文章信息
- 罗在柒, 郭辉力, 杨亚东, 杨明峰, 马兰青, 王有年
- Luo Zaiqi, Guo Huili, Yang Yadong, Yang Mingfeng, Ma Lanqin, Wang Younian
- 农杆菌介导虎杖芪合酶基因遗传转化壶瓶枣的研究
- Agrobacterium-Mediated Transformation of Resveratrol Synthase Gene (PcPKS5) into Huping Jujube (Zizyphus jujuba)
- 林业科学, 2015, 51(10): 101-109
- Scientia Silvae Sinicae, 2015, 51(10): 101-109.
- DOI: 10.11707/j.1001-7488.20151013
-
文章历史
- 收稿日期:2014-08-11
- 修回日期:2014-10-14
-
作者相关文章
2. 北京农学院 农业部都市农业(北方)重点实验室 北京 102206;
3. 贵州省林业科学研究院 贵阳 550005
2. Key Laboratory of Urban Agriculture (North) of Ministry of Agriculture Beijing University of Agriculture Beijing 102206;
3. Guizhou Academy of Forestry Guiyang 550005
枣(Ziziphus jujuba)为鼠李科枣属植物(刘孟军,1999),是中国最古老的果树之一,有3 000多年的栽培历史。枣树具有抗逆性强、早果速丰、经济效益和生态效益显著等特点,在我国广泛栽培,是我国主要经济林树种之一,栽培面积(133.3万hm2)和年产量(246.3万t)均占世界的95%以上,在全球枣产品国际贸易市场中占据绝对主导地位(国家林业局,2007)。随着枣树种植面积不断增加,枣树栽培管理方式由粗放经营向密植集约化方向转变。但近10年来,我国枣树病害面积不断增加,果实病害危害程度逐年加重,严重制约了枣果实产量、品质和商品价值。
20世纪70年代末以来,相继报道了枣炭疽病(Alternaria alternate)、枣铁皮病(A.alternate)、枣黑斑病(Isariopsis imdica var.ziziphi)、枣轮纹病(A.alternate)、冬枣烂果病(A.alternate)和金丝小枣浆烂病(A.alternate)、黑疔病(A.alternate)、枣褐皮病等危害枣果实的病害,病原涉及9个真菌属和4个细菌属,病害严重阻碍了枣产业的发展(魏天军等,2006)。
白藜芦醇(resveratrol)是具有抗真菌功能的植保素。Takaoka(1939)首次从藜芦中分离得到白藜芦醇。白藜芦醇是一种有益于人类健康的天然物质,除了红酒外,还有茶叶、花生、开心果、花生酱和巧克力等食品都含有白藜芦醇(Burns et al.,2002;Tokusoglu et al.,2005;Counet et al.,2006;Hurst et al.,2008)。
苯丙烷代谢途径分支中的芪合酶(resveratrol synthase gene,STS)是白藜芦醇生物合成的关键酶,主要是在真菌感染、机械伤害、紫外辐射等诱导因素作用下产生的(Hart,1981)。芪合酶存在于花生(Arachis hypogaea)、葡萄(Vitis vinifera)和松欧洲赤松(Pinus sylvestris)等植物体内(Sotheeswaran et al.,1993)。研究证明,STS和查尔酮合酶(chalcone synthase,CHS),均能够催化3个Malonyl-CoA分子和1分子CoA-tethered phenylpropanoid,分别合成白藜芦醇和另外一种物质,即STS和CHS能够催化相同的底物生成不同的产物(Rolfs et al.,1984)。因此,遗传转化外源STS基因在植物代谢途径中能够与CHS竞争相同底物催化合成白藜芦醇物质,增强植物自身抗病性和提高果品品质。虎杖根中白藜醇含量最高,已从中分离出与白藜醇代谢相关的芪合酶基因(Guo et al.,2013),并对其功能进行分析(Ma et al.,2009a; 2009b)。
白藜芦醇在农业方面的应用主要是体现在抗真菌活性方面(Jeandet et al.,1995; Adrian et al.,2006),由于植物叶片和浆果等遭受机械损伤或病源入侵等胁迫反应,白藜芦醇扮演植保素的功能得以体现(Langcake et al.,1976)。近些年来,国内外已在多种植物上分离出STS基因并论证了它们的抗真菌活性(Giorcelli et al.,2004),异源成功表达转STS基因的植物有烟草(Nicotiana tabacum)(Hain et al.,1990; 1993)、苹果(Malus domestica)(Szankowski et al.,2003)、苜蓿(Medicago sativa)(Hipskind et al.,2000)、木瓜(Carica papaya)(Zhu et al.,2004)、银白杨(Populus alba)(Giorcelli et al.,2004)、水稻(Oryza sativa)(Stark-Lorenzen et al.,1997)、大麦(Hordeum vulgare)(Leckband et al.,1998)、小麦(Triticum aestivum cv. “Florida” and cv. “Combi”)(Serazetdinova et al.,2005)和生菜(Lactuca sativa)(Liu et al.,2006)等。
