文章信息
- 崔朝宇, 王园秀, 蒋军喜, 欧阳慧, 秦双林, 黄婷
- Cui Chaoyu, Wang Yuanxiu, Jiang Junxi, Ouyang Hui, Qin Shuanglin, Huang Ting
- 美国红枫褐斑病病原菌鉴定
- Identification of the Pathogen Causing Brown Spot Disease of Acer rubrum ‘October Glory’
- 林业科学, 2015, 51(10): 142-147
- Scientia Silvae Sinicae, 2015, 51(10): 142-147.
- DOI: 10.11707/j.1001-7488.20151018
-
文章历史
- 收稿日期:2014-07-08
- 修回日期:2014-08-22
-
作者相关文章
2. 江西农业大学生物科学与工程学院 南昌 330045
2. College of Bioscience and Bioengineering, Jiangxi Agricultural University Nanchang 330045
美国红枫(Acer rubrum),又名红花槭,原产于美国北部及加拿大南部,其树型优美,秋季红叶纯正,叶色秀丽,是目前欧美地区最受欢迎的道路绿化美化树种之一(Abrams et al.,1998; 李晶等,2006)。我国自2000年从美国引入该树种以来,由于其适应性较强,很快在我国北方地区得到推广种植,现主要分布于我国北方的辽宁、山东等地,而在我国南方分布较少(张健等,2009; 任杰等,2013)。
近年来通过对美国红枫人工改良,培育出‘秋日火焰’(A.urbrum ‘Autumn Flame’)、‘十月辉煌’(A.rubrum ‘October Glory’)、‘夕阳红’(A.rubrum Red Sunset)等一系列观赏性表现突出的优异品种,其中园艺品种“十月辉煌”由于具有对土壤田间性质要求低、红叶期长、变色一致性强、耐寒性稍差等特点,特别适宜在我国南方冬季偏暖的地区栽培,在我国部分南方城市引种试验中已表现出良好的性状。目前,国内对于该树种的研究多集中于其繁殖方式、生物学特性及应用方面(冯立娟等,2009; 杨帆等,2013; 赵庆柱等,2014),而对其栽培过程中的病害问题研究较少。
2014年,江西省鄱阳县东郁园林公司从美国引进多个槭树科树种。调查发现,基地种植的美国红枫‘十月辉煌’植株叶片出现大量褐色枯斑,甚至整个叶片焦枯(本文称为褐斑病),个别病株病叶率达60%以上,这种病害不仅严重影响了‘十月辉煌’的生长,还破坏了该树种作为观赏植株的美观性。为了有效控制该病害,迫切需要查明其发病原因。为此,笔者在开展病害田间调查的基础上采集样品,对其进行病菌分离培养和鉴定。
1 材料与方法 1.1 病害田间调查及症状观察于2014年4—6月,对江西省鄱阳县东郁园林公司种植基地的美国红枫褐斑病发病时期、为害程度进行定期调查,对病害症状特征及变化进行观察记载。
1.2 病原菌的分离纯化先后3次赴基地随机采集30份不同发病阶段的病叶样品,采用PDA培养基按常规组织分离法进行病菌分离,培养待菌落达到适当大小后,取其边缘菌丝进行菌种纯化,获得的各分离物用PDA斜面培养基保存备用。
1.3 致病性测定采用离体叶片接种法进行致病性测定。将各分离物转接于PDA平板,于25 ℃黑暗条件下培养10天后获得大量产孢,配制浓度为1×106个·mL-1的孢子悬浮液,用经灭菌的滤纸片蘸取悬浮液对美国红枫健康叶片进行刺伤接种,接种叶片置于25 ℃保湿培养24 h,后将相对湿度调为90%继续培养。各处理重复3次,并以接种无菌水作为对照。逐日观察接种结果,并对接种病斑进行病菌再分离,以完成柯赫氏法则验证。
1.4 病原菌形态特征观察1)自然寄主上病菌形态观察 将田间采集发病的叶片用无菌水冲洗数次,于25 ℃条件下保湿培养3天,挑取典型病斑上的小黑点进行切片,并制成临时玻片,于光学显微镜下观察病菌的载孢体和分子孢子形态,并测量载孢体及分生孢子的大小。
2)PDA培养基上病菌形态观察 将供试菌株接种于PDA平板上,25℃黑暗条件下培养,逐日观察记录病菌菌落颜色、形状、气生菌丝的疏密程度等特征。产孢后,于显微镜下观察产孢结构及分生孢子形态,并测量分生孢子大小。根据病原菌在自然寄主和PDA培养基上的形态特征,参考对拟盘多毛孢种的特征描述进行种的鉴定(赵光材等,1995;陆家云,2001;韦继光等,2005)。
