文章信息
- 刘晓晗, 王峰, 董爱荣, 陈俏丽, 刘立宏, 零雅茗, 王博文, 丁晓霞, 王世新
- Liu Xiaohan, Wang Feng, Dong Airong, Chen Qiaoli, Liu Lihong, Ling Yaming, Wang Bowen, Ding Xiaoxia, Wang Shixin
- 粗毛纤孔菌糖苷水解酶5基因克隆及蛋白质结构分析
- Gene Cloning and Protein Structural Studies of a Glycoside Hydrolase Family 5 Enzyme Gene from Inonotus hispidus
- 林业科学, 2015, 51(2): 163-168
- Scientia Silvae Sinicae, 2015, 51(2): 163-168.
- DOI: 10.11707/j.1001-7488.20150220
-
文章历史
- 收稿日期:2013-11-22
- 修回日期:2014-08-27
-
作者相关文章
粗毛纤孔菌(Inonotus hispidus)是一种主要生长在水曲柳(Fraxinus m and shurica)、榆树(Ulmus sp.)、桑树(Morus alba)等阔叶树活立木上的白腐菌(Saddler,1982),子实体1年生,单生或覆瓦状叠生,于夏季和秋季出现,晚秋后变黑。粗毛纤孔菌具药用功效,应用和开发价值高。
纤维素是地球上分布较广且蕴藏量丰富的可再生资源,充分利用纤维素可以缓解全球能源危机及粮食短缺问题。碳水化合物降解酶能将纤维素水解为单糖(单糖是进一步生产乙醇、丙酮和丁酮等化合物的原料),对有效利用纤维素具有十分重要的意义。碳水化合物降解酶分为糖苷水解酶(GHs)、糖基转移酶(GTs)、多糖裂解酶(PLs)、碳水化合物酯酶(CEs)、碳水化合物结合模块(CBMs)(Nicol et al.,2012),其中,糖苷水解酶家族GH5,6,7,8,9,10,12,26,44,45,48,51,61和74是纤维素酶(Henrissat,1991)。本研究克隆到粗毛纤孔菌糖苷水解酶家族5基因,并对该基因进行了生物信息学分析、蛋白质原核表达和酶活研究,为下一步研究打下基础。
1 材料与方法 1.1 菌种采集及纯化培养2008年于哈尔滨林业示范基地水曲柳活立木上采集木材腐朽菌子实体。该子实体担子果平伏,覆瓦状叠生,木栓质。菌盖金黄色,被粗毛,边缘钝。孔口表面金黄色,多角形,每毫米2~3个(Rubini et al.,2010)。菌管与孔口表面颜色相同,但明显比菌肉颜色浅。担孢子椭圆形,表面光滑,金黄色,有明显厚壁。ITS序列系统发育树状图表明该粗毛纤孔菌与辐射状纤孔菌亲缘关系较近。经过形态学和ITS区序列PCR扩增后序列比对,鉴定为粗毛纤孔菌(郭春宣等,2010),该菌种分离纯化后于PDA斜面长期保存。
1.2 cDNA文库构建RNA提取试验前于纤维素PDA平板(1.5%琼脂及0.5%羧甲基纤维素)诱导培养菌苔备用。应用TRIzol提取总RNA,DNase I(RNase Free)(TaKaRa,Japan)消化后,采用AMV 反转录系统(Promega,Cat. No. A3500),以Oligo(dT)18为引物反转录第1链cDNA,随机引物合成第2链cDNA(Robson et al.,1989)。双链cDNA加接EcoR I 接头(400 ng·μL-1),进行末端的磷酸化及 Xho I 酶切后连接至pBlueScriptII载体并转化至大肠埃希菌(Escherichia coli)感受态细胞DH5α。菌落PCR法筛选阳性克隆送上海生物工程公司测序。
1.3 基因全长克隆应用NCBI BLASTN 2.2.16在线比对测序结果。BLASTX/BLAST分析,检测糖苷水解酶家族5基因阳性序列。根据该序列设计3′RACE引物(In-GH5-5′R:5′-CCT TGC GGA TCC CGT CGA TC-3′)和5′RACE引物(In-GH5-3′F:5′-TGA GTT CGG TGT ATG GAG -3′),采用Clontech公司的Marathon RACE试剂盒(Cat. No. 634913)进行基因全长克隆,克隆产物分别送上海生物工程公司测序,测序所得3′和5′序列拼接后命名为IhGH5-1,并提交NCBI注册。