目前,有关枣树遗传转化与品种改良的研究不多且进展缓慢。何业华等(2002; 2004)首先建立了根癌农杆菌(Agrobacterium tumefactions)介导的枣树遗传转化系统,并获得了反义ACC合成酶基因转化枣树。黄建(2006)将S6PDH基因导入枣树,获得遗传转化植株。Gu等(2008)成功采用农杆菌介导遗传转化沾化冬枣(Z.jujuba ‘Dongzao’)。但是虎杖芪合酶作为一个超家族基因,目前仅看到Pan等(2012)将PcRS(DQ900615)遗传转化拟南芥(Arabidopsis thaliana),异源表达得到反式白藜芦醇苷并使遗传转化植株获得抗真菌活性。
壶瓶枣(Z. jujuba ‘Huping’)作为山西太谷的地理标识品种,经济价值高,产业发展前景好,但病害发生严重。白藜芦醇在虎杖根中含量较高,前期实验从虎杖(Polygonum cuspidatum Sieb.et Zucc.)根中分离得到白藜芦醇芪合酶基因PcPKS5(EU647245),该基因与PcPKS1、PcPKS3同属于含有3个内含子的CHS家族基因,具有高效合成白藜芦醇功能(Ma et al.,2009a,2009b; Guo et al.,2013)。本研究将虎杖芪合酶基因PcPKS5遗传转化壶瓶枣,以期提高其抗病性和改善果品品质。
1 材料与方法 1.1 试验材料壶瓶枣无性系材料系山西农业大学王玉国教授惠赠。受体材料的准备:壶瓶枣茎在培养基MS+6-BA2.0mg·L-1+IBA0.5mg·L-1中分化出丛生芽(图 5A),用于制备受体材料。无性系再生体系增殖系数平均达11.0,其中通过茎段顶端和底端诱导愈伤组织分化芽为3~4个,再生体系生根率达97.0%以上,根系发达(图 5B)。
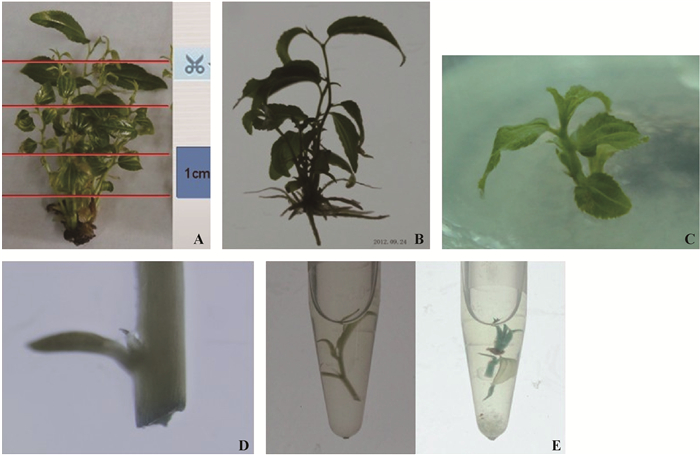 |
图 5 受体材料遗化转化进程 Fig. 5 Experimental progress of genetic trasformation A:受体材料Plant material;B:壶瓶枣生根植株Huping jujube rooting plants;C:获得的转化植株Plants of genetic transformation ;D:共培养2天外植体GUS染色Histochemistry GUS on explants in co-cultured for 2 days;E:转化苗GUS染色对照Histochemistry GUS on genetic transformed seedlings. |
培养条件为温度(25±1)℃,14 h光照/10 h黑暗,光强2 000 lx。
虎杖芪合酶基因(EU647245)、植物表达载体p3301-121、农杆菌EHA105为本实验室保存。各种DNA聚合酶、内切酶、连接酶,质粒提取试剂盒、胶回收试剂盒、植物DNA提取试剂盒等试剂耗材均由Takara公司提供,引物合成和分子测序工作由生工生物工程(上海)有限公司完成。
1.2 试验方法 1.2.1 植物表达载体的构建选择植物表达载体上酶切位点,设计带酶切位点XbaI、BamHI引物,扩增目的片段,连接得到p3301-121-PcSTS重组质粒,转化到农杆菌EHA105,并进行阳性检测和测序验证。
引物F1(5′ to 3′)ATT CTC Tag AAT ggC AgC TTC AAC TgA AgA,F2(5′ to 3′)TAT Agg ATC CAA TgA Tgg gCA CAC TTC gTA,PCR反应条件: 94 ℃起始变性4 min; 94 ℃变性30 s,58 ℃复性30 s,72 ℃延伸1 min,35个循环; 72 ℃延伸8 min。PCR 反应体积为 25 μL。
1.2.2 遗传转化体系条件与抗性植株筛选选择1.1节中培养40天的丛生芽为受体材料,将其剪成1.0 cm左右茎段;将植物表达载体的工程菌活化培养,获OD600值为0.4~0.8的菌液,离心后用液体植物培养基重悬后用于侵染受体材料,与侵染材料置于较低浓度琼脂糖的MS培养基中共培养。
选择乙酰丁香酮(acetosyringone,AS)浓度、抗生素类型及浓度、抗除草剂的抗敏实验临界浓度3个因子进行试验,比较各因子对丛生芽分化的影响。各试验中培养基浓度分别是,羧苄青霉素(carbenicillin,Cb)、头孢霉素(cefotaxine,Cef)分别为200,400和600 mg·L-1,乙酰丁香酮为30,60和90 mg·L-1,Basta为1.0,2.0,3.0,4.0,6.0,8.0,10.0和20.0 mg·L-1。每个试验分别设3组,每组均接瓶苗10瓶,每瓶5株。
