文章信息
- 李朝婵, 乙引, 全文选, 田红红
- Li Chaochan, Yi Yin, Quan Wenxuan, Tian Honghong
- 野生高山杜鹃群落林内自然挥发的化感成分
- The Natural Volatile Components of Allelochemicals in the Wild Alpine Rhododendron Community
- 林业科学, 2015, 51(12): 35-44
- Scientia Silvae Sinicae, 2015, 51(12): 35-44.
- DOI: 10.11707/j.1001-7488.20151205
-
文章历史
- 收稿日期:2015-06-30
- 修回日期:2015-10-13
-
作者相关文章
2. 贵州师范大学贵州省植物生理与发育调控重点实验室, 贵阳 550001
2. Guizhou Provincial Key Laboratory of Plant Physiology and Developmental Regulation, Guizhou Normal University Guiyang 550001
植物化感作用在植物生态系统中起着重要的作用,特别是在林业可持续发展中具有重要意义。化感作用又称为他感、异株相克作用,最早是由奥地利的Molish教授于1937年提出(Willis,2007),后美国科学家Rice将其定义为“一种植物通过它产生并释放于环境的生化物质对另一植物产生直接或间接的毒害作用”(Rice,1974; 1984),是植物通过向环境中释放化学物质,而对周围植物(包括微生物)产生直接或间接的作用(An et al.,2003)。植物通过化感作用对其他植物(包括同一物种的其他个体)产生作用,从而增强本物种对其他物种的竞争力或自身对其他个体的竞争力,抑制生境内的其他植物的种子萌芽率、幼苗的正常生长,具有调节种群结构(Emden,1995; Viles et al.,1996; Inderjit et al.,2000)、抵御环境胁迫(Loreto et al.,2004; Tang et al.,1995)和作为重要的化学信息传递物质的作用(Pophof et al.,2005; Theis et al.,2003)。化感作用已被证明在森林中发挥着至关重要的作用,为森林更新模式提供了新的解释。黑核桃(Juglans nigra)可以产生化感物质胡桃醌,从而使其他植物无法生长(Jose,2002)。桉树(Eucalyptus)的落叶和根系分泌物对一些植物及土壤微生物具有化感作用(Sasikumar et al.,2001; 王小雪等,2011)。Muller等(1964)通过对南加州海岸灌木释放的挥发物质的研究,从而揭示了挥发性化感物质在化感作用中的价值。目前,国内外针对杜鹃属(Rhododendron)植物化感的研究较少。杜鹃属植物群落中存在明显的化感作用,杜鹃属植物生长的土地如果被用作农田,作物生长将受到严重抑制(Rice,1983)。有研究表明,因为杜鹃属植物产生的化学物质抑制了其他物种的发芽和生长,同时杜鹃花吸引并垄断传粉的昆虫,对附近的其他物种产生影响。杜鹃属植物入侵的地方,该地区的生物多样性下降,包括植物物种、昆虫和其他无脊椎动物的损失。Day等(1988)研究认为,阿巴拉契亚山脉南部的极大杜鹃(R. maximum)林下化感物质抑制幼苗和冠层生长; Nilsen等(1999)研究了极大杜鹃枯落物层浸提液中的化感物质对种子萌发、幼苗根生长的抑制作用。Chou等(2010)和Wang等(2013)对台湾杜鹃(R. formosanum)的化感作用进行了深入研究和探讨,重点研究了微生物在台湾杜鹃化感效应中的作用。周艳等(2015)对枯落叶对种子萌发和幼苗生长的影响进行研究,确定了贵州百里杜鹃景区迷人杜鹃(R. agastum)的化感自毒作用。本研究从贵州百里杜鹃景区马缨杜鹃(R. delavayi)、迷人杜鹃和露珠杜鹃(R. irroratum)3大群落状况入手,分析探讨了3个高山杜鹃群落林内气体的化感物质成分,为百里杜鹃林区天然更新提供科学依据。
1 材料与方法 1.1 研究区概况百里杜鹃国家森林公园成立于1993年,是贵州最早获批成立的国家级森林公园,也是省级自然保护区。公园的主体植被——杜鹃林带,是迄今为止发现的,在地球同一纬度、中低海拔地区面积最大的天然杜鹃林。无论从科学考察研究还是从旅游开发观光角度而言,百里杜鹃林区都具有极高的价值。海拔在1 060~2 200 m,地处东经105°52′—106°03′,北纬27°10′—27°20′。年平均温度11.8 ℃,年平均积温400 ℃,最冷月(1月)均温为5 ℃,最热月(7月)均温21 ℃,极端最高温度32 ℃,极端最低温度为-9.3 ℃。年降水量1 000~1 100 mm,全年雨日多达220.5天,春夏降水量占70%,水热同季。阴天多,晴天少,云雾多,全年日照时数仅1 335.5 h,林区年平均相对湿度为84%,4月份平均相对湿度最小,只有79%,12月份平均相对湿度为89%,表现出冬湿春干。森林公园内形成了以迷人杜鹃、马缨杜鹃、露珠杜鹃等为优势树种的森林群落,林内伴生植物主要为乔木树种的幼苗及灌木、草本。