1.4 生物信息学分析及纤维素酶基因鉴定ORF-Finder(Open Reading Frame Finder of NCBI)分析IhGH5-1基因开放阅读框,翻译氨基酸序列并进行生物信息学分析(Wood et al.,1989)。筛选NCBI登录的糖苷水解酶家族5同源序列,Clustal W进行保守结构域区段多序列比对。应用Mega 5.05选用WAG+G模型构建最大似然树(maximum likelihood tree),自展检验(bootstrap test)重复次数为1 000。
1.5 同源建模及结构分析应用PSIPRED server对IhGH5-1进行α螺旋和β折叠的蛋白质二级结构分析(Aggelis et al.,2002)。在二级结构分析的基础上,应用SWISS-MODEL对IhGH5-1进行三维建模,应用VMD1.8.6分析IhGH5-1三维结构。同源三维结构比对,进一步修正后输出三维立体结构图。
1.6 原核表达应用Primer Premier 5.0 设计原核表达所需引物(In-GH5-Ex-F:5′-CAA GCA GTA GAG CGT ACC-3′和In-GH5-Ex-R:5′- GTT GTC GGA GAG ACG AGC T -3′)并进行PCR扩增。分别将扩增得到的IhGH5-1基因片段连接酶连接至pQE-30 UA 载体,转化大肠埃希菌JM109感受态细胞,LB平板上筛选阳性重组子。阳性重组子经IPTG(浓度1 mmol·L-1)诱导4 h。离心沉淀,生理盐水洗涤后重悬,超声波破碎菌体,离心收集上清液和沉淀,SDS-PAGE电泳检测表达量(Muthezhilan et al.,2007)。
1.7 酶活测定阳性IhGH5-1重组子采用LB液体培养基发酵摇培,培养至指数期时添加IPTG(终浓度1 mmol·L-1)。发酵液4 ℃离心10 min,弃上清,洗涤菌体并转至50 mmol·L-1磷酸缓冲液。冰浴超声破碎,4 ℃离心15 min取上清液即为粗酶液。将葡萄糖80 ℃烘烤至恒重,配制母液,绘制标准曲线(O’Leary et al.,2004)。以羧甲基纤维素钠盐(CMC-Na)酶活性测定法,3,5-二硝基水杨酸(DNS)显色测定不同温度下粗酶液550 nm吸光值,并计算酶活。
2 结果与分析 2.1 基因克隆应用TRIzol提取粗毛纤孔菌总RNA(图 1),经分光光度计检验符合标准(OD260/OD280=2.0,OD260/OD230>1.8)。RNA反转录为cDNA,构建cDNA文库并测序。经BLASTN对EST测序结果进行分析,得到一条糖苷水解酶家族5基因同源序列片段,根据该序列设计引物In-GH5-5′R和In-GH5-3′F。应用RACE法克隆得到5′序列长度为770 bp(图 1泳道3),3′长度为1 562 bp含有PolyA的序列(图 1 泳道2)。5′和3′序列拼接得到基因全长序列长1 727 bp,提交NCBI注册,注册号为KM368321。经ORF分析得到IhGH5-1氨基酸序列含有300个氨基酸,分子质量为31.226 55 kD,等电点(pI)为9.24。
 |
图 1 RNA及IhGH5-1基因全长克隆 Fig. 1 RNA and RACE of IhGH5-1 gene M:DNA Marker DL2 000;1:粗毛纤孔菌RNA RNA of I. hispidus ; 2:IhGH5-1基因3′序列克隆Clone 3′sequence of IhGH5-1 gene by RACE; 3:IhGH5-1基因5′序列克隆Clone 5′sequence of IhGH5-1 gene by RACE. |
筛选NCBI收录的真菌IhGH5-1同源序列4条(表 1),应用Clustal W程序对同源序列保守结构域进行多序列比对。另下载NCBI登录的担子菌(Basidomycota)和子囊菌(Ascomycota)IhGH5-1同源序列70条,采用PAUP 4.0和Modetest 3.06依据赤池信息量准则(Akaike Information Criterion)选出WAG+G为进化的最适模型构建最大似然树(图 2B)。结构域分析表明IhGH5-1具有保守的催化结构域(Catalytic Domain)(图 2A)。最大似然树分析表明多数子囊菌糖苷水解酶家族5聚在一起,多数担子菌糖苷水解酶家族5聚在一起。但也有子囊菌糖苷水解酶家族5的系统进化与菌物进化不完全一致,粗毛纤孔菌IhGH5-1蛋白质与太瑞斯梭孢壳霉(Thielavia terrestris)和球毛壳菌(Chaetomium globosum)等子囊菌的糖苷水解酶亲缘关系更近。