受体材料预培养3天后进行遗传转化。遗传转化过程设定农杆菌菌液浓度梯度OD600值为0.4,0.6,0.8,每个梯度侵染时间分别为5,10,15 min,共培养时间2,3,4天,然后转入含Cb 400 mg·L-1的丛生芽培养基中培育,每隔1周转入新培养基作除菌处理。试验共设18个处理,每个处理40瓶,每瓶接种材料10个。
将分化丛生芽转接入含Cb 400 mg·L-1、Basta 10.0 mg·L-1的丛生芽培养基进行筛选,培养3周后将抗性植株转入含Cb 400 mg·L-1的丛生芽培养基中正常培养,再培养3周后再次转入含Cb 400 mg·L-1,Basta 10.0 mg·L-1的丛生芽培养基中重复筛选阳性植株。
1.2.3 阳性植株的检测取阳性植株经梯度90%丙酮固定,β-糖醛酸苷酶(β-glucuroidase,GUS)染色,37 ℃过夜,经梯度乙醇脱色后FAA固定液保存,观测染色情况,以野生型材料作阴性对照。
gDNA的提取采用eZNA Plant DNA Mini Kit 试剂盒,PCR反应体系方法同1.2.1。
RNA的提取采用奥莱博试剂盒法:制备20 μL反应体系[1 μg总RNA,1 μL Anchored Oligo(dT)18,10 μL 2× TS Reaction Mix,1 μL TransScript Enzyme Mix,RNase-free水补至20 μL];42 ℃孵育30 min,85 ℃孵育5 min终止反应。RT-PCR体系及程序:参照TransScript First-Str and cDNA Synthesis Supermix 试剂盒说明书制备50 μL反应体系(2 μL cDNA,1 μL F Prime,1μL R Prime,5 μL 10x Buffer,4 μL 2.5 mmol·L-1 dNTPs,0.5μL Enzyme,ddH2O补至50 μL);94 ℃预变性5 min,94 ℃ 30 s,58 ℃ 30 s,72 ℃1 min,35个循环,72 ℃延伸10 min终止反应。取10 μL PCR产物1%琼脂糖电泳检测。
1.2.4 基因表达效率比较从转化植株分别提取总RNA,反转录(RNA浓度约1 000 ng·μL-1,取1.0 μL制备20.0 μL反转录体系,RNA浓度降为约50.0 ng·uL-1)获得cDNA模板,荧光实时定量PCR试剂为Cat:CW0956 2 × Ultra SYBR Mixture(Lot:30910K),反应条件: 95 ℃起始变性10 min; 95 ℃变性20 s,60 ℃复性、延伸1 min,40个循环; 溶解曲线条件:95 ℃变性1 min,60 ℃复性、延伸20 s。内参基因为ZjH3(Ziziphus jujuba H3,GenBank登录号:EU916201)基因(孟玉平,2010),ZjH3引物 F1:GAACAGTGGCTCTGAGG GAAAT,F2:gagggaaatcgctc aggatt。试验分别设计负对照处理。PcSTS F1:ACCTCACCCACCTCAAGCA CAAAT;PcSTS F2:aaaacccgaatatcggtgcg。
1.2.5 白藜芦醇的提取与HPLC分析以野生型植株作为对照,称取1.5 g(鲜质量)植物材料,液氮匀浆,转入40 mL 80%甲醇溶液中冷浸过夜,40 ℃超声40 min,4 000 r·min-1离心10 min,上清过孔径0.22 μm的有机滤膜,加入3倍体积的石油醚萃取小极性化合物,用乙酸乙酯萃取有机相物质,旋转回蒸乙酸乙酯,用1 mL 80%甲醇溶解,待测。HPLC条件为分离柱类型为Sun Fire TM C18 5 μm,4.6 mm×150 mm Column。柱温设定25.0 ℃,检测波长为306 nm,流动相为乙醇和水,流速为0.5 mL·min-1,增速为10%~53% 10 min,53%~56% 30 min,56%~70% 10 min。
转基因植物叶片中白藜芦醇的 ESI-MS 验证:HPLC 过程中,多次收集转基因植物提取物中与标样有相同吸收峰时段的化合物,蒸干后溶于 50%的甲醇溶液,用电喷雾(ESI)电离源电离检测,仪器型号为日本岛津公司的LCMS2010 型质谱仪。
2 结果与分析 2.1 表达载体的构建与工程菌株制备将TOP10菌株在Kan50 mg·L-1 LB培养基中活化3次,提取质粒作为模板,对上、下游分别为XbaI,BamH I位点的引物进行PCR扩增,获得目标序列(图 1a)。
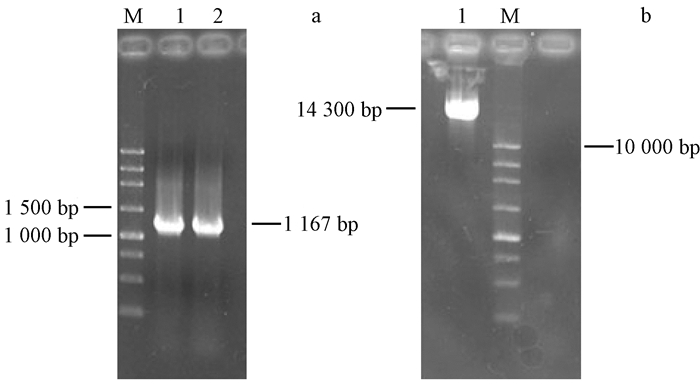 |
图 1 目标基因PCR扩增及p3301-121质粒电泳 Fig. 1 Electrophoretogram of the STS gene and plant expression vector |