1.2 研究方法 1.2.1 研究样地调查采用常规群落调查法对百里杜鹃林区内露珠杜鹃、迷人杜鹃、马缨杜鹃三大主要纯林群落进行生态学调查,记录样地面积、经纬度、海拔、坡向、坡位、坡度、郁闭度、伴生树种等因子并取样。调查样地选在普底乡醉九牛和黑石头,土壤类型为硅质黄壤和煤层土,调查时间为2014年12月—2015年1月的种子成熟散布期,在10 m×10 m样方内进行每木调查,记录物种名、株高、基径、冠幅、株数等,藤本及草本层的调查包括物种名、数量等。针对群落更新的调查包括林下幼苗数量、高度及1 m高度以下的萌枝数等。样地情况见表 1。
|
|
本研究采用α多样性测度(钱迎倩等,1994),共选取了3类,通过BIO-DAP程序进行相关多样性指数计算,包括:物种优势度指数、物种多样性指数和物种均匀度指数,计算公式如下:
Berger-Parker优势度指数: D=Nmax/N。
式中: Nmax为优势种的个体数,N为全部物种的个体数。Simpson多样性指数: D=1-ΣPi2。
式中: Pi为第i个物种的个体数占群落中总个体数的比例。Pielou均匀度指数: E=H/lnS。
式中: H为实际观察的物种多样性指数,S为群落中的总物种数。 1.2.2 仪器与采样采用开放式采样、集气袋采集法。选择在天气晴好无风的一天内进行,试验中将大气采样仪呈品字型放置于林内,离地高度0.3 m,采样仪两两间隔3 m 以上。11: 00—14: 00,3 个重复,采样时间60 min,气体流量为200 mL·min-1。采样仪为QC-1 型大气采样仪(北京市劳动保护科学研究所生产),采样袋为铝箔采样袋。插入PDMS纤维头的手动进样器至采样袋中,在室温萃取保持30 min 取出,快速将SPME针管插入气相色谱仪进样口,热解析3 min推手柄杆,伸出纤维头,热脱附样品进色谱柱。
1.2.3 GC/MS 测定条件Agilent7890A/5975C气相色谱-质谱联用仪(美国安捷伦公司); 手动固相微萃取装置(美国 Supelco 公司),萃取纤维头为70 μm PDMS。
色谱柱为 Agilent HP-5MS毛细管柱(30 m×0.25 mm×0.25 μm),柱温40 ℃(保留2 min),以4 ℃·min-1 升温至230 ℃,气化室温度250 ℃; 载气为高纯He(99.999%),柱前压7.62 psi,载气流量1.0 mL·min-1,不分流进样,延迟时间为1 min。离子源为EI源,离子源温度230 ℃,四极杆温度150 ℃,电子能量70 eV,发射电流34.6 μA,倍增器电压1 052 V,接口温度280 ℃,质量范围20~450 amu。
1.2.4 数据计算及处理对总离子流图中的各峰经RTLPEST3.L和NIST05.L进行检索,并结合图谱分析,鉴定各种化学成分,按峰面积归一化法计算各峰的相对百分含量。
2 结果与分析 2.1 3个杜鹃群落乔木层生物多样性特征由表 2可知,3个群落的优势种的优势度顺序为迷人杜鹃>露珠杜鹃>马缨杜鹃,多样性指数顺序为露珠杜鹃>迷人杜鹃>马缨杜鹃,各群落均匀度指数为0.66~0.77。马缨杜鹃优势群落具有最低的优势度和物种多样性,但是具有最高的均匀度; 露珠杜鹃优势群落具有最高的物种多样性; 迷人杜鹃群落虽然有最高的优势度,可均匀度却是最低的,这可能是由于不同的植物之间出现的种间和种内竞争及密度制约因素,导致竞争性弱的物种比例减少,从而引起群落的均匀度降低(钟彦龙等,2009)。
|
|
3种高山杜鹃林内的其他物种,如青冈(Cyclobalanopsis glauca)和白栎(Quercus fabri)有幼苗出现,原因可能是它们的种子大、传布距离不远、种子内含物质丰富,郁闭的环境有利于栎类种子的发芽生长,其天然有性繁殖更新相对容易。乔木层的阔叶树种将对杜鹃群落的天然更新和持续稳定造成一定程度上的威胁(李苇洁,2006)。
2.2 3个杜鹃群落林内气体固相微萃取的化感物质3个杜鹃群落林内气体固相微萃取的气相色谱总离子流见图 1,基于一系列前人研究鉴定有化感效应的次级代谢产物。露珠杜鹃林内气体中一共有17种具有化感潜力的化合物,包括烷烃类、萜烯类、酮类和酚类(表 3); 其中相对含量最高的成分是2,2,4,6,6-五甲基庚烷(30.81%),较高的成分是5-乙基-2,2,3-三甲基庚烷(19.87%)、癸烷(11.71%)和十二烷(10.00%)。迷人杜鹃林内气体中一共有13种具有化感潜力的化合物,包括烷烃类、萜烯类、酯类和有机酸类(表 4); 其中,相对含量最高的成分是2,5-二甲基-2,5-过氧化二氢己烷(20.23%),较高的成分是5-乙基-2,2,3-三甲基正庚烷(18.13%)、十二烷(14.30%)和2,2,7,7-四甲基辛烷(10.20%)。马缨杜鹃林内气体化感物质有11种,包括烷烃类、酚类、酯类和有机酸(表 5); 其中相对含量最高的成分是癸烷(22.92%),较高的成分是2,2,4,6,6-五甲基庚烷(18.11%)、γ-丁内酯(16.82%)和棕榈酸(11.65%)。
