文章信息
- 王建勇, 谭晓风, 陈鸿鹏, 曾艳玲, 龙洪旭, 梅芳芳, 刘凯
- Wang Jianyong, Tan Xiaofeng, Chen Hongpeng, Zeng Yanling, Long Hongxu, Mei Fangfang, Liu Kai
- 油茶丙二酰单酰CoA:ACP转酰基酶基因的克隆与原核表达
- Cloning and Prokaryotic Expression of a Malonyl-CoA:ACP Transacylase Gene from Camellia oleifera
- 林业科学, 2015, 51(3): 148-154
- Scientia Silvae Sinicae, 2015, 51(3): 148-154.
- DOI: 10.11707/j.1001-7488.20150319
-
文章历史
- 收稿日期:2014-06-10
- 修回日期:2014-10-05
-
作者相关文章
2. 国家林业局桉树研究开发中心 湛江 524022;
3. 华中师范大学生命科学学院 武汉 430079;
4. 广西林业科学研究院 南宁 530001
2. China Eucalypt Research Centre Zhanjiang 524022;
3. College of Life Sciences, Central China Normal University Wuhan 430079;
4. Guangxi Academy of Forestry Nanning 530001
根据脂肪酸中FAS复合体的存在形式,生物体中存在2种脂肪酸代谢系统(FASⅠ和FASⅡ),其中FAS复合体以融合的形式存在于胞浆中的代谢途径称之为FASⅠ代谢途径,以独立的单功能的蛋白形式存在的则称为FASⅡ代谢途径(Mehboob et al.,2010;Izumizono et al.,2011)。丙二酰单酰CoA:ACP转酰酶(Malonyl-CoA:ACP transacylase,MCAT)是由MCAT基因编码的一种巯基酶,是FASⅡ脂肪酸代谢途径中的FAS复合体组成酶之一,在脂肪酸合成途径中起装载作用。目前认为MCAT是通过ping-pong bibi机制把丙二酰团从丙二酰单酰CoA上的硫酯连接部位转移到ACP的磷酸泛酰巯基乙胺臂上,从而形成丙二酰单酰ACP,该过程是通过两步反应完成(White et al.,2005;Li et al.,2007;Zhang et al.,2007;Arthur et al.,2009)。MCAT催化生成的丙二酰单酰ACP不仅是脂肪酸合成过程中的核心物质,还是酮酯酰ACP合成酶(KASⅠ/Ⅱ/Ⅲ)的底物,说明MCAT参与了脂肪酸代谢过程(Prigge et al.,2003;Arthur et al.,2009)。MCAT可能还参与多聚酮的生物合成,因此MCAT被认为是脂肪酸和多聚酮2个代谢途径的结合点(Hong et al.,2010a;2010b; Zhou et al.,2010)。自Verwoert等(1992)首次从大肠杆菌(Escherichia coli)中克隆到MCAT基因以来,已经陆续从细菌、顶复门(Apicomplexa)原虫(Apicomplexa)和植物[如玉米(Zea mays)、欧洲油菜(Brassica napus)、拟南芥(Arabidopsis thaliana)和花生(Arachis hypogaea)]等生物中发现了MCAT基因的存在(孙铭飞等,2011)。通过多重序列比对分析发现,这些基因所编码的氨基酸序列具有较高的相似性,各物种之间的同源性在20%~33%之间。在结构上,不同物种的MCAT也是极其相似的,每一个MCAT单体具有2个亚结构域:N端是由多个β折叠和α螺旋组成的大亚基结构域,C端是一个类似于铁氧还原蛋白折叠的小亚基结构域(White et al.,2005)。尽管低等的原核生物同真核生物在进化关系上差异很大,但由于它们的脂肪酸代谢同属于FASⅡ代谢途径,通过同源重组互补置换试验证实,不同物种之间的MCAT在结构和功能上是高度相似的(Zhang et al.,1998; Simon et al.,1998; Soto et al.,2002)。
油茶(Camellia oleifera)是我国重要的木本食用油料树种,茶油主要由油酸、亚油酸等不饱和脂肪酸组成,其含量一般在90%以上,是一种优质食用油(廖书娟等,2005; 胡芳名等,2006)。但是,油茶种仁含油率较低且易遭逆性环境等的影响,这些都严重影响和制约了油茶产业的快速发展。现代分子生物学技术(如基因工程育种)给油茶的利用与发展开创了崭新的局面。对油茶优质基因的分离、克隆及功能的研究对于油茶育种具有重要意义。本文以本实验室构建的油茶转录组数据库为基础,首次克隆获得油茶MCAT基因的全长cDNA序列,构建其原核表达载体在宿主细胞BL21(DE3)中成功诱导表达目的蛋白,并对重组蛋白进行了初步的酶活性分析,这些工作将为今后进一步深入开展和揭示油茶MCAT基因在油脂合成的代谢过程的作用奠定基础。