将目标基因片段与植物表达载体分别进行XbaI,BamH I双酶切,经T4连接酶连接,得到环状表达质粒(图 2),转化到TOP10菌株,酶切、测序分析验证序列正确后,将构建的质粒转化到农杆菌EHA105感受态,菌液PCR检测为阳性,获得侵染工程菌株。
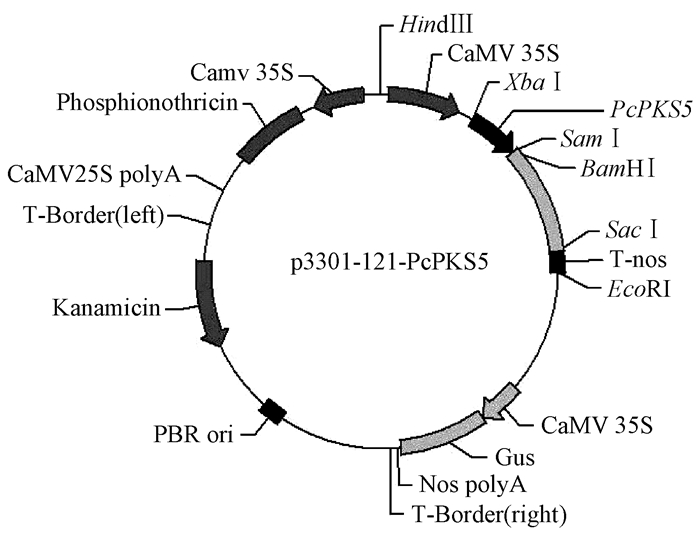 |
图 2 构建植物表达载体 Fig. 2 Construction of plant expression vector of the STS gene |
比较2种抗生素对受体材料再生体系影响,结果如表 1所示。显著性检验表明Cb丛生芽增殖效果优于Cef,选择Cb作为遗传转化体系抑菌的抗生素。
|
|
AS能够促进外植体丛生芽分化(表 2),AS浓度为90 mg·L-1时与其他浓度效果间存在显著差异。
|
|
Basta主要作用是破坏光合系统。当外植体吸收培养基中Basta后,初期主要表现在茎尖和叶尖出现褐化,随着Basta浓度的加大和培养时间的延长,褐化程度加深,植株死亡。培养3周后可知,Basta浓度小于2.0 mg·L-1时对植株基本没有伤害,浓度4.0 mg·L-1时有50%致死率,浓度达到10.0 mg·L-1时致死率达100%。因此,Basta临界浓度为4.0mg·L-1(图 3)。
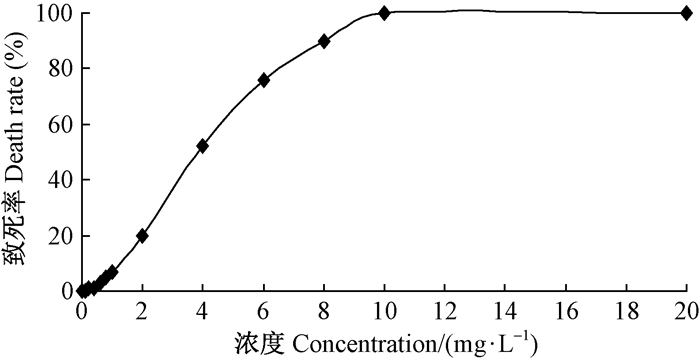 |
图 3 不同Basta浓度的致死率 Fig. 3 Death rate under different Basta concentration |
遗传转化过程中,随着侵染时间的推移,GUS活性随之增加。如图 4A所示,当时间为15 min时,GUS活性达到97.0%,20 min时GUS活性接近100%。所以,遗传转化过程侵染时间确定为15 min适宜。试验过程中取样进行GUS染色检测,如果如图 5D所示。
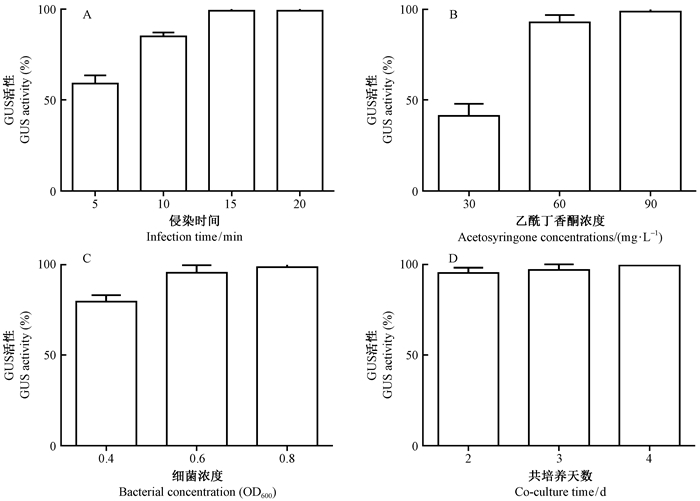 |
图 4 遗传转化过程中不同影响因子下的Gus活性 Fig. 4 Gus activity under different factor in the genetic transformation |
乙酰丁香酮在遗传转化过程中能够诱导脓杆菌Vir基因的活化,促进外源基因的整合,但随着乙酰丁香酮浓度增大,其物质本身对再生体系的增殖有抑制作用。当乙酰丁香酮浓度为60 mg·L-1时,GUS活性达到92.0%,浓度为90 mg·L-1时,GUS活性增至96.0%(图 4B)。因此,遗传转化过程选用乙酰丁香酮浓度为60 mg·L-1为宜。
侵染农杆菌浓度作为遗传转化过程中重要的因子之一。随着农杆菌浓度的增大,GUS活性随之增强,如图 4C 所示,细菌浓度OD600从0.4~0.6过程中,增幅明显,OD600值从0.6~0.8过程增幅不明显,而且随着细菌浓度增大,即细菌数量虽然增加,但是细菌的侵染能力减弱,综合考虑,选择农杆菌浓度OD600值为0.6。
共培养天数是指农杆菌侵染外植体后目标基因整合到受体材料基因的用时。试验检测共培养2,3,4天时的GUS活性,结果呈递增趋势,如图 4D 所示,3个培养时间段的GUS活性依次为92.0%,97.0%,97.0%,因此,本遗传转化试验选用的共培养天数为3天。
2.4 阳性植株的检测试验共侵染外植体7 200个,产生丛生芽约72 000个,经过初筛获得抗性外植体197株,经复壮培养后复筛,最终得到抗性外植体3株(图 5C,E),遗传转化率为1.52%。
3株阳性抗性苗在高浓度Basta中能存活,且外形纤细。经GUS染色,外植体全部呈现深紫色(图 5E),表明遗传载体的GUS基因已经整合到壶瓶枣植株基因组中。从转化苗基因组DNA能够扩增出目标基因(图 6),初步证明目标基因PcSTS5已整合到壶瓶枣基因组中。获得的遗传转化苗经培养复壮后与野生型对照无明显的表型差异。
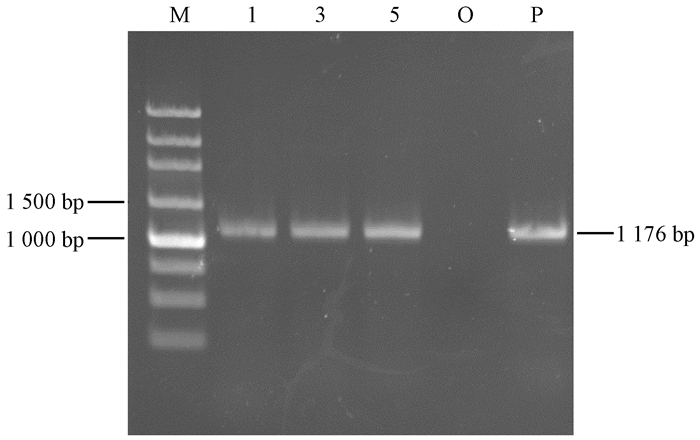 |
图 6 转化苗基因组检测 Fig. 6 PCR analysis of target genes extracted from the genetic transformation plant M:Marker 250 bp;1,3,5:转化苗Positive control; O:野生型对照Positive control; P:转化质粒Transformed plasmid (p3301-121-PcRS) |
进一步通过荧光实时定量技术比较3个不同转化植株中表达效率,结果为其中1个株系高于另外2个株系(图 7)。
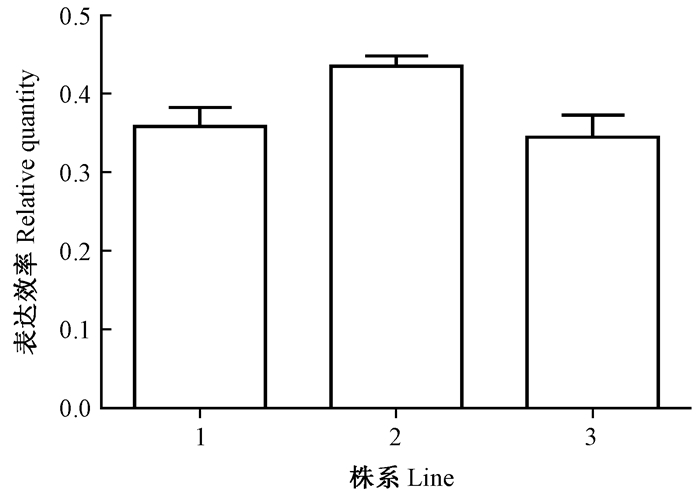 |
图 7 不同转化植株Q-PCR比较 Fig. 7 Comparison of the Q-PCR of different transgenic clones |
HPLC分析发现,保留时间为16.438~17.236 min,其中在16.920 min出现白藜芦醇吸收峰,标准曲线为Y=67 354X + 62 755(R2=0.999 8),通过共色谱初步确定转基因植物样品中存在白藜芦醇(图 8),通过标准曲线计算得到其鲜重白藜芦醇含量为0.45 μg·g-1。多次收集出峰时间内的HPLC物质,经LCMS分析得到吸收光谱的m/z为228.9,吸收值与白藜芦醇标样一致,证实所得产物为白藜芦醇(图 9)。而作为对照的野生型植株在相同的提取分离条件下未检测到白藜芦醇的吸收峰(图 10)。
