文章信息
- 欧阳芳群, 付国赞, 王军辉, 马建伟, 安三平, 王美琴, 李悦
- Yang Fangqun, Fu Guozan, Wang Junhui, Ma Jianwei, An Sanping, Wang Meiqin, Li Yue
- 欧洲云杉扦插生根进程中内源激素和多酚类物质变化
- Qualitative Analysis of Endogenesis Hormone and Polyphenol During Rooting of Cuttings in Norway Spruce (Picea abies)
- 林业科学, 2015, 51(3): 155-162
- Scientia Silvae Sinicae, 2015, 51(3): 155-162.
- DOI: 10.11707/j.1001-7488.20150320
-
文章历史
- 收稿日期:2014-06-10
- 修回日期:2014-08-16
-
作者相关文章
2. 北京林业大学 林木花卉遗传育种教育部重点实验室 北京 100083;
3. 河南林业职业学院 洛阳 471002;
4. 甘肃省小陇山林业科学研究所 天水 741002
2. The Key Laboratory for Genetics and Breeding in Forest Tree and Ornamental Plants, Beijing Foresty University Beijing 100083;
3. Henan Forestry Vocation College Luoyang 471002;
4.Hiadongshan Mountain Research Institute of Forestry Tianshui 741020
欧洲云杉(Picea abies)又名挪威云杉,速生且适应性强,木材品质好,可做纸浆材和建筑材,是重要的工业用材树种(傅紫芰,1998; 裴会明等,1998)。世界各地广为引种栽培,在美国东北部、加拿大东部和日本北海道引种的欧洲云杉生长速度比当地云杉快1倍。我国熊岳树木园引进栽培欧洲云杉已有80多年历史,青岛、内蒙古、黄山、庐山、北京、宜昌、天水等地均有引种栽培,其生长速度明显优于各种乡土云杉,开花结实正常且抗寒抗病虫害,表现出较强的适应性(傅紫芰等,1998)。云杉属树种早期生长缓慢,实生种子园和无性系种子园良种进程缓慢,严重影响经济效益和遗传改良进程(季孔庶等,1996)。随着无性繁殖技术的快速发展,转而推行无性系选择和利用的遗传改良方向。同样基因起源的云杉扦插苗比实生苗表现优良(Gemmel et al.,1991),无性系林增益比实生苗林高(Roulund et al.,1985),且采用扦插苗缩短育种周期25~30年。
欧洲云杉属愈伤组织生根型树种,母株生理条件、采穗部位、穗条类型、穗条长度和外界环境等都是影响扦插成活率的因子。随着母株年龄增加穗条扦插生根能力下降,母株年龄23年生根率为0,而5年生穗条生根率高达89.6%(靳景春等,2009);不同类型插穗的生根能力不同(胡勐鸿等,2014; 靳景春等,2009; 马建伟等,2011);外施激素IBA和ABT-1能提高9年生欧洲云杉扦插生根率(董健等,2001)。但对欧洲云杉扦插生根过程中,插穗内源激素和多酚类物质对插穗生根影响的研究未见报道。前人研究结果表明,内源激素物质对多个树种扦插生根有明显促进效应,多酚类物质是多个树种插穗生根的抑制物质(黄卓烈等,1994; 王军辉等,2006),本文通过分析5年生欧洲云杉嫩枝扦插后不同时间点插穗生根性状,以及插穗内源激素、多酚类物质含量的动态变化,探讨插穗内不同内源激素和多酚类物质对欧洲云杉嫩枝扦插生根影响,旨在为欧洲云杉扦插繁殖机理等方面的研究提供科学理论依据。
1 材料和方法 1.1 插穗来源及插穗处理试验所用插穗由“国家林木种质资源平台--云杉种质资源保存库”提供,具体位置是甘肃省小陇山林业科学研究所欧洲云杉采穗圃(105°54′37″ E、34°28′50″ N),海拔1 160 m,年降雨量600~800 mm,年均气温10.7 ℃;采穗母株生长的土壤氮62.546 mg ·kg-1,磷 32.322 mg ·kg-1,钾186.89 mg ·kg-1,土壤有机质0.64%,pH值为7.96。采穗母株为5年生,单株一级侧枝平均36.2条。从母株中部剪取一级侧枝1年生半木质化嫩枝。用利刃刀将基部削成锲形,用100 mg ·kg-1的IBA中浸泡1 h,共处理500株,扦插深度3.5 cm左右,插后轻按,随插随淋水,使插穗与插壤紧密结合。
