文章信息
- 高冬妮, 范晓旭, 赵丹
- Gao Dongni, Fan Xiaoxu, Zhao Dan
- 产漆酶半知真菌Myrothecium verrucariaNF-08菌株的分离及产酶研究
- Isolation of a Deuteromycete Fungus Myrothecium verrucaria NF-08 and Its Laccase Production
- 林业科学, 2015, 51(1): 80-87
- Scientia Silvae Sinicae, 2015, 51(1): 80-87.
- DOI: 10.11707/j.1001-7488.20150109
-
文章历史
- 收稿日期:2013-11-17
- 修回日期:2014-06-04
-
作者相关文章
2. 农业微生物技术教育部工程研究中心 哈尔滨 150500
2. Engineering Research Center of Agricultural Microbiology Technology, Ministry of Education Harbin 150500
漆酶(laccase,EC 1.10.3.2)即对苯二酚: 双氧氧化还原酶(benzenediol:dioxygen oxidoreductase),是蓝多铜氧化酶(blue multi-copper oxidases)家族中最古老的一员,因最早发现于日本紫胶漆树(Rhus vernicifera)中而得名(Yoshida,1883)。漆酶的底物范围十分宽泛,能催化多种酚类、胺类及芳香族化合物的氧化,同时伴随一步四电子反应,将氧分子还原为水。漆酶广泛存在于自然界动植物及微生物中,具有多种生理及生态学功能,目前已在环境修复(Kuddus et al.,2013)、污染物降解(Daâssi et al.,2013)、染料脱色与解毒(Zheng et al.,2013)、纺织印染(王石磊等,2010)、制浆造纸(Martín-Sampedro et al.,2011)、分析监测(Casero et al.,2013)、智能包装(Elegir et al.,2008)及生物电子设备(Milton et al.,2013)等领域广泛应用。
微生物漆酶具有来源广泛、易于纯化、底物宽泛、作用条件温和等优点,是漆酶理论及应用研究的主要来源。建立产漆酶微生物的筛选方法,开发产酶微生物种质资源,提高微生物产酶水平,是漆酶研究中的基础与重点内容。目前微生物漆酶中研究最广泛而深入的是担子菌亚门(Basidiomycotina)白腐真菌(white rot fungi)漆酶(Cabana et al.,2009; Fonseca et al.,2010)。与之相比,半知真菌因生活史相对简单、生长周期短、易于遗传转化和实现大规模发酵等优点,现已成为漆酶来源研究的新热点,主要集中在种质资源发掘、产酶条件优化等方面。现已报道的产漆酶半知真菌有拟盘多毛孢属(Pestalotiopsis sp.)(Hao et al.,2007; Feng et al.,2013)、棘孢木霉(Trichoderma asperellum)(陈今朝等,2010)、拟青霉属(Paecilomyces sp.)(Kluczek-Turpeinen et al.,2003; Liu et al.,2010)等,但产酶活性普遍低于白腐真菌。
本研究从凉水国家级自然保护区土壤样品中筛选产漆酶真菌菌株,并对其培养基组分、发酵条件及诱导底物进行优化,旨在获得具有发酵周期短、产酶活性高的产酶菌株,并提高其产酶水平。这有助于完善产漆酶真菌的筛选方法,拓展漆酶微生物来源,为漆酶的大规模发酵生产和漆酶工业应用奠定基础。
1 材料与方法 1.1 采样地概况凉水国家级自然保护区位于黑龙江省伊春市带岭区,128°48′30″—128°55′50″E,47°07′39″—47°14′22″N,区内以红松(Pinus koraiensis)为主的针阔混交林生态系统占保护区总面积的64%。保护区地带性土壤为暗棕壤,非地带性土壤有草甸土、沼泽土和泥炭土。2009年11月,在该保护区采集土壤样品,采样深度为10~15 cm。土壤样品装入事先灭菌的密实袋中,标记采样点树种及地理坐标。
1.2 培养基1)菌种纯化与保藏采用马铃薯葡萄糖(PDA)固体培养基(赵敏等,2011)。
2)富集培养基: 木质素磺酸钠0.3 g·L-1,K2HPO4 1.0 g·L-1,NaCl 0.5 g·L-1,MgSO4·7H2O 0.3 g·L-1,NaNO3 2.5 g·L-1,CaCl2 0.1 g·L-1,FeCl3 0.01 g·L-1,pH值7.4,121 ℃灭菌15 min。
3)分离培养基: 在融化后冷却的固体PDA培养基中过滤除菌加入终浓度为0.04%愈创木酚,过滤除菌加入终浓度为50 μg·mL-1的氨苄青霉素和链霉素用于抑制细菌生长。
4)复筛培养基: 在灭过菌的液体PDA培养基中加入CuSO4·5H2O,使Cu2+终浓度为0.05 mmol·L-1。
5)发酵培养基: 在液体PDA培养基中加入终浓度为10 g·L-1蛋白胨,终浓度为0.1 mmol·L-1 Cu2+。
1.3 供试菌株的分离与鉴定1)产漆酶真菌的富集、分离与筛选: 准确称取5 g土壤样品40 ℃干燥2 h以去除细菌。将土壤转移至含有若干玻璃珠的50 mL·(250 mL)-1无菌水中,30 ℃,140 r·min-1振荡过夜。取5 mL悬液接入150 mL·(250 mL)-1富集培养基中,30 ℃,140 r·min-1振荡5天。将富集液适当稀释涂布在分离培养基平板上,30 ℃恒温静置正置培养。将显黄红色的菌落转接到PDA平板上。