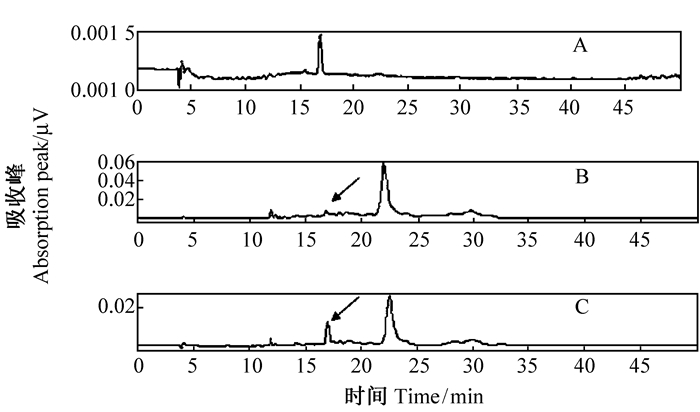 |
图 8 白藜芦醇HPLC分析 Fig. 8 Analysis of resveratrol by HPLC A.白藜芦醇标品Resveratrol reference standard; B.遗传转化植株Transgenic plants; C.共轭结果Conjugated of the same amount both resveratrol reference standard and transgenic plants. |
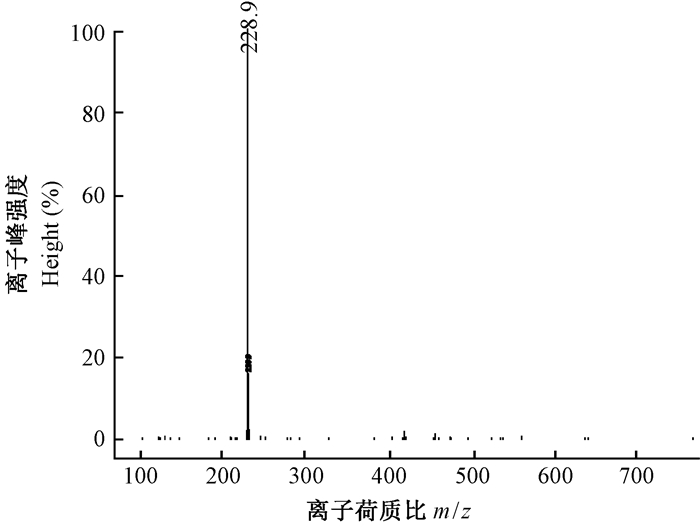 |
图 9 HPLC目标产物LCMS分析 Fig. 9 LCMS analysis of the target product by HPLC |
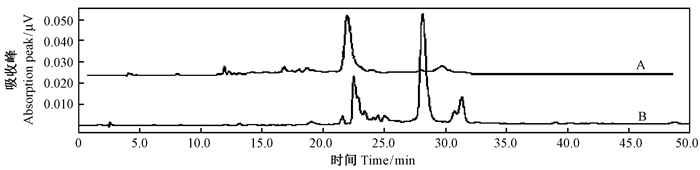 |
图 10 转化植株与野生型植株HPLC目标产物对比 Fig. 10 Comparison of target product in the transgenic plants and wild-type plants by HPLC 峰值时间Peak fime:16.928 min;A.转化植株Transgenic plants;B.野生型植株Wild plants. |
本试验通过优化壶瓶枣优良无性系,从其茎段增殖中获得再生体系,并建立了遗传转化体系,将虎杖芪合酶基因PcPKS5遗传转化壶瓶枣并对转化植株进行了鉴定。
壶瓶枣PCPKS5基因遗传转化体系的建立,为枣树遗传转化外源基因表达机制提供了技术方法和材料。试验成功的关键是获得了茎段高效的再生体系,能够在截口产生愈伤组织并分化得到丛生芽,遗传转化体系中抗生素Cb优于Cef,AS对转化效率表现不明显,Basta致死临界浓度为4.0 mg·L-1;其次是明确菌体的侵染时间和菌体浓度以提高侵染效率,菌液浓度为OD600=0.6时侵染15 min效果最好,共培养时间2天、含60 mg·L-1乙酰丁香酮的培养基效果较优。试验中遗传转化率仅为1.52%,遗传转化效率还比较低,其原因除遗传转化体系还需进一步优化外,还应注意初筛、复壮和复筛环节过程复杂,导致部分外植体死亡。
值得一提的是壶瓶枣转化外源基因植株中能够代谢产生白藜芦醇,实现了外源基因的遗传转化表达,白藜芦醇鲜质量含量为0.45 μg·g-1。另外,从产生的白藜芦醇含量来看,相对其他研究结果含量偏低,究其原因主要为枣树本身作为植物表达载体,是否有其特殊的代谢路径仍需深入研究;其二是有可能受启动子因素的影响;再次是缺乏必要的如微生物入侵、紫外照射和机械损伤等外界刺激。理论上由于白藜芦醇生物合成途径和步骤较多,单纯过量表达关键酶芪合酶基因虽然对白藜芦醇的含量可能会产生一定的促进作用,但是要想通过枣树中的表达载体大幅提高白藜芦醇含量,今后的试验还应探索多个基因共转化,从而实现融合基因“邻近效应”,使枣树中积累大量的白藜芦醇代谢产物。
遗传转化STS基因的植物具有很强的抵抗各种真菌感染的能力(Giorcelli et al.,2004;Halls et al.,2008;Delaunois et al.,2009)。在转入STS基因的甜薯(Ipomoea batatas)(Pan,2012)、生菜(Liu et al.,2006)和拟南芥(Arabidopsis thaliana)(Liu et al.,2012)等植株中均得到白藜芦醇,另外在苜蓿(Medicago sativa)(Kobayashi et al.,2009)、烟草(Nicotiana tabacum)(Condori et al.,2009)等得到白藜芦醇苷等衍生物,且所有的转化植株均有抗真菌的活力。本试验还需进一步检测转化植株的抗真菌活性及深入研究相关机制。
| [1] |
国家林业局. 2007.中国林业统计年鉴.北京:中国林业出版社. (State Forestry Administration. China Forestry Statistical Yearbook 2007. Beijing: China Forestry Publishing House. [in Chinese])(  1) 1)
|
| [2] |
何业华,林良斌,熊兴华,等.2002.根癌农杆菌介导的枣树遗传转化系统的建立.分子植物育种,1(5):683-686. (He Y H, Lin L B, Xiong X H, et al. 2002. Studies of development of efficient genetic transformation of Zizyphus jujuba. Molecular Plant Breeding, 1(5), 683-686 [in Chinese]).(  1) 1)