1.2 扦插网袋容器的制作及激素来源该试验所用的网袋基质是泥炭和炭化稻壳按体积比1 :1搅拌混匀后,再倒入灌装机中生产成直径40~50 mm的基质肠。然后用3‰的高锰酸钾溶液喷湿消毒,再用全自动切割机将其切成约10 cm长的短肠段,摆放到58 cm×23 cm的育苗托盘中放置于苗床待用。外施激素是商用吲哚丁酸IBA。
1.3 插床及生根条件扦插工作于2009年6月下旬,在甘肃省天水市小陇山林业科学研究所一露地进行。用钢丝网做成的钢架苗床,将苗床摆放成直径15 m,高60 cm的近圆形架空插床。插床上放置已摆放好短肠段的育苗托盘。摆放整齐后全面喷洒500倍多菌灵药液进行灭菌,用量为1 000 mL ·m-2。采用悬臂转动式自动喷雾装置保持湿度。根外施肥用0.2%尿素和0.3%磷酸二氢钾混合液,用量0.5 kg ·m-2,在傍晚停止喷雾后进行。
1.4 生根性状与调查统计分析扦插后30,40,50,55,60,65,70,75和80天分别取观测区20株插穗调查愈伤组织形成和根系发育情况,包括生根率、生根数量和单根长度,共调查9次。评价生根性状采用根系效果指数,根系效果指数=平均根长 × 根系数量/扦插穗条总数(朱湘渝等,1991)。
0,37,45,67和72天取插穗的针叶和45,67和72天插穗基部或基部愈伤组织液氮速冻后-80 ℃冰箱保存,用于分析内源激素含量和多酚类物质含量。每个时间点采插条9株,每3株混样作为1重复,合计3个重复。
植物内源激素分析方法:精密称取新鲜植物样品1.0 g,剪细,加入少量石英砂于研钵中迅速研细后用20 mL冰甲醇(40 ℃)将样品传至具塞三角瓶中,摇匀后放在超声波中振荡1 h(不断加冰块,保持超声波水温不超过40 ℃,取出后放入40 ℃冰箱过夜过滤,残渣中再加10 mL冰甲醇,超声0.5 h后,过滤,合并滤液,减压、浓缩至1 ml后待机分析。色谱条件:选用Waters 244型 HPLC;色谱柱为Diamosil C18(0.4 cm × 25 cm)。ZT的流动相是15% CH3和85% H2O(用H3PO4调pH至3.5),流速为0.8 mL ·min-1,UV 254 nm检测,外标法定量;而GA3和IAA流动相是30% CH3OH,15% CH3OH和55% H2O(用H3PO4调pH至3.5),流速为0.8mL ·min-1,UV 254 nm检测,外标法定量。
多酚类物质分析方法:样品处理:精密称取植物样品2 g(精确至0.001 g),剪细,加入石英砂少许放入研钵中研细,转移至50 mL三角瓶中,加25 mL 80% CH3OH后加塞摇匀,水浴上回流2 h后过滤,滤液经0.45 um 滤膜过滤,清液待分析。色谱条件:选用Waters 244型 HPLC,色谱柱为Diamosil C18(0.4 cm×25 cm),流动相为 40% CH3OH,5% CH3CN和55% H2O(用H3PO4调pH至3.5),流速为0.8 mL ·min-1,检测器为 UV 254 nm,外标法定量。
采用Sigmaplot 10.0软件作图。
2 结果与分析 2.1 欧洲云杉嫩枝扦插生根特点及生根进程欧洲云杉嫩枝插穗生根类型有3种(图 1):愈伤组织生根,占95%以上;皮部生根;愈伤组织和皮部双向生根。
 |
图 1 欧洲云杉嫩枝插穗3种生根类型 Fig.1 Three rooting types of shoot cuttings in Norway spruce |
欧洲云杉嫩枝插穗经100 mg ·kg-1的IBA浸泡1 h处理后插入网袋基质中,30天后已有一半以上(55%)的穗条形成愈伤组织;40天后,有85%的扦插苗形成愈伤组织;经过50天,愈伤组织全部形成(100%),并且有10%的插穗已经生根。50天前是愈伤组织形成阶段,40天以后开始有部分分化生根。65天后生根率达70%,75,80天调查结果一致,生根率达90%,其中5%插穗腐烂,5%插穗基部愈伤组织膨大,但未分化成根。55~70天是插穗基部愈伤组织膨大活跃期。扦插后50~55天,60~65天和70~75天为愈伤组织分化生根的3个高峰期,生根率为90%(图 2)。超过此时期尚未分化生根的穗条容易导致愈伤组织老化不能生根或者腐烂。65~75天平均生根数急剧增加,75天的平均生根数为10.7,比65天增加76.64%。75天以后生根数量不变,不过根系仍然在生长,且扦插后根系效果指数持续增高,由0增加至0.7,80天时有所降低(图 3)。
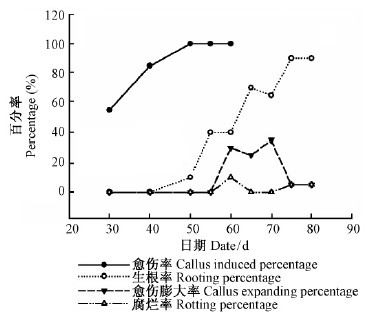 |