分别以1 mmol·L-1 ABTS[2,2-连氮-双(3-乙基苯并噻唑-6-磺酸),2,2-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid)]和1 mmol·L-1 SGZ[4-羟基-3,5-二甲氧基-苯甲醛连氮,4-hy-droxy-3,5-dimethoxybenzaldehyde azine,即丁香醛联氮,syringaldazine]定性初筛PDA平板上的纯化菌株,选取显色速度快且颜色明显的菌株,在PDA平板上培养10天,制备成直径1 cm菌饼,接入复筛培养基进行摇瓶发酵复筛,30 ℃,140 r·min-1振荡培养。第2天起测定漆酶活力,选取产酶活性高且发酵周期短的菌株进行后续研究。每个试验处理设置3个重复。
2)供试菌株的形态学鉴定:观察供试菌株在固体培养基上的菌落形态,制片观察菌丝、分生孢子、分生孢子梗等显微形态。
3)供试菌株rDNA-ITS序列扩增:以pH7.0磷酸缓冲液洗涤供试菌菌丝体,-80 ℃冷冻过夜后以液氮研磨。以CTAB法提取的基因组(吴发红等,2009)为模板,以rDNA-ITS通用引物(ITS1和ITS4)进行PCR扩增(燕勇等,2008)。引物合成及片段测序由上海生工生物工程有限公司完成。测序结果提交至GenBank并利用BLAST比对分析。
1.4 漆酶活性及蛋白测定1)发酵液10 000 r·min-1 离心10 min,上清即为粗酶液。漆酶活性测定在Niladevi等(2009)的方法基础上稍作改良: 50 μL 适当稀释粗酶液,1 mL 1 mmol·L-1 ABTS,2.95 mL pH4.0磷酸氢二钠-柠檬酸缓冲液。1个酶活单位(U)定义为每分钟催化1 μmol ABTS氧化所需的酶量。
2)参照Lowry等(1951)的方法测定蛋白含量。
1.5 菌株生长测量定发酵液10 000 r·min-1 离心10 min,沉淀以pH4.0磷酸缓冲液洗涤3次,转移至纱网上40 ℃烘干至恒质量,即为菌丝干质量(dry cell weight,DCW)。
1.6 产酶条件1)种子液制备: 将适当稀释的供试菌孢子悬液涂布在PDA平板培养至菌丝铺满,以无菌打孔器制备直径1 cm的菌饼。以2%[菌饼数/培养基体积(mL)]接种量接入发酵培养基,30 ℃,140 r·min-1 振荡培养3天,培养物即为种子液。
2)产酶处理设定: 以发酵培养基为基础,考察2%不同碳源及最佳碳源的不同浓度对菌株产漆酶的影响,考察1%不同氮源及最佳氮源的不同浓度对菌株产漆酶的影响,分别考察培养基初始pH为3.0~8.0及自然时菌株产漆酶情况,分别考察装液量、接种量、培养温度及摇床转速对菌株产漆酶的影响,分别考察9种常规漆酶底物对菌株产酶的诱导情况。每个批次的发酵试验培养基及条件均设为上一批次的最优参数,每个试验处理均设置3个重复。
1.7 数据处理利用JMP9.0.2软件进行方差分析及多重比较分析,并用Sigmaplot10.0软件绘图。所有计算结果均用mean±SE表示,统计检验的显著水平设定为0.05。
2 结果与分析 2.1 产漆酶菌株的分离与鉴定漆酶能够将愈创木酚氧化为黄红色,在分离培养基平板上选取显色菌落,转接至PDA平板上培养。利用漆酶分别将ABTS和SGZ氧化为绿色和粉红色的特异性反应,对分离到的菌落定性初筛,同时以摇瓶发酵复筛。菌株NF-08[来源于黄菠萝树(Phellodendron amurense)下土壤样品,128°53′89″E,47°10′76″N]在初筛平板上显色明显快速,复筛摇瓶中产酶活力高、周期短,确定为供试菌株。
菌株NF-08菌落形态如图 1A所示,菌丝为白色绒毛状; 分生孢子由浅到深从淡黄色变为墨绿色。显微形态如图 1B所示,分生孢子呈宽梭状,无色至浅绿色; 分生孢子梗单生或丛生,顶端帚状分枝显微形态; 边缘菌丝为无色至浅褐色卷曲状分枝; 分生孢子座为座状或浅杯状。形态学特征表明菌株NF-08为半知菌亚门(Deuteromycotina)漆斑菌属(Myrothecium sp.)真菌(吴文平,1991; 陆家云,2001)。菌株NF-08 rDNA-ITS部分序列长度538 bp,提交至GenBank获得序列登录号HM347342。BLAST同源性比对结果显示,该菌株与2株疣孢漆斑菌(Myrothecium verrucaria)(序列登录号分别为GQ131886和FJ235085)rDNA-ITS序列相似性均为99%。综合形态学特征和rDNA-ITS序列分析结果,菌株NF-08鉴定为疣孢漆斑菌。
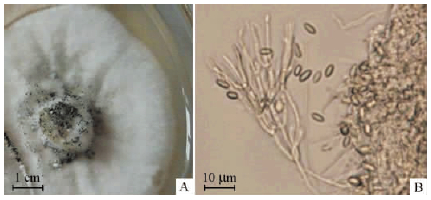 |
图 1 菌株NF-08的形态特征 Fig. 1 Morphological properties of strain NF-08 A.菌落Colony; B 分生孢子Conidiophore. |