|
| [3] |
何业华,熊兴华,林顺权,等.2004.根癌农杆菌介导反义ACC合成酶基因对枣树的转化.湖南农业大学学报,30(1):33-36. (He Y H, Xiong X H, Lin S Q, et al. 2004. Transformation of Zizyphus jujuba hetau with antisense acc synthase gene. Journal of Hunan Agricultural University, 30(1), 33-36 [in Chinese]).(  1) 1)
|
| [4] |
黄 建.2006.农杆菌介导S6PDH基因转化枣树的研究.杨凌:西北农林科技大学硕士学位论文. (Huang J. 2006. Study on agrobacterium-mediated transformation of Zyziphus jujuba with S6PDH gene. Yanglin:Ms thesis of Northwest Agricultural and Forest University [in Chinese]).(  1) 1)
|
| [5] |
刘孟军.1999.枣属植物分类学研究进展.园艺学报,26(5):302-308. (Liu M J.1999. Advances in Taxonomy Study on the Genus Ziziphus. Acta Horticulturae, 26 (5):302-308 [in Chinese]).(  1) 1)
|
| [6] |
孟玉平,曹秋芬,孙海峰. 2010.枣树内参基因ZjH3的克隆与筛选.生物技术通报,27(11):101-107. (Meng Y P, Cao Q F, Sun H F.Cloning and selection of housekeeping gene ZjH3 for Ziziphus jujuba. Biotechnology Bulletin, 27(11):101-107 [in Chinese]).(  1) 1)
|
| [7] |
魏天军, 魏象廷. 2006.中国枣果实病害研究进展.西北农业学报,15(1): 88-94. (Wei T J, Wei X T. 2006. Advances in research on diseases of Chinese jujube fruits (Zizyphus jujuba Mill.). Acta Agriculturae Boreali-Occidentalis Sinica, 15(1):88-94 [in Chinese]).(  1) 1)
|
| [8] |
Adrian M, Jeandet P.2006. Trans-resveratrol as an antifungal agent//Aggarwal B B, Shishodia S. Resveratrol in Health and Disease. CRC Press, 475-497.( 1) 1)
|
| [9] |
Burns J, Yokota T, Ashihara H, et al. 2002 Plant foods and herbal sources of resveratrol. J Agric Food Chem, 50(11):3337-3340.( 1) 1)
|
| [10] |
Condori J, Medrano G, Sivakumar G, et al. 2009. Functional characterization of stilbene synthase gene using a transient expression system in planta. Plant Cell Rep, 28(4):589-599.( 1) 1)
|
| [11] |
Counet C, Callemien D, Collin S.2006.Chocolate and cocoa: new sources of trans-resveratrol and trans-piceid. Food Chem, 98:649-657.( 1) 1)
|
| [12] |
Delaunois B, Cordelier S, Conreux A, et al.2009.Molecular engineering of resveratrol in plants. Plant Biotechnol J, 7:2-12.( 1) 1)
|
| [13] |
Giorcelli A, Sparvoli F, Mattivi F, et al.2004.Expression of the stilbene synthase (StSy) gene from grapevine in transgenic white poplar results in high accumulation of the antioxidant resveratrol glucosides. Transgenic Res, 13:203-214.( 3) 3)