图 2 欧洲云杉扦插生根进程中愈伤率、生根率、愈伤膨大率和腐烂率 Fig.2 The change of callus percentage,rooting percentage, inflating callus percentage and rotting percentage |
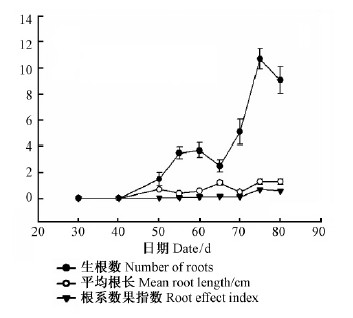 |
图 3 欧洲云杉扦插生根进程中生根数、平均根长和根系效果指数的变化 Fig.3 The change of number of roots,mean root length and root effect index during rooting process of shoot cuttings in Norway spruce |
插穗生根过程其针叶內源激素含量的动态变化如图 4所示。多种内源激素的消长促进不定根的形成。内源IAA含量的变化规律整体上是先下降后上升再下降。即扦插后至45天愈伤组织形成阶段呈下降趋势,从扦插前的116.7 ng ·100 g-1先降至83.1 ng ·100 g-1(37天),再降至61.7 ng ·100 g-1(45天),共降低了47.13%。45天到67天属愈伤组织膨大分化期,IAA含量逐渐上升,67天的时候IAA平均含量为79.8 ng ·100 g-1,促进愈伤组织分化生根。67天后愈伤组织膨大分化已基本接近完成,IAA含量急速下降,降低了32.83%。
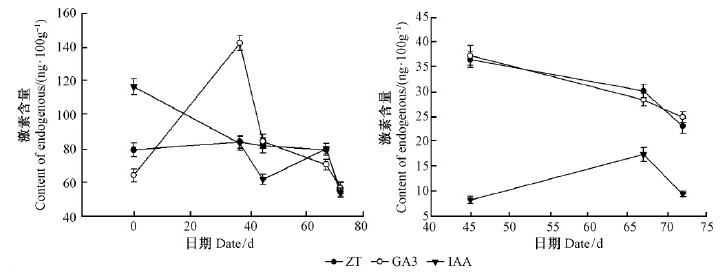 |
图 4 欧洲云杉嫩枝插穗生根进程中内源激素变化 Fig.4 The change of endogenous hormones during rooting process of shoot cuttings in Norway spruce (A.针叶Needles;;B.插穗基部韧皮部Phloem of cuttings base)。下同。The same below. |
针叶内源GA3含量动态变化规律则是先上升后急速下降、而后缓慢下降。即扦插后37天愈伤组织形成前中阶段GA3含量迅速上升,从64.1 ng ·100 g-1增加到142.5 ng ·100 g-1,增幅55.02%;37天以后愈伤组织膨大分化直至生根阶段GA3含量先急速下降,下降率为40.91%;而后缓慢降低,从45~67天和67~72天分别降低了16.27%和19.57%。针叶内源玉米素ZT的变化比较平稳,扦插后至67天,ZT的含量约为80 ng ·100 g-1,67~72天以29.97%速率降低,降低为55.6 ng ·100 g-1。
整体上看,基部韧皮部的内源激素变化趋势(图 4B)同针叶内,只是各种内源激素含量远远低于针叶内,尤其是67天测得的IAA含量,针叶内IAA含量(79.8 ng ·100 g-1)是基部韧皮部内(17.4 ng ·100 g-1)是4.58~7.52倍;针叶内玉米素ZT含量(79.4 ng ·100 g-1)是基部韧皮部内(30.2 ng ·100 g-1)的2.24~2.41倍;针叶内GA3含量(70.5 ng ·100 g-1)是基部韧皮部内(28.4 ng ·100 g-1)的2.26~2.48倍。
从针叶内源激素间的比值来看(图 4A),扦插前至扦插后37天,GA3/IAA迅速上升至1.71,比扦插前高出67.97%;ZT/IAA上升幅度偏小,其值为1.01,比扦插前高出32.78%。37~45天GA3/IAA转为下降趋势,而ZT/IAA继续保持上升趋势。45天之后两者的变化趋势一致,先降低后升高。ZT/GA3和GA3/IAA的变化趋势正好相反(图 5A)。扦插基部韧皮部内源激素间的比值45~72天的变化趋势和针叶内基部相同,只是67天测得的针叶内ZT/GA3的值(1.13)是高于GA3/IAA(0.88)和ZT/IAA(0.99),而基部韧皮部内ZT/ GA3(1.06)却低于GA3/IAA(1.63)和ZT/IAA(1.74)。
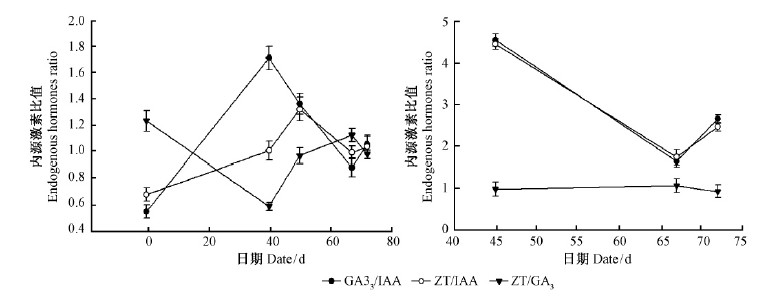 |
图 5 欧洲云杉嫩枝插穗生根进程中内源激素比值变化 Fig.5 The change of endogenous hormones ratio during rooting process of shoot cuttings in Norway spruce |