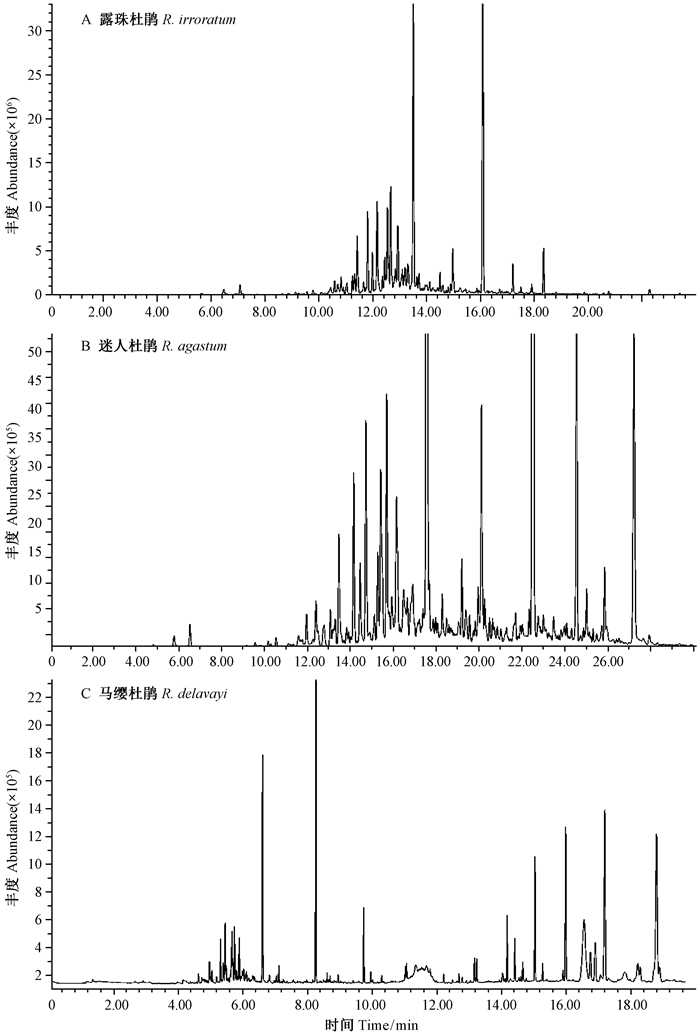 |
图 1 3种高山杜鹃群落林内挥发性气体成分GC/MS总离子流 Fig. 1 The total ions chromatograms of volatile gas components from three alpine Rhododendron communities by GC/MS |
|
|
|
|
|
|
3个杜鹃群落林内气体化感成分中,均以烷烃类及其衍生物为主,这些化感物质中的大多数已被确认为具有明显的化感作用(Rice,1984)。酯类物质具有明显的抑制植物幼苗生长的作用(崔翠等,2013),有机酸对土壤微生物、真菌数量及酶活性有显著影响(周宝利等,2010; 冀晓青等,2012)。
3 结论本文基于群落调查和气相色谱质谱联用技术分析了贵州西北部野生高山杜鹃群落生物多样性与林内挥发性化感成分。对野生高山杜鹃群落天然更新障碍进行深入探讨和分析,得出以下结论:
1)高山杜鹃是百里杜鹃国家森林公园生态系统的主体,露珠杜鹃、迷人杜鹃和马缨杜鹃是云贵高原亚热带高山常绿阔叶林组成中重要的建群种和优势种,3个杜鹃群落均具有较高的优势度和均匀度,但是多样性指数较低,存在天然更新障碍。
2)高山杜鹃种子库形成期的群落林内自然挥发途径的化感物质种类丰富,通过气相色谱质谱鉴定,主要有烷烃类、萜烯类、酮类、酚类、有机酸类、酯类等成分,挥发性化感物质的毒害作用是高山杜鹃种子萌发、幼苗建立困难的原因之一。加强人为干预促进高山杜鹃实生苗更新是改善群落结构的必要和有效措施。
4 讨论 4.1 化感作用与群落生物多样性化感作用是影响森林天然更新的重要因子,对于群落更新失败往往从林内光照、水分和养分条件考虑较多,对生化因子的作用估计不足(翟明普等,1993)。本研究中,3个杜鹃群落均具有较高的优势度和均匀度,但是多样性指数较低,这与实地调查和前人的研究成果较为一致(陈训等,2003; 刘应珍等,2008; 刘纪岗等,2012)。
本研究SPME-GC/MS结果显示,3个高山杜鹃纯林内气体中含有丰富的化感物质成分,这可能与杜鹃属植物的组织中含有显著数量的苯酚和其他潜在的有毒化感物质有关(Yang et al.,1978; Wang et al.,1981)。一旦杜鹃侵入一个地区,原生植物的生存将受到威胁,同时杜鹃冠层的水平增长将持续下去,其他植物幼苗很难在林冠下建立,直至杜鹃完全占主导地位。杜鹃植物组织含有的化感物质导致林下植物种类减少,一旦原生植物消失,将造成群落生物多样性降低和杜鹃花区土壤的贫瘠(温美娟等,2009)。
作者在实地踏查中,仅在黑石头样方外围裸地发现2株3~5年生马缨杜鹃幼苗,且均生长于裸露的人为干扰过的路边,林下未发现实生的高山杜鹃幼树(图 2)。通过对高山杜鹃植株1 m以下萌发枝条进行调查,林下萌枝率极低(数据未发表)。本研究中的3个样地均具有层次结构简单的现象存在,群落的郁闭度达到90%以上,在如此郁闭的条件下,高山杜鹃的种子天然更新过程很难完成。幼苗的建立过程通常是植物生活史中最脆弱的阶段(Nakashizuka,2001),很多物种,如连香树(Cercidiphyllum japonicum)、薄叶润楠(Machilus leptophylla)等,其实生苗均需要开敞地的存在(Tang et al.,2002; Sakio et al.,2002)。这可能也是高山杜鹃种子天然更新的瓶颈,萌根从而成为主要的更新模式,并成为其早期种群得以建立的关键。
 |
图 2 3种高山杜鹃群落林下植被状况 Fig. 2 The understory vegetation of three alpine Rhododendron communities A.露珠杜鹃R.irroratum; B.迷人杜鹃 R.agastum; C.马缨杜鹃R.delavayi. |
在研究区百里杜鹃国家级森林公园内,高山杜鹃群落内有性繁殖及天然更新均出现严重的障碍,幼龄种群缺失严重,形成巨大的可持续发展障碍,繁殖障碍将导致植物种群的遗传多样性降低和生产力下降(高贤明等,2003)。因此,在人类对高山杜鹃群落生境及周边地貌的改变难以逆转的情况下,如何通过深入研究化感作用对群落天然更新的影响,采取人为措施降低化感作用的干扰、促进高山杜鹃群落内的实生苗更新,是一个亟待研究的课题。
4.2 化感物质的测定与自然挥发途径本研究在高山杜鹃种子成熟散布期进行近地面气体采样,研究种子库形成期的群落林内自然挥发途径的化感物质。除烷烃类,露珠杜鹃群落中有萜烯类、酮类、酚类,在迷人杜鹃群落中有萜烯类、有机酸类、酯类,马缨杜鹃群落中有有机酸类、酯类,酚类。这些物质均属于化感类物质或具有抑制作用,其中酯类物质等在较小浓度下即具有较强的化感作用潜力(Macias,1995)。由于化感作用在自然界存在的复杂性,尽可能模仿自然界的条件可以使研究结果更具有生态学意义(曾任森,1999)。本研究采用的固相微萃取(SPME)是一种新型的无溶剂样品预处理技术,可以提取不同类型的有机分析物(Spietelun et al.,2010),该技术集采样、萃取、浓缩于一体,所需样品量少,具有简便快速、无污染等特点,重现性和精密度都非常好(Gyorgy et al.,2004; Ouyang et al.,2006)。