1.5 rDNA-ITS的PCR扩增与序列分析1)病原菌总DNA提取 将供试菌株转接于PDA平板上,于25℃活化培养4天后,挑取直径5 mm的菌丝块接种于PD培养液中,25 ℃、150 r·min-1振荡培养3~4天,收集菌体,采用CTAB法提取病菌基因组DNA。
2)rDNA-ITS序列扩增与分析 利用真菌核糖体基因转录间隔区(ITS)通用引物ITS1(5′-TCCGTAGG TGAACCTGCGG-3′)和ITS4(5′-TCCTCCGCTTATT GATATGC-3′)对病菌ITS和5.8S rDNA区段进行PCR扩增,PCR反应在25 μL体系中进行,依次加入以下试剂: 引物ITS1和ITS4各1 μL(10 μmol · L-1),2×Taq PCR MasterMix 12.5 μL,模板DNA 2 ng,用ddH2O补足25 μL,各组分混匀后按以下程序进行PCR扩增: 94℃预变性3 min; 94℃变性30 s,60℃退火30 s,72℃延伸1 min,共35个循环; 最后72℃延伸10 min。PCR产物交由上海生工生物工程有限公司进行序列测定。
对测得的供试菌株核糖体DNA-ITS区段的序列,运用GenBank核酸数据库(http://www.ncbi.nlm.nih.gov)中BLAST工具软件,在核酸序列数据库中搜索同源DNA序列并进行比较分析,根据其BLAST搜索和比较的结果,判断所获菌株的种类或其近缘种。并利用MegA 5.2软件中的邻位加入法(neighbor-joining,NJ)构建系统发育树。
2 结果与分析 2.1 发病调查及症状描述美国红枫褐斑病只感染叶片,一般老叶较新叶发病严重,在5月中旬开始零星发生,6月中下旬后进入发病盛期,此时重病株病叶率达60%以上。发病初期,在叶片表面形成浅褐色圆形小斑点,后逐渐扩展为圆形、椭圆形或不规则形大斑,病部中央黄褐色,边缘黑褐色,周围有黄色晕圈,后期病斑常联合成大斑造成叶片大面积枯死(图 1A),在多雨潮湿的条件下,在病斑上常形成黑色小点,即病原菌的分生孢子盘。
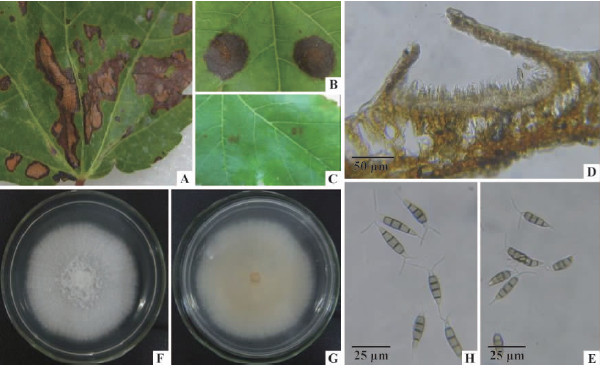 |
图 1 美国红枫褐斑病症状及病菌形态 Fig. 1 Symptoms of red maple brown spot disease and morphology of the pathogen of Pestalotiopsis microspora A: 田间发病症状Symptoms in the field; B-C: 接种7 天后叶片发病症状( B: 接种叶片; C: 对照叶片)Symptom of inoculated leaf( B: Leaf inoculated with P.microspora; C: Leaf inoculated with sterile water); D: 分生孢子盘Acervulus; E: 自然寄主上的分生孢子Conidia on host leaf; F-G: 小孢拟盘多毛孢在PDA 上25 ℃ 培养5 天后的菌落特征( F: 菌落正面图; G: 菌落背面图)Colony characteristics of P.microspora on PDA at 25 ℃ after 5 days( F: Front; G: Reverse); H: PDA 培养基上的分生孢子Conidia on PDA medium. |
通过对采集自基地的30个典型褐斑病病叶样品进行病菌分离,共分离获得25个真菌菌株,这些菌株经纯化后在PDA平板上培养性状表现一致。选择代表性菌株PM-1、PM-2作为供试菌株进行致病性测定,结果显示红枫叶片刺伤接种发病率为100%(6/6),叶片在接种3天后开始发病,从刺伤伤口处形成褐色小点,随后小褐点逐渐扩大成褐色病斑,在接种7天后病斑扩大至1~2 cm,此时叶片接种发病症状与田间植株自然发病症状相似(图 1B),对接种病斑进行病菌再分离,均分离到与原接种菌株培养性状一致的真菌; 而对照处理则未出现任何发病症状(图 1C),符合柯赫氏法则。