1 材料与方法 1.1 油茶种子总RNA的提取及单链cDNA合成以油茶优良品种‘华硕’(谭晓风等,2011)的9月份近成熟种子为材料,提取种胚总RNA(见Invitrogen RNA提取试剂盒操作手册),检测总RNA的纯度与浓度,并以此为模板反转录合成单链cDNA[方法详见3′ RACE(Invitrogen),5′ RACE(Clontech)与Fermentas试剂盒说明书]。
1.2 MCAT基因的克隆与生物信息学分析BLAST比对后,根据初步确定的MCAT Unigene序列,设计特异引物(表 1),采用RACE技术,克隆油茶MCAT基因。
|
|
以油茶种胚的cDNA为模板,采用普通Taq酶扩增Unigene和RACE序列(25 μL),采用高保真酶Pfu扩增基因全长序列(50 μL),反应体系参照各自说明书。Touch Down PCR循环扩增条件采用95 ℃ 预变性5 min;95 ℃ 变性30 s,退火30 s [退火温度从高于(Tm+4)℃开始,每7个循环依次降低退火温度2 ℃直至低于(Tm -4)℃],72 ℃延伸1.5 min;72 ℃延伸10 min;4 ℃保存。PCR产物回收、克隆、转化,鉴定重组子并测序。利用本地和在线软件Vector NTI 10.3,GENDOC,ProtParam,TargetP 1.1 Server,ProtScale,InterProScan,SOPMA和SWISS-MODEL,预测分析基因的理化性质、结构与功能。
1.3 基因的原核表达以获得的全长克隆质粒为模板,通过特异引物(表 1)扩增结合酶切连接构建到原核表达载体pET30a的多克隆位点,将表达载体质粒导入BL21(DE3),37 ℃培养至OD600值约为0.5~0.8后,在28 ℃和IPTG终浓度为1 mmol ·L-1的条件下诱导表达(诱导总时间为12 h),每隔2 h取菌液进行SDS-PAGE(12%)分析。
1.4 重组蛋白的酶活性测定冷冻离心收集菌体,用0.1 mol ·L-1Tris-HCl(pH 7.4)的缓冲液洗涤2次后重悬于Lysis Buffer(0.1 mol ·L-1Tris-HCl,0.6 mol ·L-1NaCl,1.0 mmol ·L-1PSMF,5.0 mg ·mL-1溶菌酶,pH 7.4)中,冰浴充分搅拌后,超声破碎细胞(380 W,工作1 s,停2 s,工作6 min),离心(12 000 r ·min-1,20 min,4 b℃)后,上清液即为粗酶液,Ni柱纯化后进行SDS-PAGE(12%)分析,并用Br and ford方法进行浓度测定。采用α酮戊二酸脱氢酶(KDH)偶联系统(Molnos et al.,2003),测定重组蛋白在不同pH(4.5-8.5)和温度(15~45 ℃)条件下的酶活性,相同条件每个反应重复3次。酶活性单位U定义为单位时间(min)内生成1 nmol NADH所用的酶量。
2 结果与分析 2.1 CoMCAT基因cDNA全长克隆与生物信息学分析通过RACE技术,获得全长为1 867 bp的目的片段,5′ UTR和3′ UTR分别为93 bp和643 bp,终止密码子后有PolyA尾巴结构,根据序列同源性将其命名为CoMCAT,GenBank登录号KJ910337。 该基因编码区为1 131 bp,编码376个氨基酸,分子量为 39.98 kDa,理论等电点为6.84,带负电荷残基(Asp + Glu)数为42,带正电荷残基(Arg + Lys)数42,分子式为C1771H2846N482O542S13,不稳定指数(Ⅱ)为28.49,属于典型的稳定蛋白。整个蛋白的疏水指数为-2.256~2.078,是亲水性蛋白,没有比较明显的跨膜区。经TargetP 1.1 Server软件预测,该蛋白具有58个氨基酸长度的叶绿体转运肽(图 1,Ⅲ)。CoMCAT存有“GQGXQ”(图 1,Ⅰ)和“GXSXG”(图 1,Ⅱ)2个高度保守的基序。高级结构预测显示:α螺旋是CoMCAT的主要结构元件,而不规则卷曲则散布于整个蛋白质中,其中α螺旋结构49.73%,β折叠14.10%,β转角5.05%和无规则卷曲31.12%(图 2A);CoMCAT与人类线粒体丙二酰转移酶2C2N_A序列相似性最高为40.39%,图 2B为预测的CoMCAT三维空间模型。
 |
图 1 CoMCAT与其他植物MCAT蛋白质序列的多重比较 Fig.1 Multiple comparisons between CoMCAT and other MCATs 1: 油茶Camellia oleifera(KJ910337); 2: 大豆Glycine max(NP_001238312); 3: 番茄Solanum lycopersicum(XP_004228628); 4: 黄瓜Cucumis sativus(XP_004145242); 5: 可可Theobroma cacao(XP_007016988); 6: 辣椒Capsicum annuum(ACF17665); 7: 烟草Nicotiana tabacum(AFE88235); 8: 马铃薯Solanum tuberosum(XP_006348452); 9: 花生Arachis hypogaea(ACJ07138). Ι: GQGXQ 基序GQGXQ motif; ΙΙ: GXSXG 基序GXSXG motif; Ш: CoMCAT的叶绿体转运肽序列A chloroplast transit peptide of CoMCAT. |