在群落中优势树种的化感作用方面,研究认为优势树种产生的化感物质不断积累,从而影响其林下植被的种子发芽、生长(Souto et al.,1994; Lodhi,1978),林下荒芜的主要原因是化感作用导致(Lodhi,1976)。化感物质的释放方式是研究化感作用的关键,释放方式取决于其化学性质(Mandave,1985)。美国红栎(Quercus rubra)树叶排放单萜和异戊二烯(Loreto et al.,1990; Sharkey et al.,1991),辐射松(Pinus radiata)枯落物产生的气体高浓度时抑制黑麦草(Lolium perenne)种子发芽(Lill et al.,1979)。高山杜鹃优势群落内植物每天都在释放挥发性气体,虽然每种挥发性气体的相对含量较小,但一些被认定为化感物质的成分,比如烷烃类、萜烯类、有机酸类、酚类、酮类、酯类等对种子萌发和幼苗生长都存在较强的抑制作用。这些化感物质在自然状态下多大浓度下才会产生化感效应,化感物质如何协同生境条件(土壤、光照、温度、水分等)发挥作用,有待于进一步的研究与分析。
| [1] |
陈训,巫华美. 2003.中国贵州杜鹃花.贵阳:贵州科技出版社. (Chen X, Wu H M. 2003. China Guizhou Azalea. Guiyang:Guizhou Science and Technology Press.[in Chinese])(  1) 1)
|
| [2] |
崔翠,蔡靖,张硕新. 2013.核桃根系分泌物化感物质的分离与鉴定.林业科学, 49(2):54-60. (Cui C, Cai J, Zhang S X. 2013. Isolation and identification of the allelochemicals in walnut (Juglans regia) root exudates. Scientia Silvae Sinicae, 49(2):54-60[in Chinese]).(  1) 1)
|
| [3] |
高贤明,杜晓军,王中磊. 2003.北京东灵山区两种生境条件下辽东栎幼苗补充与建立的比较.植物生态学报, 27(3):404-411. (Gao X M, Du X J,Wang Z L. 2003. Comparison of seedling recruitment and establishment of Quercus wutaishanica in two habitats in Donglin mountainous area, Beijing. Chinese Journal of Plant Ecology, 27(3):404-411[in Chinese]).(  1) 1)
|
| [4] |
冀晓青,韩笑天,杨佰娟,等. 2012.强壮前沟藻化感物质分析.生态学报, 32(6):1745-1754. (Ji X Q, Han X T, Yang B J, et al. 2012. Analysis on allelochemicals in the cell-free filtrates of Amphidinium carterae. Acta Ecologica Sinica, 32(6):1745-1754[in Chinese]).(  1) 1)
|
| [5] |
李苇洁. 2006.马缨杜鹃生态学特性与繁殖技术研究.贵阳:贵州大学硕士学位论文. (Li W J. 2006. Studies on the ecology characteristics and propagation techniques of Rhododendron delavayi Franch. Guiyang:MS thesis of Guizhou University[in Chinese]).(  1) 1)
|
| [6] |
刘纪岗,谢元贵,杨小庆. 2012.贵州百里杜鹃大草原景区迷人杜鹃群落特征研究.广东农业科学, 39(9):46-48. (Liu J G, Xie Y G, Yang X Q. 2012. Study on community characteristics of Rhododendron agastum in the prairie scenic area of Baili Rhododendron protection zone in Guizhou. Guangdong Agricultural Sciences, 39(9):46-48[in Chinese]).(  1) 1)
|
| [7] |