|
|
 |
图 2 IhGH5-1同源序列比对及进化树 Fig. 2 Homologous sequence alignment and phylogenetic tree A:IhGH5-1及其同源序列保守结构域比对The conserved domain of IhGH5-1 and homologous sequence alignment; B:IhGH5-1及其同源序列最大似然树 The maximum likelihood tree of IhGH5-1 and homologous sequence. |
以PDB注册结构1h1nB为模板进行同源建模后,以“Cartoon”模式,从氮末端(N-terminus)到碳末端(C-terminus)展示粗毛纤孔菌IhGH5-1三维结构(图 3A)。粗毛纤孔菌IhGH5-1含有7个α螺旋(图 3A A1~A7),4个β折叠(图 3A B1~B4)。三维比对表明粗毛纤孔菌IhGH5-1三维结构与其他真菌糖苷水解酶家族5蛋白空间结构相近(图 3B),进化关系与最大似然树分析相符(图 3C)。
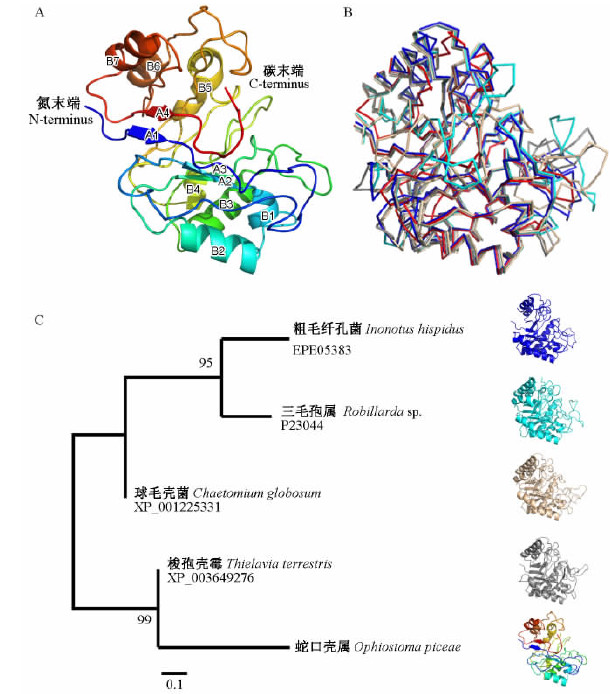 |
图 3 真菌糖苷水解酶家族5蛋白空间结构 Fig. 3 The protein spatial structure of fungal glycoside hydrolase family 5 A:IhGH5-1三维结构Three-dimensional structure of IhGH5-1; B:真菌糖苷水解酶家族5蛋白空间结构比对The protein structure alignment of fungal glycoside hydrolase family 5; C:真菌糖苷水解酶家族5蛋白进化分析The phylogenetic analysis of fungal glycoside hydrolase family 5 |
阳性IhGH5-1重组子经IPTG(浓度1 mmol·L-1)诱导4 h,样品经超声波破碎后进行菌液离心,收集上清液。经SDS-PAGE电泳检测,在约30 kD处检测到目的条带(图 4A),表明粗毛纤孔菌糖苷水解酶已成功表达。以羧甲基纤维素钠盐(CMC-Na)酶活性测定法测定粗酶液酶活,葡萄糖标准曲线符合线性相关(图 4B),65 ℃时该酶相对活性最高(图 4C)。
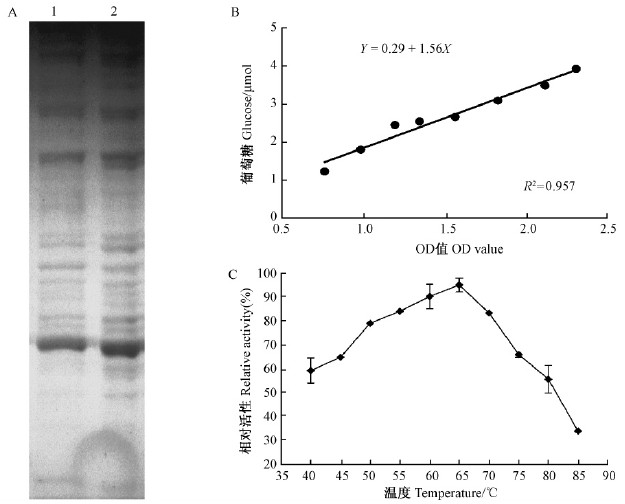 |
图 4 SDS-PAGE电泳及酶活测定 Fig. 4 Electrophoresis and enzymatic assays of SDS-PAGE A: SDS-PAGE电泳SDS-PAGE electrophoresis; B: 葡萄糖标准曲线Standard curve of glucose; C: 不同温度条件下相对酶活Relative activity under different temperature conditions. |
纤维素是地球上含量最丰富、分布最广、合成量最高的可再生碳水化合物资源,占植物干质量的1/3~1/2,是植物细胞壁的主要成分(Wilson,2011)。由于纤维素是大分子有机物,短期内不易被降解,因此,难以直接提高土壤肥力,影响田间耕作等农业生产。长期以来,焚烧秸秆等农作物副产品,不但造成了环境污染,同时也是有机资源的浪费(Guo et al.,2008)。我国20%植物纤维素成为不可回收的垃圾。纤维素酶可将纤维素这种宝贵的资源水解为葡萄糖,进而生产乙醇、丁醇、丙酮等有机燃料和化工原料(Petre et al.,1999),在能源、纺织、食品、洗涤剂、饲料加工和造纸等领域的应用前景广阔(李慧蓉,2005)。纤维素酶是可以将纤维素降解为葡萄糖的多组分酶系的总称,能水解天然纤维素的纤维素酶都是复杂的多酶体系。纤维素酶依功能不同可分为3大类:葡萄糖内切酶(endo-1,4-β-D,glucanase,E.C 3.2.1.4,来自真菌简称EG,来自细菌简称Len)、葡萄糖外切酶(exo-1,4-β-D,glucanase,E.C 3.2.1.91)和β-葡萄糖苷酶(β-1,4-glucanase.E.C 3.2.1.21,简称BG)。
