S rRNA,52~223 bp为ITS1区,224~380 bp为5.8S rRNA,381~537 bp为 ITS2区,538~601为28S rRNA(图 4)。它们之间的各个区域片段大小及定位是一致的,18S rRNA和28S rRNA片段大小略有不同,这是由扩增时所用引物的差异引起的。
 |
图 4 牛心柿分离物rDNA-ITS基因序列区域定位 Fig. 4 The regional assignment of rDNA-ITS sequence of anthracnose pathogen from beef heart persimmon |
系统进化树(图 5)显示,山东牛心柿分离物与次郎甜柿分离物(JQ957543)、浙江无核柿(AY787483和AY791890)和新西兰分离物(GQ329687,GQ329688和GQ329690)处在进化树的同一分支上(图 5),说明山东牛心柿分离物属于柿树炭疽菌(Colletotrichum horii),并将山东牛心柿炭疽病病原rDNA-ITS序列提交到GenBank数据库(基因登录号:KF010811)。
 |
图 5 基于rDNA-ITS基因序列构建的牛心柿分离物系统进化树 Fig. 5 Phylogenetic tree of anthracnose pathogen from beef heart persimmon based on rDNA-ITS sequences |
牛心柿分离物和次郎甜杮分离物对柿、桃、苹果和梨的致病性如表 1所示:2种分离物对桃、苹果和梨均不致病,对杮树有致病性且对不同柿树品种的致病性一致。柿树叶片上的病斑比果实上的病斑小,而且不同的接种方式,所产生的病斑差异性较大:菌饼-非伤口接种在叶片上不产生病斑,在果实上产生的病斑也明显小于菌饼-非伤口接种;孢子悬浮液接种的效果介于伤口接种和非伤口接种之间(表 1)。取发病组织重新分离病原,均得到与原接种菌性状一致的培养物。
|
|
柿树炭疽病作为一种毁灭性病害,对柿树产业的发展造成了严重威胁。对病害的诊断及病原菌的鉴定是进行病害防治的前提。柿树炭疽病病原曾被鉴定为柿盘长孢菌(Gloeosporium kaki Hori)(Hori,1910a;1910b; Ito,1911)和柿树炭疽病菌(Colletotrichum kaki)(Maffei,1921)。Von Arx(1957; 1970)认为Hori和Maffei发现的病原菌是一致的,它们与胶胞炭疽菌(Colletotrichum gloeosporioides)为同物异名。根据植物命名法准则,柿树炭疽病菌正式统一为柿盘长孢菌,并在一段时间内得到普遍应用(Hu et al.,2006),有些文献也将柿树炭疽病菌鉴定为胶胞炭疽菌(张敬泽等,2005)。Weir等(2010)研究了来自中国、日本和新西兰的标本,认为Hori和Maffei发现的病原菌是不同的,基于分子和形态学证据,将柿树炭疽病菌命名为柿树炭疽菌(Colletotrichum horii),成为C. Gloeosporioides sensu lato复合种的成员之一。因此,浙江无核柿树炭疽病菌也被重新归类到柿树炭疽菌(C.horii)(Xie et al.,2010),此名自此为柿树炭疽病的病原菌被正式确定为柿树炭疽菌(Colletotrichum horii)(Xie et al.,2010; Kwon et al.,2013)。
本研究中,山东牛心柿嫩梢、叶片和果实上出现的病斑与张敬泽等(2005)描述的基本一致,据此,将所采样品的病害确定为柿树炭疽病。基于PDA培养基上的菌落形态特征和分生孢子形态特征,将山东牛心柿炭疽病的病原确定为Colletotrichum sp.。序列比对结果显示,从3个柿子园感病牛心柿材料中分离获得的牛心柿分离物与次郎甜柿分离物的rDNA-ITS基因序列完全一致,并且与浙江无核柿(AY787483)同源性为100%,说明柿树炭疽病菌专性较强,因地理距离变异率几乎为零。系统进化树分析结果显示,牛心柿分离物与次郎甜柿分离物(JQ957543)、浙江无核柿(AY787483和AY791890)以及新西兰分离物(GQ329687,GQ329688和GQ329690)亲缘关系最近,处于同一分支,因此,将山东牛心柿炭疽菌鉴定为柿树炭疽菌。
通过离体接种试验,笔者测定了牛心柿分离物和次郎甜柿分离物对柿、桃、苹果和梨的致病性。由于枝条上的病斑面积不便计算,因此,本研究仅在果实和叶片上进行离体接种。结果显示,两个分离物对桃、苹果和均不致病,而对柿子的致病性完全一致,说明柿炭疽病菌有一定的专性寄生性;rDNA-ITS序列分析也说明9个分离物基因序列完全一致,这也从侧面支持了离体接种试验的结果。离体的菌饼-伤口接种在叶片上产生的病斑较小,这与田间叶片上症状较轻的现象一致。下一步笔者将对更多柿园的样品进行分析,以便对柿树炭疽病进行系统有序的研究,为该病的防治提供理论依据和技术支持。
| [1] |
邓维萍, 杨敏, 杜飞, 等. 2013. 云南葡萄产区葡萄炭疽病病原鉴定及致病力分析. 植物保护学报, 40(1):61-67. (Deng W L, Yang M, Du F, et al. 2013. Identification of the pathogen causing grape anthracnose in Yunnan and its pathogenicity. Acta Phytophylacica Sinica, 40(1):61-67[in Chinese]).(  1) 1)