刘应珍,郭曼,邹天才,等. 2008.百里杜鹃三种杜鹃群落类型物种多样性调查分析. 2008年中国植物园学术年会论文集. (Liu Y Z, Guo M, Zou T C, et al. 2008. Investigation and analysis on species diversity of three Rhododendron communities in Hundred-mile Azalea. China Botanical Garden Annual Conference Proceedings 2008[in Chinese]).(  1) 1)
|
| [8] |
钱迎倩,马克平. 1994.生物多样性研究的原理与方法.北京:中国科学技术出版社. (Qian Y Q, Ma K P. 1994. Principles and methodologies of biodiversity studies. Beijing:China Science and Technology Press.[in Chinese])(  1) 1)
|
| [9] |
王小雪,刘芸,邵呈龙,等. 2011. 5种经济植物对幼龄尾巨桉叶片提取液的化感敏感性.林业科学, 47(11):188-193. (Wang X X, Liu Y, Shao C L, et al. 2011. Allelopathic sensitivity of five economic species to aqueous leaf extract of Eucalyptus urophylla×E. grandis with different ages. Scientia Silvae Sinicae, 47(11):188-193[in Chinese]).(  1) 1)
|
| [10] |
温美娟. 2009.合欢山地区台湾高山杜鹃之族群遗传与玉山杜鹃相生相克作用.台湾:中国医药大学硕士学位论文. (Wen M J. 2009. Population genetics of Rhododendron rubropilosum Hayata var. taiwanalpinum and allelopathy of Rhododendron pseudochrysanthum Hayata at Mt. Hohuan. Taiwan:MS thesis of China Medical University.[in Chinese])(  1) 1)
|
| [11] |
曾任森. 1999.化感作用研究中的生物测定方法综述.应用生态学报, 10(1):123-126. (Zeng R S.1999. Review on bioassay methods for allelopathy research. Chinese Journal of Applied Ecology, 10(1):123-126[in Chinese]).(  1) 1)
|
| [12] |
翟明普,贾黎明. 1993.森林植物间的他感作用.北京林业大学学报, 15(3):138-147. (Zhai M P, Jia L M.1993. Allelopathy of forest plants. Journal of Beijing Forestry University, 15(3):138-147[in Chinese]).(  1) 1)
|
| [13] |
张爱加,袁照年,陈冬梅,等. 2010.甘蔗根际土壤化感潜力评价及其化感物质分析.中国生态农业学报, 18(5):1013-1017. (Zhang A J, Yuan Z N, Chen D M, et al. 2010. Analysis of allelochemicals and allelopathic effect of rhizosphere soils of newly planted and ratoon sugarcane (Saccharum officenarum L.). Chinese Journal of Eco-Agriculture, 18(5):1013-1017[in Chinese]). |
| [14] |
钟彦龙,吕光辉,傅德平. 2009.克拉玛依植物群落物种多样性研究.干旱区资源与环境, 23(6):127-131. (Zhong Y L, Lü G H, Fu D P. 2009. Species diversity of the plant community in Karamay. Journal of Arid Land Resources and Environment, 23(6):127-131[in Chinese]).(  1) 1)
|
| [15] |