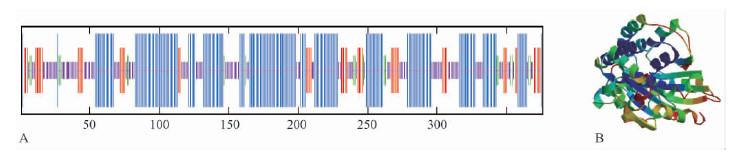 |
图 2 CoMCAT蛋白质的结构预测 Fig.2 Structure predictions of CoMCAT A: 二级结构;B:三级结构。A: Secondary structure;B:3D structure. |
在28 ℃条件和不同诱导时间下,相比于未加诱导剂的菌株,诱导的宿主菌株有1条清晰的分子量约为40 kDa的条带,随着时间延长,目的条带颜色加深(图 3A),说明如图 4所构建的表达载体pET30a-CoMCAT是正确的;Ni柱纯化后,有条单一的分子量约为40 kDa的条带(图 3B),这同预测的分子量大小相一致,说明CoMCAT基因在BL21(DE3)中得到了表达。Br and ford方法测定,蛋白纯化后的浓度约为125 μg ·mL-1。
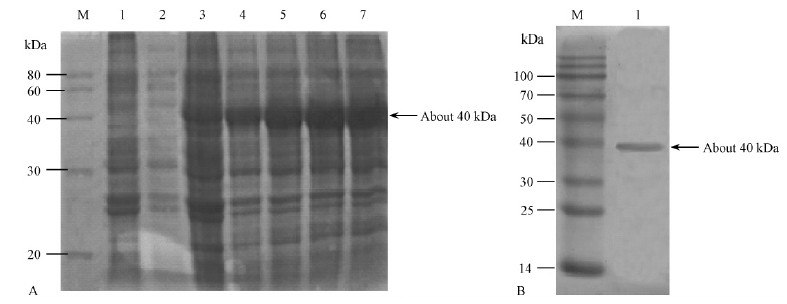 |
图 3 pET30a-CoMCAT表达蛋白的SDS-PAGE分析 Fig.3 SDS-PAGE analysis of MCAT protein expression in BL21(DE3) transformed with pET30a-CoMCAT A:不同诱导时间的菌液蛋白检测(M:蛋白相对分子标量;1:未经IPTG诱导的重组菌总蛋白;2-7: IPTG分别诱导2,4,6,8,10,12 h重组菌总蛋白);B:纯化后的蛋白检测。 A: Detection of crude proteins in different time (M: Protein marker; 1: Total protein of BL21(DE3); 2-7: Crude proteins of pET30a-CoMCAT induced for 2,4,6,8,10,12 hours); B: Detection of purified protein. |
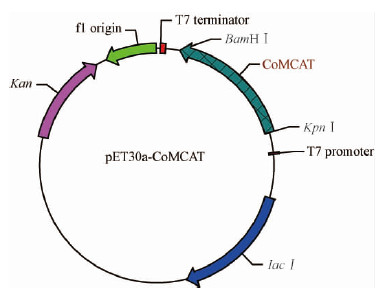 |
图 4 重组表达载体pET30a-CoMCAT Fig.4 Recombinant vector of pET30a-CoMCAT |
不同温度和pH条件下所表达CoMCAT酶活性分析见图 5,当温度在30 ℃和pH为7.0时,该蛋白的活性最高,且当二者超过最佳值之后,其活性均表现出急骤下降,说明高温和偏碱性不利于CoMCAT的活性表现。经计算,所获得的重组蛋白CoMCAT的比酶活性约为2.384 U ·μg-1。
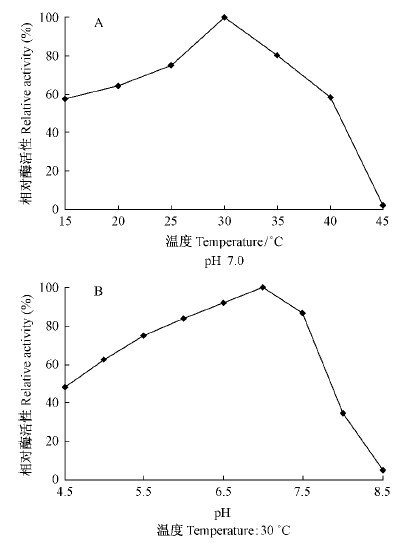 |
图 5 不同温度(A)和pH(B)对重组蛋白CoMCAT酶活性的影响 Fig.5 Effects of temperatures(A)and pH(B) on enzymatic activity of recombinant CoMCAT |
自Serre等(1994)报道了大肠杆菌MCAT的晶体结构以来,目前已经解析了多种生物MCAT的晶体结构(Keatinge-Clay et al.,2003;Li et al.,2007;Zhang et al.,2007;Hong et al.,2010b)。同源比对发现,CoMCAT存在“GQGXQ”和“GXSXG”2个高度保守的基序,一个位于 malonyl-CoA结合位点上,另外一个位于ACP结合位点上,这同已报道的MCAT氨基酸序列特征相一致(Li et al.,2007;Zhang et al.,2007);通过结构信息分析证实,这2个基序与MCAT的催化功能密切相关(Hong et al.,2010b)。经在线软件预测CoMCAT蛋白序列具有58个氨基酸长度的叶绿体转运肽,这同已报道的MCAT基因序列的5′ 端具有编码质体(plastid)靶向的引导肽结论相一致(孙铭飞等,2010; Goodman et al.,2007)。该基因是从近成熟的种子中分离的,通常种子的脂质合成部位是plastid(分为白色体、有色体和叶绿体,均由前质体发育分化而来),根据油茶‘华硕’种子的油脂合成规律(曾艳玲等,2014),该基因可能先在前质体中表达,然后才被转运至叶绿体而发挥油脂合成作用。
在原核表达系统中,温度和pH是影响蛋白可溶性和酶活性的最为明显因素(Sørensen et al.,2005),不利的条件不仅会引起重组蛋白多肽的错误折叠而形成包涵体结构[包涵体表达蛋白在复性后几乎完全没有或仅有很低的活性(孙铭飞等,2010)],还影响酶与底物的正确或高效的结合等而影响酶的功能,这提示所表达的蛋白若用于研究其结构或功能,需要探索和优化表达条件。本研究所获得的重组蛋白CoMCAT的最佳催化功能条件为30 ℃和pH 7.0,这为今后研究MCAT蛋白的功能奠定了实验条件基础。目前,虽然MCAT的研究取得了诸多的进展,但是必须看到的是这些成果主要集中于少数的原核生物和顶复门寄生虫上,在其他物种上的研究几乎是一片空白。未来针对MCAT的研究热点将主要集中在以下方面:由于原核生物、顶复门原虫和大部分植物的FASⅡ代谢酶在空间结构和酶学特征上与高等动物的FASⅠ代谢酶明显不同,这可能为研究和开发抗病原体药物提供理想靶向位点(Lu et al.,2008;Chan et al.,2010;Du et al.,2010;Zhang et al.,2007),而MCAT是FASⅡ系统的关键酶,对MCAT的研究,特别是MCAT在促进丙二酰单酰CoA转化丙二酰单酰ACP的作用机制的研究将会越来越成为抗菌、抗原虫及抗真菌药等研究的焦点问题之一;有研究证实,在大肠杆菌中过量表达MCAT基因能提高脂肪酸的产量(Zhang et al.,2012),这为MCAT基因应用于油料作物的遗传改良与育种指明了方向。