如图 2所示,疣孢漆斑菌NF-08在发酵培养基中产漆酶与菌丝生长及胞外总蛋白进程基本同步。发酵初期菌丝生长较慢,发酵液中漆酶活性和胞外蛋白总量都较低。随着菌丝进入快速生长期,菌丝生长和胞外蛋白总量在第7天达到最大值,分别为0.72 mg·mL-1和1.25 mg·mL-1。发酵液漆酶活性在发酵第6天达到最高峰9.28 U·mL-1,疣孢漆斑菌NF-08菌丝生长与漆酶产生部分偶联。漆酶基因在真菌生活史周期的各个阶段均有表达(Mansur et al.,1998),如冷季型香菇(Lentinula edodes)(Ohga et al.,2001)和双孢蘑菇(Agaricus bisporus)(Ohga et al.,1999),在个体生长及子实体形成阶段,菌丝体生长时均伴随漆酶基因表达。赵敏等(2008)曾报道同种产漆酶真菌疣孢漆斑菌NF-05发酵第5天达到产酶高峰8.38 U·mL-1,胞外漆酶分泌与菌体生长部分偶联。本研究中,以ABTS和SGZ检测疣孢漆斑菌NF-08平板菌落时,边缘新生菌丝即可分别快速显现绿色和粉红色,也证明菌丝生长与胞外漆酶分泌偶联。疣孢漆斑菌NF-08在基础发酵培养基中第6天达到产酶高峰,略晚于疣孢漆斑菌NF-05,但酶活峰值提高了10.7%,显著高于疣孢漆斑菌NF-05(P<0.000 1),显示出发酵产漆酶的工业应用潜力。此后,随着菌丝生长衰弱,菌丝自溶,发酵液浑浊且伴有轻微刺鼻气味,漆酶活性和胞外蛋白量均逐渐下降。后续产酶条件研究均选取在发酵第6天测定发酵液漆酶活性。
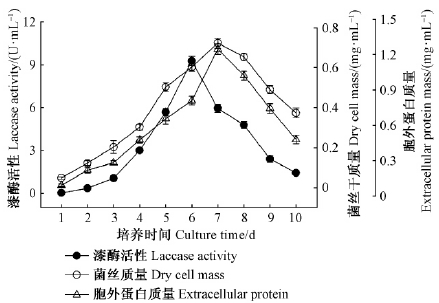 |
图 2 疣孢漆斑菌NF-08生长及产酶进程 Fig. 2 Time course of growth, laccase activity and extracellular protein of M.verrucaria NF-08 |
碳、氮源种类及浓度对疣孢漆斑菌NF-08胞外漆酶产生均有显著影响(P<0.000 1)。由表 1可知,以废糖蜜、可溶性淀粉、木质素磺酸钠、乳糖、甘油和麦芽糖为碳源时,疣孢漆斑菌NF-08漆酶活性均在0.5 U·mL-1以下。疣孢漆斑菌NF-08利用蔗糖、果糖和甘露醇时产酶效率显著提高。葡萄糖是供试碳源中的最优碳源,浓度为4%时,发酵液漆酶活性达到10.60 U·mL-1,且浓度在2%~5%之间时,酶活水平显著高于其他供试浓度。葡萄糖是微生物代谢过程中最易利用的单糖之一,也是半知真菌拟盘多毛孢属产漆酶的最优碳源(Hao et al.,2007)。
|
|
表 2结果表明,在7种供试氮源中,有机氮源显著优于无机碳源,其中蛋白胨最好,与黄乾明等(2006)对粗毛栓菌(Trametes gallica)产漆酶最优氮源的研究结果一致。蛋白胨浓度为3.5%时,疣孢漆斑菌NF-08发酵液漆酶活性最高为12.27 U·mL-1,比基础产酶培养基中的酶活(9.28 U·mL-1)提高了32.2%。
|
|
培养基初始pH对疣孢漆斑菌NF-08胞外漆酶产生有显著影响(P<0.000 1)。如图 3所示,初始pH从3上升至7,发酵液漆酶活性显著提高至13.40 U·mL-1,显著高于pH自然的对照处理(11.31 U·mL-1)。当初始pH升高至8,疣孢漆斑菌NF-08产漆酶水平逐渐下降,pH高于10的碱性范围内,发酵液上清几乎检测不到漆酶活性。
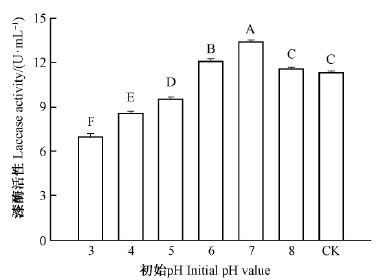 |
图 3 培养基初始pH对疣孢漆斑菌NF-08产漆酶的影响 Fig. 3 Effects of the initial pH on laccase activity produced by M.verrucaria NF-08 |
装液量与接种量对疣孢漆斑菌NF-08胞外漆酶产生均有显著影响(P<0.000 1)。如表 3所示,装液量过多或过少均不利于漆酶的产生。装液量为60 mL·(250 mL)-1时发酵液漆酶活性显著最高为13.50 U·mL-1。接种量过少,菌体生长不足,接种量过多,培养基中营养匮乏。接种量为4%和6%时发酵液漆酶活性显著高于其他接种量且无显著差异。
|
|
培养温度和摇床转速对供试菌发酵产漆酶均有显著影响(P<0.000 1)。如表 4所示,温度过低或过高均不利于疣孢漆斑菌NF-08产酶,30 ℃培养时发酵液漆酶活性最高,为14.67 U·mL-1。摇床转速决定发酵液溶氧水平,显著影响菌株产酶。转速较低使溶氧不足,转速过高则增大菌丝所受剪切力,均不利于菌株生长和产酶。摇床转速为140 r·min-1时发酵液漆酶活性达到最大值14.64 U·mL-1。
|
|
供试漆酶底物对疣孢漆斑菌NF-08产酶有显著影响(P<0.000 1)。多重比较结果如图 4所示,加入丁香醛连氮、对苯二酚、愈创木酚和ABTS的处理与对照组无显著差异; 1-萘酚对供试菌产酶有显著抑制作用; 阿魏酸、单宁酸和没食子酸对产酶有显著诱导作用,其中没食子酸诱导效果最好,最大酶活达16.82 U·mL-1,比对照提高了15.04%。
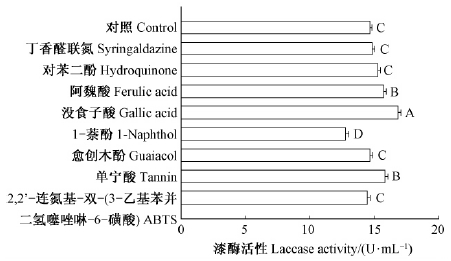 |
图 4 漆酶底物对疣孢漆斑菌NF-08产漆酶的影响 Fig. 4 Effects of laccase substrates on laccase activity produced by M.verrucaria NF-08 |
漆酶能催化生态系统中木质素的合成与分解,文中以木质素磺酸钠为唯一碳源的无机盐培养基可对产漆酶的微生物进行高效富集。愈创木酚作为漆酶催化底物,虽然对微生物生长有毒性而减缓其生长,但低浓度条件下能够增加选择压力从而提高分离效率(Viswanath et al.,2008; 陶君等,2008)。纯化愈创木酚-PDA选择性平板上的阳性菌落,以ABTS和SGZ初筛检测,快速、灵敏地筛选到酶活较高的产酶菌株,同时排除了酚氧化酶产生菌的干扰。