周宝利,韩琳,尹玉玲,等. 2010.化感物质棕榈酸对茄子根际土壤微生物组成及微生物量的影响.沈阳农业大学学报, 41(3):275-278. (Zhou B L, Han L, Yin Y L, et al. 2010. Effects of allelochemicals hexadecanoic acid on soil microbial composition and biomass in rhizosphere of eggplant. Journal of Shenyang Agricultural University, 41(3):275-278[in Chinese]).(  1) 1)
|
| [16] |
周艳,陈训,韦小丽,等. 2015.凋落物对迷人杜鹃幼苗更新和种子萌发的影响.林业科学, 51(3):65-74. (Zhou Y, Chen X, Wei X L, et al. 2015. Effects of litter on the seedling regeneration and seed germination of Rhododendron agastum. Scientia Silvae Sinicae, 51(3):65-74[in Chinese]).(  1) 1)
|
| [17] |
An M, Liu D L, Johnson I R, et al. 2003. Mathematical modelling of allelopathy:Ⅱ. The dynamics of allelochemicals from living plants in the environment. Ecological Modelling, 161(2):53-66.( 1) 1)
|
| [18] |
Chou S C, Huang C H, Hsu T W, et al. 2010. Allelopathic potential of Rhododendron formosanum Hemsl in Taiwan. Allelopathy Journal, 25(1):73-91.( 1) 1)
|
| [19] |
Day F P, Phillips D L, Monk C D. 1988. Forest communities and patterns//Swank W T, Crossley Jr D A. Forest hydrology and ecology at Coweeta, New York, USA:Springer-Verlag,141-149.( 1) 1)
|
| [20] |
Emden H F V. 1995. Host plant-aphidophaga interactions. Agriculture Ecosystems and Environment, 52(1):3-11.( 1) 1)
|
| [21] |
Gyorgy V, Karoly V. 2004. Solid-phase microextraction:A powerful sample preparation tool prior to mass spectrometric analysis. Journal of Mass Spectrometry, 39(3):233-254.( 1) 1)
|
| [22] |
Inderjit, Weston L A. 2000. Are laboratory bioassys for allellopathy suitable prediction of field responses?. Journal of Chemical Ecology, 26(9):2111-2118.( 1) 1)
|
| [23] |
Jose S. 2002. Black walnut allelopathy:current state of the science//Mallik A U, Inderjit. Chemical ecology of plants:Allelopathy in aquatic and terrestrial ecosystems. Basel, Switzerland:Birkhauser Verlag.( 1) 1)
|
| [24] |
Lill R E, Mcwha J A, Cole A L J. 1979. The influence of volatile substances from incubated litter of Pinus radiata on seed generation. Annals of Botany, 43(1):81-85.( 1) 1)
|
| [25] |
Lodhi M A K. 1976. Role of allelopathy as expressed by dominating trees in a lowland forest in controlling the productivity and pattern of herbaceous growth. American Journal of Botany, 63(1):1-8.( 1) 1)
|
| [26] |