2.3 病原菌培养性状及形态在显微镜下观察,该病菌载孢体为盘状,散生,初埋生后突破表皮,直径108~205 μm,呈不规则开裂(图 1D); 分生孢子纺锤形,4个真隔膜,分割处缢缩不明显,共5个细胞,直或微弯,在自然寄主上大小(19.3~26.8)μm×(5.3~6.8)μm; 中间3个细胞褐色,长11.9~17.0 μm;顶细胞长圆锥形,顶生2~4根不分枝的附属丝,以2~3根为主,无色透明,长6.3~18.3 μm;基细胞无色,具短柄,长1.8~7.5 μm(图 1E)。
病菌在PDA平板上菌落圆形,初呈白色,25 ℃培养5天后,菌落正面仍为白色(图 1F),背面则因分泌色素而呈橙黄色(图 1G),气生菌丝白色绒毛状,平铺,培养6~7天即能长满整个培养皿,8天后从菌落中央形成墨汁状的黏液,为分生孢子堆; 病菌在培养基上产生的孢子形态与自然病斑上形成的分生孢子形态相一致,仅在分生孢子大小上存在微小差异,在PDA培养基上分生孢子大小为(22.5~28.8)μm×(5.3~7.5)μm;中间3个细胞长12.5~17.5 μm; 顶生附属丝以2根为主,长6.3~19.8 μm; 基细胞长2.3~8.0 μm(图 1H)。
根据病菌上述培养性状和形态大小,参照拟盘多毛孢菌分类相关文献(赵光材等,1995;陆家云,2001; 韦继光等,2005),初步将美国红枫褐斑病病原菌鉴定为小孢拟盘多毛孢(Pestalotiopsis microspora(Speg.)G.C. Zhao & N. Li)。
2.4 rDNA-ITS序列分析选择代表性菌株PM-1和PM-2,提取其基因组总DNA,采用真菌通用引物对ITS1/ITS4进行PCR扩增,均扩增出一条约600 bp的清晰条带(图 2),经测序分析,片段全长均为606 nt,2个菌株的扩增序列完全一致。将此序列(GenBank登录号为KM076766和KM076767)与GenBank中已有的核苷酸序列进行同源性比较,结果与包括小孢拟盘多毛孢、忽视拟盘多毛孢(P. neglecta)、韦司梅拟盘多毛孢(P. vismiae)、马醉木拟盘多毛孢(P. pallidotheae)、拟角状拟盘多毛孢(P. heterocornis)、木枋已拟盘多毛孢(P. cocculi)和双纤毛拟盘多毛孢(P. bicilia)在内的7种拟盘多毛孢菌40余个分离株的对应rDNA-ITS核苷酸序列同源性均为100%; 系统发育分析也表明病原菌株PM-1、PM-2与以上7种拟盘多毛孢菌均在一个分支内(自展支持率达98%),与Pestalotiopsis属其他种类遗传关系相对较远(图 3)。近年来,韦继光等(2005)根据以上7种拟盘多毛孢菌分生孢子形态和大小相互重叠,其rDNA-ITS序列之间同源性也高达98%~100%的事实,认为它们实际上应属于同一种真菌,并冠名为小孢拟盘多毛孢。显然,本文鉴定的美国红枫褐斑病病菌即为小孢拟盘多毛孢菌。
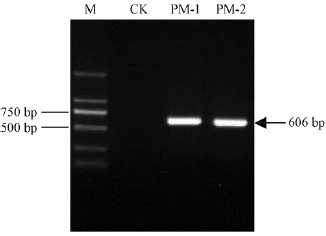 |
图 2 菌株PM-1和PM-2 rDNA-ITS区段的PCR扩增产物 Fig. 2 PCR-amplified product of rDNA-ITS of strain PM-1and PM-2 |
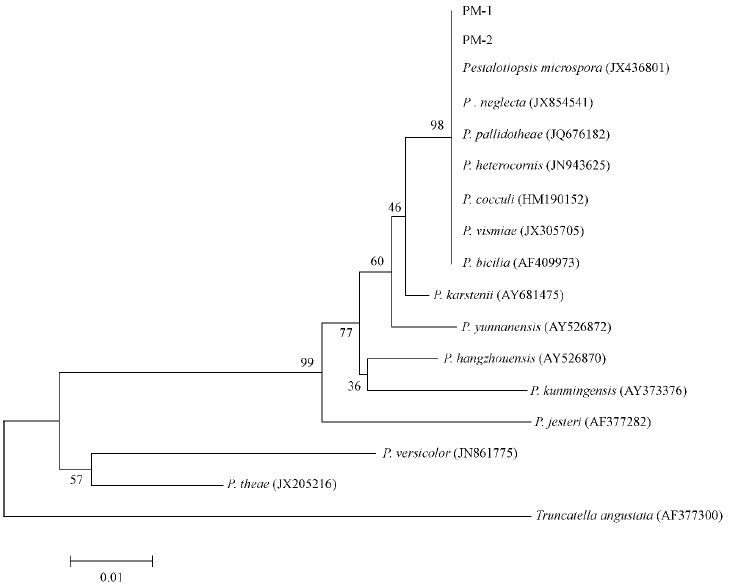 |
图 3 基于ITS序列构建的小孢拟盘多孢及该属其他真菌的系统发育树 Fig. 3 Phylogenetic tree based on ITS sequences of Pestalotiopsis microspora and other related fungi自展支持值(Bootstrap)>50%显示在各个进化分支点上.以Truncatella angustata(AF377300)ITS 序列作为外群.Only bootstrap values over50% are shown above the branches. Truncatella angustata(AF377300)was defined as the outgroup,respectively. |
本研究对美国红枫褐斑病病样进行了病菌分离,采用形态学和分子生物学技术对分离菌株进行了种类鉴定与致病性验证,结果表明引起江西省鄱阳县美国红枫褐斑病的病原菌为小孢拟盘多毛孢,属于子囊菌无性型拟盘多毛孢属真菌。这是国内外有关小孢拟盘多毛孢寄生美国红枫而引起病害的首次报道。