疣孢漆斑属菌半知真菌在植物和土壤中广泛分布,以前报道为植物病原菌(Quezado-Duval et al.,2010)和胆红素氧化酶产生菌(Han et al.,2012)。疣孢漆斑菌是目前报道的该属真菌中唯一产漆酶的种类。Sulistyaningdyah等(2004)纯化了疣孢漆斑菌24G-4碱性漆酶,并比较研究了该酶和商品漆酶的酶学性质及染料脱色特性。葛衡等(2009)报道了疣孢漆斑菌Ws2-6的原生体制备条件。本研究所在课题组在凉水保护区土壤样品中分离到该种产漆酶菌株疣孢漆斑菌NF-05,在基础发酵培养基中发酵第5天达到酶活峰值8.38 U·mL-1,纯化漆酶可催化氧化多种酚胺类底物,高效降解多种合成染料,并具有良好的电化学催化特性(赵敏等,2011; Zhao et al.,2012a; 2012b)。已报道产漆酶的半知菌发酵周期较短,但酶活不高,如Pestalotiopsis sp. J63发酵第5天酶活达到5.8 U·mL-1 (Feng et al.,2013),T.asperellum W03发酵3天半时酶活为0.24 U·mL-1(陈今朝等,2010)。常见产漆酶白腐菌虽然酶活较高,但发酵周期长。目前报道产漆酶活性最高的白腐菌株糙皮侧耳(Pleurotus ostreatus),发酵10天时漆酶活性达到100 U·mL-1(del Vecchio et al.,2012)。本研究供试菌株疣孢漆斑菌NF-08发酵6天即达到漆酶峰值酶活9.28 U·mL-1,显著早于白腐真菌且高于半知菌,是漆酶研究的良好供试菌株。
单因素优化培养基组分和培养条件是初期提高产漆酶微生物发酵水平的常用方法(王乐等,2012)。本研究中考察的碳、氮源种类及浓度、培养基初始pH、装液量、接种量、摇床转速、培养温度及漆酶底物10个单因素,对疣孢漆斑菌NF-08发酵产漆酶均有显著影响。葡萄糖是多种产漆酶真菌的最佳碳源,疣孢漆斑菌NF-08以2%~5%葡萄糖为碳源时产酶活性较高。葡萄糖浓度过高或过低均不利于供试菌产酶,与Ronne(1995)的理论一致,即真菌胞外漆酶常与菌丝生长偶联,葡萄糖浓度过低会使菌丝生长营养不足而限制漆酶分泌,浓度过高则可能导致菌株的节能响应(energy-saving response),抑制漆酶基因表达。Feng等(2013)和Mishra等(2009)等曾尝试以廉价农业副产品为培养基组分开展微生物发酵产漆酶研究。今后研究中应广泛考察疣孢漆斑菌NF-08在廉价易得的培养基组分中的产酶情况,为实现该菌株工业发酵产漆酶提供依据。真菌发酵产漆酶的最适培养基初始pH因种属而异,以微酸性居多: 黑孢漆斑菌(Myrothecium melanosporum)为5.5(王高婕等,2009),虫拟蜡菌(Ceriporiopsis subvermispor)为5.5(王超等,2013),灵芝(Ganoderma lucidum)为6.0(陶君等,2008)。沙链霉菌(Streptomyces psammoticus)在偏碱性的初始pH为7.5时产酶最高(Niladevi et al.,2009)。本研究中疣孢漆斑菌NF-08产酶最适培养基初始pH为6~7,与上述报道结果近似。底物诱导也是提高真菌漆酶活性的常用技术。本研究中没食子酸、阿魏酸和单宁酸对疣孢漆斑菌NF-08漆酶有显著诱导效果,没食子酸最为明显,这与Liu等(2009)、Niladevi等(2008)和Revankar等(2006)的研究结果一致。未来研究将针对不同漆酶底物如金属离子、酚胺类、合成染料类物质,摸索适宜疣孢漆斑菌NF-08胞外漆酶的诱导物种类及添加浓度和时间,以期提高产酶水平。
本研究从凉水自然保护区原始森林土壤样品中分离到了1株产漆酶半知真菌,根据形态学特征及rDNA-ITS序列分析结果鉴定为疣孢漆斑菌疣孢漆斑菌NF-08。与传统漆酶来源白腐真菌相比,该菌株发酵周期短且初始酶活高,发酵第6天产酶活性达到9.28 U·mL-1。经过培养基组分和培养条件的单因素优化及底物诱导,疣孢漆斑菌NF-08产酶水平比复筛得到的原始菌株提高了81.25%,达到16.82 U·mL-1,这为微生物发酵产漆酶提供了新的菌株资源。后续对疣孢漆斑菌NF-08漆酶产生、性质及基因表达调控的深入研究,将有助于了解漆酶在半知真菌生活史中的作用及生态学功能,同时拓展半知真菌漆酶的应用领域。
| [1] |
陈今朝, 王剑锋, 李江, 等. 2010. 煤附生真菌产漆酶菌株的分离鉴定及产酶特性研究. 菌物学报, 29(3): 389-396. (Chen J Z, Wang J F, Li J,et al. 2010. Identification and production of laccase-producing fungal strain isolated from coal. Mycosystema, 29(3): 389-396[in Chinese]).(  2) 2)
|
| [2] |
葛衡,张明. 2009. 产漆酶疣孢漆斑菌Ws2-6原生质体制备条件的研究. 中国酿造, (2): 53-55. (Ge H, Zhang M. 2009.Protoplast preparation of Myrothecium verrucaria Ws2-6 producing laccase. China Brewing, (2): 53-55[in Chinese]).(  1) 1)