Lodhi M A K. 1978. Allelopathic effects of decaying litter of dominant trees and their associated soil in a lowland forest community. American Journal of Botany, 65(3):340-344.( 1) 1)
|
| [27] |
Loreto F, Sharkey T D.1990. A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta, 182(4):523-531.( 1) 1)
|
| [28] |
Loreto F, Pinelli P, Manes F, et al. 2004. Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol, 24(4):361-367.( 1) 1)
|
| [29] |
Macias F A. 1995. Allelopathy in search for natural herbicide model. ACS Symposium Series, 582:310-329.( 1) 1)
|
| [30] |
Mandave N B. 1985. Chemistry and biology of allelopathic agents//Thompson A C. The chemistry of allelopathy. American Chemical Series 268. Washington, D. C.:American Chemical Society, 33-54.( 1) 1)
|
| [31] |
Muller C H, Muller W H, Haines B L. 1964. Volatile growth inhibitors production by shrubs. Science, 143(3605):471-473.( 1) 1)
|
| [32] |
Nakashizuka T. 2001. Species coexistence intemperate, mixed deciduous forests. Trends in Ecology and Evolution, 16(4):205-210.( 1) 1)
|
| [33] |
Nilsen E T, Walker J F, Miller O K, et al. 1999. Inhibition of seedling survival under Rhododendron maximum (Ericaceae):Could allelopathy be a cause? American Journal of Botany, 86(11):1597-1605.( 1) 1)
|
| [34] |
Ouyang G, Pawliszyn J. 2006. Recent developments in SPME for on-site analysis and monitoring. Tack Trends in Analytical Chemistry, 25(7):692-703.( 1) 1)
|
| [35] |
Pophof B, Stange G, Abrell L. 2005. Volatile organic compounds assignals in a plant-herbivore system:Electrophysiological responses in olfactory sensilla of the moth Cactoblastis cactorum. Chemical Senses, 30(1):51-68.( 1) 1)
|
| [36] |
Rice E L. 1983. Pest control with nature's chemicals. Norman OK:University of Oklahoma Press.( 1) 1)
|
| [37] |
Rice E L. 1984. Allelopathy. 2nd ed. Orlando Florida:Academic Press Inc, 23-28.( 2) 2)
|
| [38] |
Rice E L. 1974. Allelopathy. New York:Academic Press, 15-19.( 1) 1)
|
| [39] |
Sakio H, Kubo M, Shimano K, et al. 2002. Coexistence of three canopy tree species in a riparian forest in the Chichibu Mountains, central Japan. Folia Geobotanica, 37(1):45-61.( 1) 1)
|
| [40] |