欧洲云杉插穗生根过程,愈伤组织形成前中阶段(37天前)对羟基苯甲酸含量呈升高的趋势,由2.23 ng ·100 g-1增加至7.19 ng ·100 g-1(图 6A)。当愈伤组织形成后进入不定根形成期及以后,对羟基苯甲酸含量呈逐渐减小的趋势,减少至4.16 ng ·100 g-1。儿茶酸和总酚酸含量在欧洲云杉枝插生根过程中均呈降低的趋势。45~72天插穗基部韧皮部(图 5B)的各多酚类物质含量变化趋势同针叶内,只是各种多酚类物质含量远远低于针叶内。针叶内儿茶酸含量(7.43~10.13 ng ·100 g-1)是基部韧皮部内含量的3.89~5.36倍;针叶内对羟基苯甲酸含量(4.16~4.97 ng ·100 g-1)是基部韧皮部内的1.24~1.48倍;针叶内总酚酸含量(11.59~15.23 ng ·100 g-1)是基部韧皮部内总酚酸含量的2.23~2.84倍。
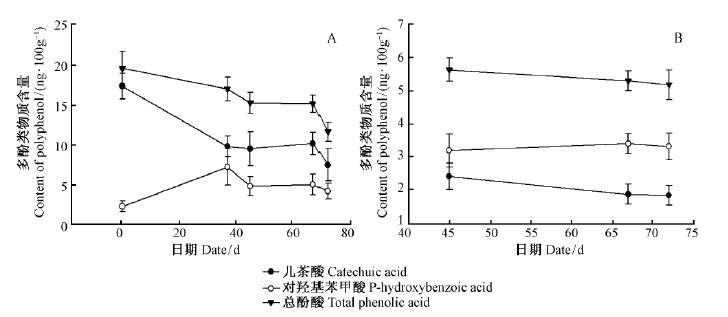 |
图 6 欧洲云杉嫩枝插穗生根进程中多酚类物质变化 Fig.6 The change of polyphenol during rooting process of shoot cuttings in Norway spruce |
从针叶内多酚类物质含量比值来看(图 7A),对羟基苯甲酸和儿茶酸与总酚酸间的变化趋势平稳,而儿茶酸/对羟基苯甲酸在扦插后至37天显著降低,降低35.01%,之后变化很小,略有上升趋势。同一时间段内插穗基部韧皮部内的多酚类物质比值(图 7B)和针叶内变化趋势一致。
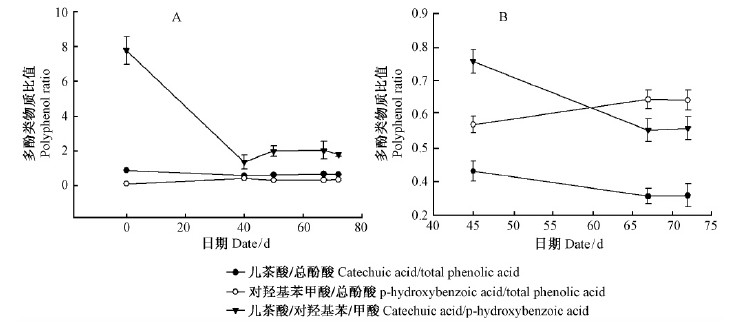 |
图 7 欧洲云杉嫩枝插穗生根进程中多酚类物质比值变化 Fig.7 The change of polyphenol ratio during rooting process of shoot cuttings in Norway spruce (A.针叶Needles; B.插穗基部韧皮部Phloem of cuttings base) |
针叶树扦插生根的过程分为3个阶段,即愈伤组织形成,根原始体分化(诱导生根),根的生长(长根)。本研究中欧洲云杉扦插后50天愈伤组织全部形成,从40天开始愈伤组织已有分化生根。扦插后50~75天为愈伤组织分化生根的高峰期,之后生根数量不变,根系仍然在生长,且扦插后根系效果指数持续增高,为长根期。
不定根形成是受许多环境因子和内在因子影响的,包括温度、光强和光质,碳水化合物和植物激素(Leakey,2004)。前人研究表明,5大类植物生长激素影响扦插生根进程,插穗生根过程中内源激素的动态平衡调控不定根发生过程(刘明国等,2011; 詹亚光等,2001)。一般生长素和乙烯促进不定根的形成,赤霉素和细胞分裂素一般表现为抑制不定根的形成;脱落酸对插条生根表现出多种效应(王乔春,1992)。IAA是促进不定根形成的主要激素(Trefois et al.,1982),IAA含量的高峰与根原基出现的高峰相一致(刘明国等,2011; 詹亚光等,2001; Trefois et al.,1982)。经研究发现,IAA与不定根原基的发生密切相关,这与新的形成层位点诱导和第1次细胞分裂的启动有关(Li et al.,1993)。生长素主要通过受体蛋白TIR1和ABP1来选择蛋白水解和细胞壁松弛(da Costa et al.,2013),以促进细胞分裂(Fogaça et al.,2005; Haissig,1970)。通过促进淀粉水解和转运糖类和营养物质到穗条基部韧皮部(Das et al.,1997)来诱导根原基的形成和生长。白桦(Betula platyphylla)插穗刚脱离母体时,IAA含量大幅度下降;根原基孕育期,IAA含量增加,积累以诱导生根,生根后下降(詹亚光等,2001)。也有研究表明,生长素通过调节茉莉酸酯体内平衡来控制拟南芥不定根形成(Gutierrez et al.,2012)。本研究中IAA含量也是一开始呈降低趋势,插穗刚刚脱离母体,IAA含量大幅降低。直至45天开始转为上升,45天的时候愈伤组织基本形成,已有5%左右的穗条开始分化生根,表明此时IAA含量的增加有利于愈伤组织分化成根原始体进而诱导生根。IAA含量在生根后呈降低趋势。大量研究证明,细胞分裂素主要抑制根原基的分化和形成(王乔春,1992)。本试验中内源激素ZT的含量持续降低,且67~72天时以约30%速率迅速降低,而这个时期正处于平均生根数急剧增加时期(图 2B),这表明内源ZT的降低有助于根原基的分化和根的形成。Bollmark等(1990)通过对欧洲云杉下胚轴插条生根研究推断乙烯加速细胞分裂素分解而促进生根。多数研究认为赤霉素抑制插条不定根的形成(Li et al.,1993)。赤霉素抑制不定根形成可能有多种途径,一是赤霉素抑制形成根原基细胞的分裂(Brian et al.,1960);二是阻碍生长素诱导的根原基的进一步生长发育(Haissig,1972)。本研究中愈伤组织形成阶段(37天前)赤霉素显著增加,抑制不定根的形成。37天以后进入不定根形成阶段,赤霉素GA3显著降低以促进不定根形成。关于赤霉素影响生根的机理,有研究表明赤霉素没有影响IAA信号途径也没有影响其上游基因,而是通过影响生长素运输从而抑制杂交杨(Populus)和拟南芥(Arabidopsis thaliana)不定根形成(Mauriat et al.,2014)。