| [1] |
胡芳名,谭晓风,刘惠民. 2006. 中国主要经济树种栽培与利用. 北京:中国林业出版社. (Hu F M, Tan X F, Liu H M. 2006. Culture and utilization of Chinese non-wood product forest trees. Beijing: China Forestry Publishing House.[in Chinese])(  1) 1)
|
| [2] |
廖书娟,吉当玲,童荣华. 2005. 茶油脂肪酸组成及其营养保健功能. 粮食与油脂,(6):7-9. (Liao S J, Ji D L, Tong H R. 2005. Study on fatty acid composition and nutrition health protection function of the oiltea Camellia seed oil. Cereals & Oils,(6):7-9[in Chinese]).(  1) 1)
|
| [3] |
孙铭飞,覃宗华,谢明权,等. 2010. 不同表达条件对Eimeria tenella丙二酰单酰辅酶A:ACP转移酶活性的影响. 畜牧兽医学报,41(9):1158-1165. (Sun M F,Qin Z H,Xie M Q,et al. 2010. Effects of different expression vectors and temperatures on the activity of Eimeria tenella malonyl CoA:ACP transacylase. Acta Veterinaria et Zootechnica Sinica, 41(9):1158-1165[in Chinese]).(  2) 2)
|
| [4] |
孙铭飞,廖申权,吴彩艳,等. 2011. 顶复门原虫与细菌Ⅱ型丙二酰单酰辅酶A:ACP转酰基酶研究进展. 动物医学进展,32(8):80-84. (Sun M F,Liao S Q,Wu C Y, et al. 2011.Advance on typeⅡ malonyl-CoA:acyl carrier protein transacylase in Apicomplexa and Bacteria. Progress in Veterinary Medicine,32(8):80-84[in Chinese]).(  1) 1)
|
| [5] |
谭晓风,袁德义,袁 军,等. 2011. 大果油茶良种'华硕'. 林业科学,47(12):184. (Tan X F,Yuan D Y,Yuan J,et al. 2011. An elite variety:Camellia oleifera 'Huashuo'. Scientia Silvae Sinicae,47(12):184[in Chinese]).(  1) 1)
|
| [6] |
曾艳玲,谭晓风,张党权,等. 2014. 油茶脂肪酸代谢途径中关键酶基因调控油脂合成的规律研究. 中国粮油学报,29(2):26-29. (Zeng Y L,Tan X F,Zhang D Q,et al. 2014. Regulation about control oil synthesis by key genes in fatty acid metabolic pathway of Camellia oleifera. Journal of the Chinese Cereals and Oils Association,29(2):26-29[in Chinese]).(  1) 1)
|
| [7] |
Arthur C J,Williams C,Pottage K. 2009. Structure and malonyl CoA:ACP transacylase binding of streptomyces coelicolor fatty acid synthase acyl carrier protein. ACS Chemical Biology,4(8):625-636.( 2) 2)
|
| [8] |
Chan D I,Tieleman D P,Vogel H J. 2010. Molecular dynamics simulations of β-ketoacyl-,β-hydroxyacyl-,and trans-2-enoyl-acyl carrier proteins of Escherichia coli. Biochemistry, 49(13): 2860-2868.( 1) 1)
|
| [9] |