Sasikumar K, Vijayalakshmi C, Parthiban K T. 2001. Allelopathic effects of four Eucalyptus species on redgram (Cajanus cajan L.). Journal of Tropical Agriculture, 39(2):134-138.( 1) 1)
|
| [41] |
Sharkey T D, Loreto F, Delwiche C F. 1991. The biochemistry of isoprene emission from leaves during photosynthesis//Sharkey T D, Holland E A, Mooney H A. Trace gas emissions from plants. San Diego:Academic Press, 153-184.( 1) 1)
|
| [42] |
Souto X C, Gonzales L, Reigosa M J. 1994. Comparative analysis of allelopathic effects produced by four forestry species during decomposition process in their soils in Galicia (NW Spain). Journal of Chemical Ecology, 20(11):3005-3015.( 1) 1)
|
| [43] |
Spietelun A, Pilarczyk M, Kloskowski A, et al. 2010. ChemInform abstract:Current trends in solid-phase microextraction (SPME) fibre coatings. Chemical Society Reviews, 39(5):4524-4537.( 1) 1)
|
| [44] |
Tang C Q, Ohsawa M. 2002. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobotanica, 37(1):93-106.( 1) 1)
|
| [45] |
Tang C S,Cai W F,Kohl K,et al. 1995. Plant stress and allelopathy. American Chemical Symposium Series, 582:142-157.( 1) 1)
|
| [46] |
Theis N, Lerdau M. 2003. The evolution of function in plant secondary metabolites. International Journal of Plant Sciences, 164(3):93-102.( 1) 1)
|
| [47] |
Viles A L, Reese R N. 1996. Allelopathic potential of Echinacea angustifolia D.C. Environmental and Experimental Botany, 36(1):39-43.( 1) 1)
|
| [48] |
Wang C M, Li T C, Jhan Y L, et al. 2013. The impact of microbial biotransformation of catechin in enhancing the allelopathic effects of Rhododendron formosanum. PLoS One, 8(12):e85162.( 1) 1)
|
| [49] |
Wang S X, Yang H R. 1981. Chemical investigation of Rhododendron habanshanense Ⅱ:The isolation and identification of (+)-catechin, hyperin, and toxicant components. Acta Botanica Sinica, 23:47-51.( 1) 1)
|
| [50] |
Willis R J. 2007. The history of allelopathy. Springer, USA:3.( 1) 1)
|
| [51] |
Yang H R, Wang S X.1978.Chemical studies of Rhododendron habanshanense I:The isolation and identification of four phenolic components. Acta Botanica Sinica, 20:355-359.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