|
| [3] |
黄乾明, 谢君, 张寒飞, 等. 2006. 漆酶高产菌株的诱变选育及其产酶条件. 菌物学报, 25(2): 263-272. (Huang Q M, Xie J, Zhang H F,et al. 2006. Mutational screening of fungal strain with high yield of laccase and enzyme production condition of the mutant. Mycosystema, 25(2): 263-272[in Chinese]).(  1) 1)
|
| [4] |
陆家云. 2001. 植物病原真菌学. 北京: 中国农业出版社. (Lu J Y. 2001. Pathegenic fungi of plant.Beijing: China Agriculture Press.[in Chinese])(  1) 1)
|
| [5] |
陶君, 马爱民, 郭爱玲, 等. 2008. 灵芝漆酶活性的测定及其产漆酶条件的优化. 食品科学, 29(3): 310-313. (Tao J, Ma A M, Guo A L,et al. 2008. Detection of laccase activity and optimization of conditions on laccase production by Ganoderma lucidum. Food Science, 29(3): 310-313[in Chinese]).(  2) 2)
|
| [6] |
王超, 余学军, 张琳琳, 等. 2013. 虫拟蜡菌固态发酵产漆酶条件优化. 中国食品学报, 13(6): 97-103. (Wang C, Yu X J, Zhang L L,et al. 2013. Optimization of the conditions for producing laccase of Ceriporiopsis Subvermispor under solid-state fermentation. Journal of Chinese Institute of Food Science and Technology, 13(6): 97-103[in Chinese]).(  1) 1)
|
| [7] |
王高婕, 曹福祥, 龙绛雪, 等. 2009. 黑孢漆斑菌漆酶活性及培养条件的优化. 中南林业科技大学学报, 29(3): 111-113. (Wang G J, Cao F X, Long J X,et al. 2009. Growth conditions of Myrothecium melanosporum for the production of laccase. Journal of Central South University of Forestry & Technology, 29(3): 111-113[in Chinese]).(  1) 1)
|
| [8] |
王乐, 陈辉, 胡霞, 等. 2012. 秦岭细粘束孢产漆酶的培养条件. 林业科学, 48(5): 164-167. (Wang L, Chen H, Hu X,et al. 2012. Laccase activity of Leptographium qinlingensis under different culture conditions. Scientia Silvae Sinicae, 48(5): 164-167[in Chinese]).(  1) 1)
|
| [9] |
王石磊, 张建波, 张建英, 等. 2010. 漆酶及其在纺织工业中的应用. 印染, 36(12): 52-55. (Wang S L, Zhang J B, Zhang J Y,et al. 2010. Laccase and its applications in textile industry. Printing and Dyeing, 36(12): 52-55[in Chinese]).(  1) 1)
|
| [10] |
吴发红, 黄东益, 黄小龙, 等. 2009. 几种真菌DNA提取方法的比较. 中国农学通报, 25(8): 62-64. (Wu F H, Huang D Y, Huang X L, et al. 2009. Comparing study on several methods for DNA extraction from endophytic fungi. Chinese Argricultural Science Bulletin, 25(8): 62-64[in Chinese]).(  1) 1)
|
| [11] |
吴文平. 1991. 河北省丝孢菌研究 Ⅲ、漆斑菌属(Myrothecium Tode:Fr.)的四个种. 河北省科学院学报, (1): 69-74. (Wu W P. 1991.Studies on hyphomycetes in HEBEI Ⅲ, species of the genus Myrothecium tode: fr on plants. Journal of Hebei Academy of Sciences, (1): 69-74[in Chinese]).(  1) 1)