|
| [14] |
Gu X. F,Meng H,Qi G,et al. 2008.Agrobacterium-mediated transformation of the winterjujube (Zizyphus jujuba Mill.). Plant Cell Tiss Organ Cult,94:23-32.( 1) 1)
|
| [15] |
Guo Y W Guo,H L, Li X, et al,.2013. Two type III polyketide synthases from Polygonum cuspidatum:gene structure, evolutionary route and metabolites. Plant Biotechnol Rep,7:371-381.( 2) 2)
|
| [16] |
Hain R, Reif H J, Krause E, et al. 1993. Disease resistance results from foreign phytoalexin expression in a novel plant.Nature,361, 153-156.( 1) 1)
|
| [17] |
Hain R, Bieseler B, Kindl H, et al. 1990. Expression of a stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin resveratrol. Plant Mol Biol, 15,325-335.( 1) 1)
|
| [18] |
Halls C, Yu O. 2008.Potential for metabolic engineering of resveratrol biosynthesis. Trends Biotechnol, 26:77-81.( 1) 1)
|
| [19] |
Hart J H. 1981. Role of phytostilbenes in decay and disease resistance. Annu Re Phytopathol, 19, 832-834.( 1) 1)
|
| [20] |
Hipskind J D, Paiva N L. 2000. Constitutive accumulation of a resveratrol- glucoside in transgenic alfalfa increases resistance to Phoma medicaginis. Mol Plant Microbe Interact, 13,551-562.( 1) 1)
|
| [21] |
Hurst W J, Glinski J A, Miller K B, et al. 2008. Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem, 56:8374-8378.( 1) 1)
|
| [22] |
Jeandet P, Bessis R, Sbaghi M, et al. 1995.Resveratrol content of wines of different ages:relationship with fungal disease pressure in the vineyard.Am J Enol Vitic,46:1-4.( 1) 1)
|
| [23] | Kobayashi S, Ding C K, Nakamura Y, et al. 2000.Kiwifruits(Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid(resveratrol-glucoside).Plant Cell Rep,19:904-910. |
| [24] |
Langcake P, Pryce R J.1976.The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol Plant Pathol, 9:77-86.( 1) 1)
|
| [25] |
Leckband G,Lorz H. 1998. Transformation and expression of a stilbene synthase gene of Vitis vinifera L. in barley and wheat for increased fungal resistance. Theor Appl Genet, 96,1001-1012.( 1) 1)
|
| [26] |
Liu S J, Hu Y L, Wang X L, et al. 2006.High Content of Resveratrol in Lettuce Transformed with a Stilbene Synthase Gene of Parthenocissus henryana. J Agric Food Chem, 54, 8082-8085.( 2) 2)