Du Y,Gisselberg J E,Johnson J D,et al. 2010. Lactococcus lactis fabH,encoding β-ketoacyl-acyl carrier protein synthase,can be functionally replaced by the Plasmodium falciparum congener. Applied and Environmental Microbiology,76(12):3959-3966.( 1) 1)
|
| [10] |
Goodman C D,McFadden G I. 2007. Fatty acid biosynthesis as a drug target in apicomplexan parasites. Current Drug Targets,8(121):15-30.( 1) 1)
|
| [11] |
Hong S K,Kim K H,Park J K,et al. 2010a. New design platform for malonyl CoA:acyl carrier protein transacylase. FEBS Letters,584(6):1240-1244.( 1) 1)
|
| [12] |
Hong S K,Kim K H,Park J K,et al. 2010b. Cloning,purification,crystallization and preliminary X-ray crystallographic analysis of MCAT from Staphylococcus aureus. Structural Biology and Crystallization Communications,66(1):20-22.( 3) 3)
|
| [13] |
Izumizono Y,Arevalo S,Koseki Y,et al. 2011. Identification of novel potential antibiotics for tuberculosis by in silico structure-based drug screening. European Journal of Medicinal Chemistry,46(5):1849-1856.( 1) 1)
|
| [14] |
Keatinge-Clay A T,Shelat A A,Savage D F,et al. 2003. Catalysis,specificity and ACP docking site of Streptomyces coelicolor malonyl CoA:ACP transacylase. Structure,11(2):147-154.( 1) 1)
|
| [15] |
Li Zexuan,Huang Yishu,Ge Jing,et al. 2007. The crystal structure of MCAT from Mycobacterium tuberculosis reveals three new catalytic models. Journal of Molecular Biology,371(4):1075-1083.( 3) 3)
|
| [16] |
Lu H,Tonge P J. 2008. Inhibitors of FabI,an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Accounts of Chemical Research,41(1):11-20.( 1) 1)
|
| [17] |
Mehboob S,Truong K,Santarsiero B D,et al. 2010. Structure of the Francisella tularensis enoyl acyl carrier protein reductase (FabI) in complex with NAD(+) and triclosan. Structural Biology and Crystallization Communications,66(11):1436-1440.( 1) 1)
|
| [18] |
Molnos J,Gardiner R,Dale G E,et al. 2003. A continuous coupled enzyme assay for bacterial Malonyl-CoA: acyl carrier protein transaeylase (FabD). Analytical Biochemistry,319(1):171-176.( 1) 1)
|
| [19] |
Prigge S T,He X,Gerena L,et al. 2003. The initiating steps of a type Ⅱ fatty acid synthase in Plasmodium falciparum are catalyzed by pfACP, pfMCAT and pfKAS Ш. Biochemistry,42(4):1160-1169.( 1) 1)