长期以来,拟盘多毛孢属真菌主要依据其分生孢子形态、寄主范围及其培养特征等性状等进行种类划分,但种间形态差异微小常常给鉴定带来困难(Hu et al.,2007; Maharachchikumbura et al.,2011)。近年来,随着分子生物学技术的快速发展,rDNA-ITS序列分析已成为真菌鉴定过程中的一种快速且有效的手段(Yuan et al.,1996; Jeewon et al.,2003)。韦继光等(2005)根据形态学特征和rDNA-ITS序列分析对拟盘多毛孢属真菌种类进行重新分类界定,认为其种的分类应先根据分生孢子淡色或暗色进行区分,然后再根据分生孢子有色胞及附属丝的特征划分到具体的种,并由此重新界定了小孢拟盘多毛孢、卡斯特尼拟盘多毛孢(P. karstenii)和异色拟盘多毛孢(P. versicolor)等种。本文的研究结果支持韦继光等(2005)的观点,并认为根据形态特征和分子系统学特征结合界定拟盘多毛孢菌的种比较科学,这也是当今真菌分类发展的趋势。
小孢拟盘多毛孢属于高温高湿型真菌(Metz et al.,2000),常在热带、亚热带地区园林植物上造成危害(张家祥,2002),已知寄主有鸡爪槭(Acer palmatum)、红叶石楠(Photiniax fraseri)、散尾葵(Chrysalidocarpus lutescens)和鹿角杜鹃(Rhododendron latoucheae)等60多种植物,主要引起植物轮斑和叶斑等病状(Guba,1961; Keith et al.,2006; Jeon et al.,2007; 李建宏等,2013; 管斌等,2013)。2014年,东郁园林公司从美国引进多个槭树科树种,包括美国红枫、糖槭(A.saccharum)和三季红红枫(A.palmatum ‘Sanguineum’)等,拟盘多毛孢褐斑病在园林公司种植基地美国红枫上的为害,也给其他槭树科植物的健康生长带来了潜在威胁,应引起足够重视。
| [1] |
冯立娟,苑兆和,尹燕雷,等. 2009. 槭属2品种叶变色期花青苷含量与相关酶活性的变化. 林业科学,45 (8): 57-60. (Feng L J,Yuan Z H,Yin Y L,et al. 2009. Anthocyanin content and the relevant enzymes activities during leaf color changing of two Acer species. Scientia Silvae Sinicae,45 (8): 57-60[in Chinese]).(  1) 1)
|
| [2] |
管 斌,徐 超,张红岩,等. 2013. 红叶石楠叶斑病病原菌分离鉴定及致病性测定研究. 西部林业科学,42 (2): 56-61. (Guan B,Xu C,Zhang H Y,et al. 2013. Identification of pathogen for leaf spot disease of Photinia fraseri and pathogenicity test. Journal of West China Forestry Science,42 (2): 56-61[in Chinese]).(  1) 1)
|
| [3] |
李建宏,谢昌平,王延丽,等. 2013. 散尾葵拟盘多毛孢叶斑病菌的鉴定. 热带农业科学,33 (2): 62-70. (Li J H,Xie C P,Wang Y L,et al. 2013. Identification of pathogen causing leaf spot of Chrysaliclocarpus lutescens. Chinese Journal of Tropical Agriculture,33 (2): 62-70[in Chinese]).(  1) 1)
|
| [4] |
李 晶,王承义. 2006. 彩叶树种美国红枫及其开发应用前景. 中国林副特产,(3):102. (Li J,Wang C Y. 2006. The cultivar of colored-leaf tree - American Red maple and its development and application foreground. Forest By-Product and Speciality in China,(3):102[in Chinese]).(  1) 1)