|
| [12] |
燕勇, 李卫平, 高雯洁, 等. 2008. rDNA-ITS序列分析在真菌鉴定中的应用. 中国卫生检验杂志, 18(10): 1958-1961. (Yan Y, Li W P, Gao J W, et al. 2008. Application of rDNA ITS sequence analysis in fungus identification rDNA-ITS. Chinese Journal of Health Laboratory Technology, 18(10): 1958-1961[in Chinese]).(  1) 1)
|
| [13] |
赵敏, 王海东, 赵丹, 等. 2011. 产漆酶疣孢漆斑菌NF-05的分离及对偶氮染料的脱色. 菌物学报, 30(4): 604-611. (Zhao M, Wang H D, Zhao D,et al. 2011. Isolation and decolorization of azo dye of a laccase-producing Myrothecium verrucaria NF-05. Mycosystema, 30(4): 604-611[in Chinese]).(  2) 2)
|
| [14] |
Cabana H, Alexandre C, Agathos S, et al. 2009. Immobilization of laccase from the white rot fungus Coriolopsis polyzona and use of the immobilized biocatalyst for the continuous elimination of endocrine disrupting chemicals. Bioresource Technology, 100 (14): 3447-3458.( 1) 1)
|
| [15] |
Casero E, Petit-Domínguez M, Vázquez L, et al. 2013. Laccase biosensors based on different enzyme immobilization strategies for phenolic compounds determination. Talanta, 115: 401-408.( 1) 1)
|
| [16] |
Daâssi D, Zouari-Mechichi H, Prieto A, et al. 2013. Purification and biochemical characterization of a new alkali-stable laccase from Trametes sp. isolated in Tunisia: role of the enzyme in olive mill waste water treatment. World Journal of Microbiology and Biotechnology, 29 (11): 2145-2155.( 1) 1)
|
| [17] |
del Vecchio C, Lettera V, Pezzella C, et al. 2012. Classical breeding in Pleurotus ostreatus: a natural approach for laccase production improvement. Biocatalysis and Biotransformation, 30 (1): 78-85.( 1) 1)
|
| [18] |
Elegir G, Kindl A, Sadocco P, et al. 2008. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzyme and Microbial Technology, 43 (2): 84-92.( 1) 1)
|
| [19] |
Feng X, Chen H, Xue D, et al. 2013. Enhancement of laccase activity by marine-derived deuteromycete Pestalotiopsis sp. J63 with agricultural residues and inducers. Chinese Journal of Chemical Engineering, 21 (10): 1182-1189.( 3) 3)
|
| [20] |
Fonseca M, Shimizu E, Zapata P, et al. 2010. Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina). Enzyme and Microbial Technology, 46 (6): 534-539.( 1) 1)
|
| [21] |
Han X, Zhao M, Lu L, et al. 2012. Purification, characterization and decolorization of bilirubin oxidase from Myrothecium verrucaria 3.2190. Fungal Biology, 116 (8): 863-871.( 1) 1)