纤维素酶在能源、食品、饲料加、纺织、洗涤剂、造纸等众多领域有着十分广阔的应用前景,此外利用生物质纤维素,制取乙醇燃料也是目前的研究热点,因此,筛选和驯化出能生产高活性纤维素酶的菌株势在必行。由于白腐菌既可以降解木质素又可以降解纤维素,因此在木质纤维素类物质降解生产中具有重要地位。目前,已经从黄孢原毛平革菌(Phanerochaete chrysosporium)、侧耳菌(Pleurotus sp.)等白腐菌中得到了能够降解纤维素的纤维素酶(Ichiro et al.,2012);本研究从另一种白腐菌——粗毛纤孔菌中克隆到了糖苷水解酶家族5基因,并进行了蛋白质结构、原核表达和活性分析,为进一步研究以便大规模的工业化应用做了铺垫。
| [1] |
郭春宣, 王峰, 董爱荣. 2010. 粗毛纤孔菌形态学鉴定及ITS序列系统发育分析.中国农学通报, 26 (3): 142-145. (Guo C X, Wang F, Dong A R. 2010. Diagnosis and ITS phylogenetic analysis of Inonotus hispidus. Chinese Agricultural Science Bulletin, 26 (3): 142-145[in Chinese]).(  1) 1)
|
| [2] |
李慧蓉. 2005. 白腐真菌生物学和生物技术.北京:化学工业出版社. (Li H R. 2005. White rot fungi biology and biotechnology. Beijing: Chemical Industry Press.[in Chinese])(  1) 1)
|
| [3] |
Aggelis G, Ehaliotis C, Nerud F, et al. 2002. Evaluation of white-rot fungi for detoxification and decolorization of effluents from the green olive debittering process. Applied Microbiology and Biotechnology, 59(2/3): 353-360.( 1) 1)
|
| [4] |
Guo R, Ding M, Zhang S L, et al. 2008. Molecular cloning and characterization of two novel cellulase genes from the mollusc Ampullaria crossean. Journal of Comparative Physiology B, 178(2): 209-215.( 1) 1)
|
| [5] |
Henrissat B. 1991. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J, 280: 309-316. ( 1) 1)
|
| [6] |
Ichiro K, Yoshiyuki H, Toshio M, et al. 2012. Direct ethanol production from cellulosic materials by the hypersaline-tolerant white-rot fungus Phlebia sp. MG-60. Bioresource Technology, 12: 137-142.( 1) 1)
|
| [7] |