|
| [20] |
Serre L,Swenson L,Green R,et al. 1994. Crystallization of the malonyl coenzyme A-acyl carrier protein transacylase from Escherichia coli. Journal of Molecular Biology,242(1):99-102.( 1) 1)
|
| [21] |
Simon J W,Slabas A R. 1998. cDNA cloning of Brassica napus malonyl-CoA:ACP transacylase (MCAT) (fab D) and complementation of an E.coli MCAT mutant. FEBS Letters,435(2/3):204-206.( 1) 1)
|
| [22] |
Soto M J,Fernndez-Pascual M,Sanjuan J,et al. 2002. A fadD mutant of Sinorhizobium meliloti shows multicellular swarming migration and is impaired in nodulation efficiency on alfalfa roots. Molecular Microbiology, 43(2):371-382.( 1) 1)
|
| [23] |
Sörensen H P,Mortensen K K. 2005. Advanced genetic strategies for recombinant protein expression in Escherichia coli. Journal of Biotechnology, 115(2):113-128.( 1) 1)
|
| [24] |
Verwoert I I,Verbree E C,Van der Linden K H,et al. 1992. Cloning,nucleotide sequence and expression of the Escherichia coli fabD gene, encoding malonyl coenzyme A-acyl carrier protein transacylase. Journal of Bacteriology, 174(9):2851-2857.( 1) 1)
|
| [25] |
White S W,Zhang J,Zhang Y M,et al. 2005. The structural biology of typeⅡfatty acid biosynthesis. Annual Review of Biochemistry,74:791-831.( 2) 2)
|
| [26] |
Zhang L,Liu W Z,Xiao J F,et al. 2007. Malonyl CoA:acyl carrier protein transacylase from Helicobacter pylori: crystal structure and its interaction with acyl carrier protein. Protein Science, 16(6):1184-1192.( 4) 4)
|
| [27] |
Zhang X J,Agrawal A,San K Y. 2012. Improving fatty acid production in Escherichia coli through the overexpression of malonyl CoA:acyl carrier protein transacylase. Biotechnology Progress, 28(1):60-65.( 1) 1)
|
| [28] |
Zhang Y,Cronan J E. 1998. Transcriptional analysis of essential genes of the Escherichia coli fatty acid biosynthesis genecluster by functional replacement with the analogous Salmonella typhimurium gene cluster. Journal of Bacteriology,180(13):3295-3303.( 1) 1)
|
| [29] |
Zhou H,Qiao K J,Gao Z Z,et al. 2010. Enzymatic synthesis of resorcylic acid lactones by cooperation of fungal iterative polyketide synthases involved in hypothemycin biosynthesis. Journal of American Chemical Society,132(13):4530-4531.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