从解剖学的角度来看,愈伤组织是产生愈伤根的前提,只有先形成愈伤组织,才能进一步分化出根。插穗基部愈伤组织中,细胞分裂素和生长素含量的消长变化同根的形成有很大关系。只有在插穗基部形成高生长素,低细胞分裂素的生理状态,才有利于根原基形成。ZT/IAA在扦插后45天呈上升趋势,45天后穗条生根,ZT/IAA下降以促进生根。扦插37天前,GA3/IAA呈迅速上升,抑制生根。生根过程中,扦插后37~67天GA3/IAA转为下降趋势,促进生根。
多酚类物质是具有酚羟基结构的一类化合物,已经证实大部分多酚类物质具有较强的抑制活性,明显抑制不定根的生长和发育(Balakrishnamurthy et al.,1988),这主要是与过氧化物酶活性相关(Rout,2006)。马尾松(Pinus massoniana)扦插苗中含有大量的多酚类化合物,是抑制马尾松扦插生根的主要内源物质之一(季孔庶等,1997)。对日本落叶松(Larix kaempferi)插穗生根研究发生生根性状与插穗内各种多酚类物质的含量呈显著或者极显著负相关关系(麻文俊等,2011)。王军辉等(2006)研究亦表明,儿茶酸、邻苯二酸对青海云杉(Picea crassifolia)硬枝插穗生根可能具有抑制作用,没食子酸和对羟基苯甲酸对插穗生根可能没有影响或影响甚微。本研究中多酚类物质含量整体呈下降趋势,有利于不定根的形成,这表明多酚类物质是欧洲云杉生根的抑制剂。不过多酚类物质对生根的影响因物种而不同,对茶树(Camellia sinensis)扦插生根的研究表明,多酚类物质是茶树生根的增强剂(Rout,2006)。
植物的内源激素和多酚类物质多在幼嫩的叶和芽内合成,并向茎基部和其他部位运输。因此欧洲云杉扦插生根进程中,针叶的内源激素和多酚类物质含量远远高于插穗基部韧皮部内,与白桦研究结果一致(詹亚光等,2001)。在扦插生根过程中,欧洲云杉穗条的内源激素和多酚类物质以及其他可溶性糖类等物质都发生了变化,此消彼长,共同促进根的发生。不定根诱导期IAA含量升高,而赤霉素、细胞分裂素和多酚类物质降低是促进插穗生根的重要原因。因此IAA对欧洲云杉的生根表现为促进作用,而赤霉素、细胞分裂素和多酚类物质则表现出抑制作用。
| [1] |
董健, 黄国学, 吴月亮, 等. 2001. 欧洲云杉嫩枝扦插育苗技术. 东北林业大学学报, 29 (4): 57-59. (Dong J, Huang G X, Wu Y L, et al. 2001. Vegetative propagation technique of Norway Spruce by softwood cuttage. Journal of Northeast Forestry University, 29 (4): 57-59[in Chinese]).(  1) 1)
|
| [2] |
傅紫芰, 李建文, 江泽平. 1998. 云杉属的分布及引种概况. 林木引种驯化与森林可持续经营: 165-174. (Fu Z J, Li J W, Jiang Z P.1998. The Distribution and Introduction of Picea. Trees Domesticated and Forest Sustainable Management: 165-174.[in Chinese])(  2) 2)
|
| [3] |
胡勐鸿, 欧阳芳群, 贾子瑞, 等. 2014. 欧洲云杉扦插生根影响因子研究与生根力优良单株选择. 林业科学, 50 (2): 42-49. (Hu M H, Ouyang F Q, Jia Z R, et al. 2014.Factors affecting rooting of Picea abies shoot cuttings and individual selection with high rooting ability. Scientia Silvae Sinicae, 50 (2): 42-49[in Chinese]).(  1) 1)