Muthezhilan R, Ashok R, Jayalakshmi S. 2007. Production and optimization of thermostable alkaline xylanase by Penicillium oxalicum in solid state fermentation. African Journal of Microbiology Research, 1(2): 20-28.( 1) 1)
|
| [8] |
Nicol P, Gill R, Fosu-Nyarko J, et al. 2012. de novo analysis and functional classification of the transcriptome of the root lesion nematode, Pratylenchus thornei, after 454 GS FLX sequencing. International journal for parasitology, 42(3): 225-237. ( 1) 1)
|
| [9] |
O'Leary J M, Radcliffe C M, Willis A C, et al. 2004. Identification and removal of O-linked and non-covalently linked sugars from recombinant protein produced using Pichia pastoris. Protein Expression and Purification, 38(2): 217-227.( 1) 1)
|
| [10] |
Petre M, Zarnea G, Adrian P, et al. 1999. Biodegradation and bioconversion of cellulose wastes using bacterial and fungal cells immobilized in radiopolymerized hydrogels. Resources, Conservation and recycling, 27(4): 309-332.( 1) 1)
|
| [11] |
Rubini M R, Dillon A J P, Kyaw C M, et al. 2010. Cloning, characterization and heterologous expression of the first Penicillium echinulatum cellulase gene. Journal of Applied Microbiology, 108(4): 1187-1198.( 1) 1)
|
| [12] |
Robson L M, Chambliss G H. 1989. Cellulases of bacterial origin. Enzyme and Microbial Technology, 11(10): 626-644.( 1) 1)
|
| [13] |
Saddler J N. 1982. Screening of highly cellulolytic fungi and the action of their cellulase enzyme systems. Enzyme and Microbial Technology, 4(6): 414-418.( 1) 1)
|
| [14] |
Wilson D B. 2011. Microbial diversity of cellulose hydrolysis. Current Opinion in Microbiology, 14(3): 259-263.( 1) 1)
|
| [15] |
Wood T M, McCRAE S I, Bhat K M. 1989. The mechanism of fungal cellulase action. Synergism between enzyme components of Penicillium pinophilum cellulase in solubilizing hydrogen bond-ordered cellulose. Biochem J, 260: 37-43.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