|
| [27] |
Ma L Q, Guo Y W, Gao D Y, et al.2009a. Identification of a Polygonum cuspidatum three-intron gene encoding a type III polyketide synthase producing both naringenin and p-hydroxybenzalacetone. Planta, 229:1077-1086.( 2) 2)
|
| [28] |
Ma L Q, Pang X B, Shen H Y, et al. 2009b.A novel type III polyketide synthase encoded by a three-intron gene from Polygonum cuspidatum. Planta,229:457-469.( 2) 2)
|
| [29] |
Pan L P, Yu S L, Chen C J,et al. 2012. Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep, 31(1):121-131.( 2) 2)
|
| [30] |
Rolfs C H, Kindl H. 1984. Stilbene synthase and chalcone synthase:two different constitutive enzymes in cultured cells of Picea excelsa.Plant Physiol,75,489-492.( 1) 1)
|
| [31] |
Serazetdinova L,Oldach K H, Lorz H. 2005. Expression of transgenic stilbene synthases in wheat causes the accumulation of unknown stilbene derivatives with antifungal activity. J Plant Physiol,162, 985-1002.( 1) 1)
|
| [32] |
Sotheeswaran S, Pasupathy V. 1993. Distribution of resveratrol oligomers in plants. Phytochemistry, 32: 1083-1092.( 1) 1)
|
| [33] |
Stark-Lorenzen P, Nelke B, Hänβler G,et al. 1997. Transfer of a grapevine stilbene synthase gene to rice (Oryza sativa L.). Plant Cell Rep, 16(10), 668-673.( 1) 1)
|
| [34] |
Szankowski I, Briviba K,Fleschhut J,et al. 2003. Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa). Plant Cell Rep, 22(2), 141-149.( 1) 1)
|
| [35] |
Takaoka Michio. 1939. The Phenolic substances of white Hellebore (Veratrum grandiflorum Loes fil.) II: Oxyresveratrol. J Fac Sci Hokkaido Imp Univ, 60(12), 1261-1264.( 1) 1)
|
| [36] |
Tokusoglu O, Unal M K, Yemis F. 2005. Determination of the phytoalexin resveratrol (3,5,4'-trihydroxystilbene) in peanuts and pistachios by high-performance liquid chromatographic diode array (HPLC-DAD) and gas chromatographymass spectrometry (GC-MS). J Agric Food Chem, 53:5003-5009.( 1) 1)
|
| [37] |
Zhu Y J,Agbayani R,Jackson M C,et al. 2004.Expression of the grapevine stilbene synthase gene VST1 in papaya provides increased resistance against diseases caused by Phytophthora palmi Vora. Planta, 220(2): 241-250.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