|
| [22] |
Hao J, Song F, Huang F, et al. 2007. Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. Journal of Industrial Microbiology and Biotechnology, 34 (3): 233-240.( 2) 2)
|
| [23] |
Kluczek-Turpeinen B, Tuomela M, Hatakka A, et al. 2003. Lignin degradation in a compost environment by the deuteromycete Paecilomyces inflatus. Applied Microbiology and Biotechnology, 61 (4): 374-379.( 1) 1)
|
| [24] |
Kuddus M, Joseph B, Wasudev-Ramteke P. 2013. Production of laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatalysis and Agricultural Biotechnology, 2(4):333-338.( 1) 1)
|
| [25] |
Liu Z, Zhang D, Hua Z, et al. 2009. A newly isolated Paecilomyces sp. WSH-L07 for laccase production: isolation, identification, and production enhancement by complex inducement. Journal of Industrial Microbiology and Biotechnology, 36 (10): 1315-1321.( 1) 1)
|
| [26] |
Liu Z Y, Zhang D X, Hua Z Z, et al. 2010. Improvement of laccase production and its properties by low-energy ion implantation. Bioprocess and Biosystems Engineering, 33 (5): 639-646.( 1) 1)
|
| [27] |
Lowry O H, Rosebrough N J, Farr A L, et al. 1951. Protein measurement with the folin-phenol reagent. Journal of Biological Chemistry, 193 (1): 265-275.( 1) 1)
|
| [28] |
Mansur M, Suarez T Gonzalez A. 1998. Differential gene expression in the laccase gene family from basidiomycete I-62 (CECT 20197). Applied and Environmental Microbiology, 64 (2): 771-774.( 1) 1)
|
| [29] |
Martín-Sampedro R, Eugenio M, Carbajo J, et al. 2011. Combination of steam explosion and laccase-mediator treatments prior to Eucalyptus globulus kraft pulping. Bioresource Technology, 102 (14): 7183-7189.( 1) 1)
|
| [30] |
Milton R D, Giroud F, Thumser A E, et al. 2013. Hydrogen peroxide produced by glucose oxidase affects the performance of laccase cathodes in glucose/oxygen fuel cells: FAD-dependent glucose dehydrogenase as a replacement. Physical Chemistry Chemical Physics, 15 (44): 19371-19379.( 1) 1)
|
| [31] |
Mishra A, Kumar S. 2009. Kinetic studies of laccase enzyme of Coriolus versicolor MTCC 138 in an inexpensive culture medium. Biochemical Engineering Journal, 46 (3): 252-256.( 1) 1)
|
| [32] |
Niladevi K N, Prema P. 2008. Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresource Technology, 99 (11): 4583-4589.( 1) 1)