|
| [4] |
黄卓烈, 林韶湘. 1994. 桉树体内的生根抑制物质研究综述. 林业科学研究, 7 (3): 319-324. (Huang Z L, Lin S X. 1994. Review on the rooting inhibition substances inEucalyptus. Forest Research, 7 (3): 319-324[in Chinese]).(  1) 1)
|
| [5] |
季孔庶, 王章荣, 陈天华, 等. 1997. 马尾松插穗内源抑制物质的研究. 林业科学, 33(2): 142-151. (Ji K S, Wang Z R, Chen T H, et al. 1997.Study on the endogenous inhibitors in masson pine ( Pinus Massoniana Lamb.) Cuttings. Scientia Silvae Sinicae, 33(2): 142-151[in Chinese]).(  1) 1)
|
| [6] |
季孔庶, 王章荣, 王明庥, 等. 1996. 针叶树种扦插繁殖的研究进展及其对策. 世界林业研究, 9(4): 17-22. (Ji K S, Wang Z R, Wang M X, et al. 1996.Progress and contermeasures in conifrer vegetatine propagation. Word Forestry Research, 9(4): 17-22[in Chinese]).(  1) 1)
|
| [7] |
靳景春, 胡勐鸿, 张宋智, 等. 2009. 欧洲云杉扦插生根特性的研究. 西北林学院学报, 24(5): 70-73. (Ji J C, Hu M H, Zhang Z Z, et al. 2009.Rooting capability of twigs of Picea abies. Journal of Northeast Forestry University, 24(5): 70-73[in Chinese]).(  1) 1)
|
| [8] |
刘明国, 王玲, 董胜君, 等. 2011. 北美香柏插穗生根过程中内源激素的变化. 沈阳农业大学学报, 41(5): 555-559. (Li M G, Wang L, Dong S J, et al. 2011. Endogenous hormone variation in cuttings of Thuja occidentalis L. in the period of adventitious root formation. Journal of Shenyang Agricultural University, 41(5): 555-559[in Chinese]).(  2) 2)
|
| [9] |
麻文俊, 王军辉, 张守攻, 等. 2011. 日本落叶松无性系扦插生根过程中多酚类物质研究. 北京林业大学学报, 33(1): 150-154. (Ma W J, Wang J H, Zhang S G, et al. 2011.Qualitative analysis of phenolic compounds in the Japanese larch during the rooting of cuttings. Journal of Beijing Forestry University, 33(1): 150-154[in Chinese]).(  1) 1)
|
| [10] |
马建伟, 安三平, 杨炜, 等. 2011. 欧洲云杉的扦插基质选择和穗条效应研究. 广西植物, 31(4): 479-484. (Ma J W, An S P, Yang W, et al. 2011.Research on media selection and cutting effects in the cuttage of Picea abies. Guihaia, 31(4): 479-484[in Chinese]).(  1) 1)
|
| [11] |