|
| [5] |
陆家云. 2001. 植物病原真菌学. 北京: 中国农业出版社. (Lu J Y. 2001. Plant Pathogenic Mycology. Beijing: China Agricultural Press.[in Chinese])(  2) 2)
|
| [6] |
任 杰,丁增成,唐 菲,等. 2013. 加拿大红枫的引种及繁育技术研究. 中国农学通报,29 (01): 37-41. (Ren J,Ding Z C,Tang F,et al. 2013. Study on introduction and propagation techniques of Red Maple(Acer rubrum L.). Chinese Agricultural Science Bulletin,29 (01): 37-41[in Chinese]).(  1) 1)
|
| [7] |
韦继光,徐 同,郭良栋,等. 2005. 根据形态学和分子系统学特征界定拟盘多毛孢属的种. 广西农业生物科学,24 (4): 304-313. (Wei J G,Xu T,Guo L D,et al. 2005. Delimitation of Pestalotiopsis species based on morphological and moecular phylogenetic characters. Journal of Guangxi Agricultural and Biological Science,24 (4): 304-313[in Chinese]).(  5) 5)
|
| [8] |
杨 帆,李 静,姚雪晗,等. 2013. 2种引种彩叶植物光合日变化及其影响因子研究. 安徽农业大学学报,40 (5): 746-750. (Yang F,Li J,Yao X H,et al. 2013. Durinal variation of photosynthesis of two kinds of introduced colored leaves plants and its influencing factors. Journal of Anhui Agricultural University,40 (5): 746-750[in Chinese]).(  1) 1)
|
| [9] |
张家祥. 2002. 中国南方拟盘多毛孢属(Pestalotiopsis)真菌及其分布性状的研究. 杭州: 浙江大学硕士学位论文. (Zhang J X. 2002. Identification of Pestalotiopsis spp. from southern China and study on the species-recognizing characters. Hangzhou: MS thesis of Zhejiang University[in Chinese]).(  1) 1)
|
| [10] |
张 健,李玉娟,李 敏,等. 2009. 典型彩叶树种美国红枫研究技术综述. 广西农学报,24 (2): 55-59. (Zhang J,Li Y J,Li M,et al. 2009. The overview of technique research on Acer rubrum of American colorful-leaf trees. Journal of Guangxi Agriculture,24 (2): 55-59[in Chinese]).(  1) 1)
|
| [11] |
赵光材,李 楠. 1995. 拟盘多毛孢属在云南的34个种. 东北林业大学学报,23 (4): 21-27. (Zhao G C,Li N. 1995. Thirty-four species of Pestalotiopsis in Yunnan. Journal of Northeast Forestry University,23 (4): 21-27[in Chinese]).(  2) 2)
|
| [12] |