|
| [33] |
Niladevi K N, Sukumaran R K, Jacob N, et al. 2009. Optimization of laccase production from a novel strain-Streptomyces psammoticus using response surface methodology. Microbiological Research, 164 (1): 105-113.( 2) 2)
|
| [34] |
Ohga S, Royse D. 2001. Transcriptional regulation of laccase and cellulase genes during growth and fruiting of Lentinula edodes on supplemented sawdust. FEMS Microbiology Letters, 201 (1): 111-115.( 1) 1)
|
| [35] |
Ohga S, Smith M, Thurston C, et al. 1999. Transcriptional regulation of laccase and cellulase genes in the mycelium of Agaricus bisporus during fruit body development on a solid substrate. Mycological Research, 103 (12): 1557-1560.( 1) 1)
|
| [36] |
Quezado-Duval A, Henz G, Paz-Lima M, et al. 2010. New hosts of Myrothecium spp. in Brazil and a preliminary in vitro assay of fungicides. Brazilian Journal of Microbiology, 41 (1): 246-252.( 1) 1)
|
| [37] |
Revankar M, Lele S. 2006. Enhanced production of laccase using a new isolate of white rot fungus WR-1. Process Biochemistry, 41 (3): 581-588.( 1) 1)
|
| [38] |
Ronne H. 1995. Glucose repression in fungi. Trends in Genetics, 11 (1): 12-17.( 1) 1)
|
| [39] |
Sulistyaningdyah W T, Ogawa J, Tanaka H, et al. 2004. Characterization of alkaliphilic laccase activity in the culture supernatant of Myrothecium verrucaria 24G-4 in comparison with bilirubin oxidase. FEMS Microbiology Letters, 230 (2): 209-215.( 1) 1)
|
| [40] |
Viswanath B, Chandra M, Pallavi H, et al. 2008. Screening and assessment of laccase producing fungi isolated from different environmental samples. African Journal of Biotechnology, 7 (8): 1129-1133.( 1) 1)
|
| [41] |
Yoshida H. 1883. Chemistry of lacquer (urushi). Journal of Chemical Society, 43: 472-486.( 1) 1)
|
| [42] |
Zhao D, Cui D Z, Zhang X, et al. 2012a. Oxidation of aromatic compounds and bioelectrocatalysis of peroxide by a novel white laccase from Myrothecium verrucaria NF-05. Catalysis Communications, 31: 48-51.( 1) 1)
|
| [43] |
Zhao D, Zhang X, Cui D Z, et al. 2012b. Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes. PloS one, 7 (6): e38817.( 1) 1)
|
| [44] |
Zheng M, Chi Y, Yi H, et al. 2013. Decolorization of Alizarin Red and other synthetic dyes by a recombinant laccase from Pichia pastoris. Biotechnology Letters, 1-7.( 1) 1)
|
 2015, Vol. 51
2015, Vol. 51