裴会明, 解建民. 1998. 麦积山植物园裸子植物引种栽培. 北京:中国环境科学出版社, 133-136. (Pei H M, Jie J M. 1998. Introduction and Planting of gymnospermae in botanic garden of Maji Mountain. Beijing China Environmental Science Press, 133-136[in Chinese]).(  1) 1)
|
| [12] |
王军辉, 张建国, 张守攻, 等. 2006. 青海云杉硬枝扦插的激素, 年龄和位置效应研究. 西北农林科技大学学报:自然科学版, 34(7):65-71. (Wang J H, Zhang J G, Zhang S G, et al. 2006.Research of Hormone, Age and Position Effect of Hardwood Cutting in Picea crassifolia Kom. Journal of Northwest Science-Technology Universtiy of Agriculture and Forstry, 34(7):65-71[in Chinese]).(  2) 2)
|
| [13] |
王乔春. 1992. 植物激素与插条不定根的形成 (综述). 四川农业大学学报, 10(1): 33-39. (Wang Q C. 1992.Plant hormones and adventitous root formation in cuttings. Journal of Sichuan Agricultural University, 10(1): 33-39[in Chinese]).(  2) 2)
|
| [14] |
詹亚光, 杨传平. 2001. 白桦插穗生根的内源激素和营养物质. 东北林业大学学报, 29(4): 1-4. (Zhan Y G, Yang C P. 2001.Endogenous hormones and nutritive material in softwood cuttings of Betula platyphylla during Rooting. Journal of Northeast Forestry University, 29(4): 1-4[in Chinese]).(  4) 4)
|
| [15] |
朱湘渝, 王瑞玲, 黄东森. 1991. 欧美杨新无性系生根性研究. 林业科学, 27(2): 163-167. (Zhu X Y, Wang R L, Huang D S.1991. Study on rooting of new Euramerican poplar clone. Scientia Silvae Sinicae, 27(2): 163-167[in Chinese]).(  1) 1)
|
| [16] |
Balakrishnamurthy G, Rao V.1988. Changes in phenols during rhizogenesis in rose (Rosa bourboniana Desp). Current Science, 57(17): 960-962.( 1) 1)
|
| [17] |
Bollmark M, Eliasson L.1990. Ethylene accelerates the breakdown of cytokinins and thereby stimulates rooting in Norway spruce hypocotyl cuttings. Physiologia Plantarum, 80 (4): 534-540.( 1) 1)
|
| [18] |
Brian P, Hemming H, Lowe D.1960. Inhibition of rooting of cuttings by gibberellic acid: with one figure in the text. Annals of Botany, 24 (4): 407-419.( 1) 1)
|
| [19] |
da Costa CT, de Almeida M R, Ruedell C M, et al. 2013. When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Frontiers in plant science 4: Article 133,1-19.( 1) 1)
|
| [20] |