赵庆柱,张占彪,邱玉宾,等. 2014. 不同植物生长调节剂对'夕阳红'槭扦插生根、生长和光合的影响. 中国农学通报,30 (10): 52-56. (Zhao Q Z,Zhang Z B,Qiu Y B,et al. 2014. Effect of different plant growth regulator on cutting rooting,growth and leaf photosynthetic of Acer rubrum'Red Sunset'. Chinese Agricultural Science Bulletin,30 (10): 52-56[in Chinese]).(  1) 1)
|
| [13] |
Abrams M D. 1998. The red maple paradox. Bioscience,48 (5): 355-364.( 1) 1)
|
| [14] |
Guba E F. 1961. Monograph of Pestalotia and Monochaetia. Cambridge: Harvard University Press.( 1) 1)
|
| [15] |
Hu H L,Jeewon R,Zhou D Q,et al. 2007. Phylogenetic diversity of endophytic Pestalotiopsis species in Pinus armandii and Ribes spp.: evidence from rDNA and β-tubulin gene phylogenies. Fungal Diversity,24: 1-22.( 1) 1)
|
| [16] |
Jeewon R,Liew C Y E,Simpson J A,et al. 2003. Phylogenetic significance of morphological characters in the taxonomy of Pestalotiopsis species. Molecular Phylogenetics and Evolution,27(3): 372-383.( 1) 1)
|
| [17] |
Jeon Y H,Kim S G,Kim Y H. 2007. First report on leaf blight of Lindera obtusiloba cause by Pestalotiopsis microspora in Korea. Plant Pathology,56(2): 349.( 1) 1)
|
| [18] |
Keith L M,Velasquez M E,Zee F T. 2006. Identification and characterization of Pestalotiopsis spp. causing scab disease of guava,Psidium guajava in Hawaii. Plant Disease,90(1): 16-23.( 1) 1)
|
| [19] |
Maharachchikumbura S S N,Guo L D,Chukeatirote E,et al. 2011.Pestalotipsis-morphology,phylogeny,biochemistry and diversity. Fungal Diversity,50(1): 167-187.( 1) 1)
|
| [20] |
Metz A M,Haddad A,Worapong J,et al. 2000. Induction of the sexual stage of Pestalotiopsis microspora,a taxol-producing fungus. Microbiology, 146 (8): 2079-2089.( 1) 1)
|
| [21] |
Yuan Y M,Kuper P,Doyle J J. 1996. Infrageneric phylogeny of genus Gentiana inferred from nucleotide sequences of internal transcribed spacers of the nuclear ribosomal DNA. American Journal of Botany,83(3): 641.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