|
| [2] |
方仲达. 1998. 植病研究方法.3版. 北京: 中国农业出版社. (Fang Z D. 1998. Plant disease research methods. 3rd ed. Beijing: China Agriculture Press.[in Chinese])(  1) 1)
|
| [3] |
刘开启, 牟惠芳, 刘凤英. 1988. 柿炭疽病的研究. 山东农业大学学报, 19: 69-71. (Liu K Q, Mu H F, Liu F Y. 1988. Studies on persimmon anthracnose (Gloeosporium Kaki Hori). Journal of Shandong Agricultural University, 19: 69-71[in Chinese]).(  2) 2)
|
| [4] |
余贤美, 侯长明, 王洁, 等. 2014. 次郞甜柿炭疽病菌的分离鉴定及其rDNA-ITS 序列分析. 经济林研究, 32(1): 45-50. (Yu X M, Hou C M, Wang J, et al. 2014. Isolation, identification and rDNA-ITS sequence analysis of pathogen of Jiro persimmon anthracnose. Nonwood Forest Research, 32(1): 45-50[in Chinese]).(  2) 2)
|
| [5] |
张敬泽, 徐同, 何黎平. 2005. 浙江无核柿炭疽病病菌鉴定及附着胞形成过程中的核相变化. 菌物学报, 24(1): 116-122. (Zhang J Z, Xu T, He L P. 2005. Anthracnose pathogen on Diospyros kaki cv. Wuheshi and its nuclear behavior in process of appressorium formation. Mycosystema, 24(1): 116-122[in Chinese]).(  5) 5)
|
| [6] |
Arx J A Von. 1957. Die Ariten der Gattung Colletotrichum Cda. Phytopathologische Zeitschrift, 29: 413-468.( 1) 1)
|
| [7] |
Arx J A Von. 1970. A revision of fungi classified as Gloeosporium. Bibliotheca Mycologica, 24: 1-203.( 1) 1)
|
| [8] |
Cai L, Hyde K D, Taylor P W J, et al. 2009. A polyphasic approach for studying Colletotrichum. Fungal Diversity, 39:183-204.( 1) 1)
|
| [9] |
Cannon P F, Buddie A G, Bridge P D. 2008. The typification of Colletotrichum gloeosporioides. Mycotaxon, 104: 189-204.( 1) 1)
|
| [10] |
Du Sh N, Bai G Sh, Zhang Sh J, et al. 2010. Identification and control of persimmon anthracnose. Plant Disease and Pests, 1(1): 40-42.( 1) 1)
|
| [11] |
Gardes M, Bruns T D. 1996. ITS-RFLP matching for identification of fungi// Clapp J P. Species diagnostics protocol. Totowa, New Jersey: Humana Press.( 1) 1)
|
| [12] |
Hori S. 1910a. Kaki no Shinbyogai Tansobyo. Engei no Tomo, 6(1): 58-61.( 2) 2)
|
| [13] |
Hori S. 1910b. Kaki no Shinbyogai Tansobyo. Engei no Tomo, 6(2): 21-24.( 2) 2)
|
| [14] |
Hu S C, Li M, Wang J G, et al. 2006. IFB-Lactam-1, a natural compound with anti-Gloeosporium kaki Hori activity. Acta Crystallogr. Section E: Struct Rep Online, 62: 5777-5778.( 1) 1)
|
| [15] |
Hyde K D, Cai L, Cannon P F, et al. 2009. Colletotrichum-names in current use. Fungal Diversity, 39: 147-182.( 1) 1)
|
| [16] |