Das P, Basak U, Das A.1997. Metabolic changes during rooting in pre-girdled stem cuttings and air-layers of Heritiera. Botanical Bulletin of Academia Sinica 38: 91-95.( 1) 1)
|
| [21] |
Fogaça C M, Fett-Neto A G. 2005. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regulation, 45 (1): 1-10.( 1) 1)
|
| [22] |
Gemmel P, Örlander G, Högberg K.1991. Norway spruce cuttings perform better than seedlings of the same genetic origin. Silvae Genet, 40: 198-202.( 1) 1)
|
| [23] |
Gutierrez L, Mongelard G, Floková K, et al.2012. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. The Plant Cell Online 24 (6): 2515-2527.( 1) 1)
|
| [24] |
Haissig B E.1970. Influence of indole-3-acetic acid on adventitious root primordia of brittle willow. Planta, 95 (1): 27-35( 1) 1)
|
| [25] |
Haissig B E.1972. Meristematic activity during adventitious root primordium development influences of endogenous auxin and applied gibberellic acid. Plant physiology, 49 (6): 886-892.( 1) 1)
|
| [26] |
Leakey R R.2004. Physiology of vegetative reproduction. Encyclopedia Forest Sciences, 1655-1668.( 1) 1)
|
| [27] |
Li H, Pan R.1993. Hormone control of adventitious rooting in mung bean stem cutings. //XV International Botanical Congress, Japan Pacifico Yokohama, 1993.( 2) 2)
|
| [28] |
Mauriat M, Petterle A, Bellini C, et al.2014. Gibberellins inhibit adventitious rooting in hybrid aspen and Arabidopsis by affecting auxin transport. The Plant Journal, 78(3): 372-384.( 1) 1)
|
| [29] |
Roulund H, Wellendorf H, Werner M.1985. A clonal experiment in Norway spruce (Picea abies (L.) Karst): 15 years' results. Akademisk Forlag,Copenhagen, Denmark.( 1) 1)
|
| [30] |
Rout G R.2006. Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant growth regulation, 48 (2): 111-117.( 2) 2)
|
| [31] |
Trefois R, Brunner T.1982. Influence of the endogenous auxinic content on the response to propagation by cuttings and on the dwarfing effect of some types of Prunus. Botanikai kozlemenyek-Botanical Publication,69(314):197-204.( 2) 2)
|
 2015, Vol. 51
2015, Vol. 51