Ito S. 1911. Gloeosporiose of the Japanese persimmon. Botanic Magazine (Tokyo), 25: 197-202.( 2) 2)
|
| [17] |
Kitagawa H, Glucina P. 1984. Persimmon culture in New Zealand. DSIR Information Series 159. Science Information Publishing Centre: Wellington, New Zealand.( 1) 1)
|
| [18] |
Kwon J H, Kim J, Chio O, et al. 2013. Anthracnose caused by Colletotrichum horii on sweet persimmon in Korea: Dissemination of conidia and disease development. Journal of Phytopathology,161(7-8):497-502.( 1) 1)
|
| [19] |
Lee J H, Han K S, Lee S C, et al. 2004. Early detection of epiphytic anthracnose inoculum on phyllosphere of Disopyros kaki var. domestica. Plant Pathology Journal, 20: 247- 251.( 1) 1)
|
| [20] |
Maffei L. 1921. Una malattia delle foglie del "Kaki" dovuta al Colletotrichum kaki m. sp. Rivista di Patologia Vegetale, 11: 116-117.( 1) 1)
|
| [21] |
Shenoy B D, Jeewon R, Lam W H, et al. 2007. Morpho-molecular characterization and epitypification of Colletotrichum capsici (Glomerallaceae, Sordariomycetes), the causative agent of anthracnose in chilli. Fungal Diversity, 27: 197-211.( 1) 1)
|
| [22] |
Than P P, Prihastuti H, Phoulivong S, et al. 2008. Review: Chilli anthracnose disease caused by Colletotrichum species. Journal of Zhejiang University, 9:764-778.( 1) 1)
|
| [23] |
Weir B S, Johnston P R. 2010. Characterisation and neotypification of Gloeosporium kaki Hori as Colletotrichum horii nom. nov. Mycotaxon, 111: 209-219.( 2) 2)
|
| [24] |
White T J, Bruns T, Lee S. 1990.Analysis of phytogenetic relationships by amplification and direct sequencing of ribosomal RNA genes// Innis M A, Gelfand D H, Sninsky J J, et al. PCR protocols: A guide to methods and applications. New York: Academic Press.( 1) 1)
|
| [25] |
Xie L, Zhang J Z, Cai L, et al. 2010. Biology of Colletotrichum horii, the causal agent of persimmon anthracnose. Mycology, 1(4): 242-253.( 2) 2)
|
| [26] |
Yonemori K, Honsho S, Kanzaki S, et al. 2008. Sequence analyses of the ITS regions and the matK gene for determining phylogenetic relationships of Diospyros kaki (persimmon) with other wild Diospyros (Ebenaceae) species. Tree Genetics and Genomics, 4: 149-158.( 1) 1)
|
| [27] |
Zhang J Z. 2008. Anthracnose of persimmon caused by Colletotrichum gloeosporioides in China. Asian and Australasian Journal of Plant Science and Biotechnology, 2(2): 50-54.( 2) 2)
|
 2015, Vol. 51
2015, Vol. 51

