文章信息
- 李金航, 齐秀慧, 徐程扬, 王畅, 刘海轩, 孙鹏
- Li Jinhang, Qi Xiuhui, Xu Chengyang, Wang Chang, Liu Haixuan, Sun Peng
- 黄栌幼苗叶片气体交换对干旱胁迫的短期响应
- Short-Term Responses of Leaf Gas Exchange Characteristics to Drought Stress of Cotinus coggygria Seedlings
- 林业科学, 2015, 51(1): 29-41
- Scientia Silvae Sinicae, 2015, 51(1): 29-41.
- DOI: 10.11707/j.1001-7488.20150104
-
文章历史
- 收稿日期:2014-04-15
- 修回日期:2014-07-20
-
作者相关文章
光合作用是植物生长发育和产量构成的重要基础(许大全,2002; 苏华等,2012; 季杨等,2013),而土壤水分是限制陆生植物光合速率的重要环境因子(Flexas et al.,2009; Keenan et al.,2010; Martin-StPaul et al.,2012; 王荣荣等,2013),光合生理可塑性是树种适应干旱胁迫环境的机制(Keenan et al.,2010; Pinheiro et al., 2011; 田治国等,2011; Hommel et al.,2014),受胁迫程度和胁迫时间等因素共同影响(Chaves et al.,2009)。轻度干旱胁迫可导致植物叶片气孔部分关闭(夏江宝等,2007; Chaves et al.,2009),严重干旱胁迫可导致叶片光合结构受损(马富举等,2012)、光化学代谢过程受阻(Flexas et al.,2002)、叶肉细胞光合能力显著降低(Yordanov et al.,2000)。叶片气孔导度下降(Grassi et al.,2005; Flexas et al.,2009)、大气进入叶绿体羧化位点的CO2浓度降低(Campos et al.,2014)是干旱胁迫环境中叶片光合生产力降低的重要原因,而干旱胁迫诱发的植物光保护机制与光化学代谢共同竞争叶片所吸收的光能量(张亚黎等,2008; Pinheiro et al.,2011; Campos et al.,2014)也导致叶片光合碳同化速率大幅下降。气孔导度和叶肉导度的调控是干旱环境中植物平衡细胞失水与CO2吸收的有效方式(冯玉龙等,2003; Galmés et al.,2007; Hommel et al.,2014),轻度干旱胁迫或干旱胁迫初期,植物的净光合速率主要受气孔关闭影响(Flexas et al.,2002; Ramalho et al.,2014),而叶肉扩散限制成为严重干旱胁迫环境中叶片净光合速率下降的主要原因(崔晓阳等,2004; 夏江宝等,2007; Chaves et al.,2009; Flexas et al.,2012; 季杨等,2013; Muir et al.,2014; Tomás et al.,2013)。
黄栌(Cotinus coggygria),属于漆树科(Anacardiaceae)黄栌属(Cotinus)落叶灌木或小乔木,是我国华北地区重要的秋季彩叶树种,被广泛应用于风景林建设(陈书文等,2005; 李海龙等,2009)。黄栌根系发达、萌蘖力强,耐干旱、瘠薄及碱性土壤,是干瘠山区造林绿化的优良先锋树种(陆秀君等,2001; 李红云等,2006)。目前国内外学者在黄栌组培快繁技术(陈书文等,2005; Pacholczak et al.,2005; Metivier et al.,2007)、容器育苗技术(陆秀君等,2001)、叶片色素(Valianou et al.,2009; 葛雨萱等,2011; Mantzouris et al.,2011)、化学成分及药理(Matic et al.,2011; 张天龙等,2011)、病虫害防治(葛瑾等,2007; 杜万光等,2011)等方面开展了大量研究,有关黄栌光合生理及水分生理的研究主要集中于光合动态(尤扬等,2009)、水分与光合关系(刘刚等,2010)、空气湿度与光合关系(杨吉华等,2002)、蒸腾耗水(周平等,2002; 鲍玉海等,2005; 王瑞辉等,2009)等方面,不同产地间抗旱机制研究方面仅有根系形态特征分异性的报道(李金航等,2014),不同产地叶片光合作用对干旱胁迫的响应方面则未见报道。因此,本研究采用大田控水试验方法,探讨华北地区不同产地黄栌幼苗叶片气体交换对干旱胁迫的响应规律,以期为筛选抗旱能力较强的黄栌造林绿化材料奠定理论基础。
1 材料与方法 1.1 试验地概况试验地设在北京林业大学实验林场的普昭院苗圃(39°54′ N,116°28′ E)。北京市属典型的暖温带半湿润大陆性季风气候,年平均气温12.1 ℃,年平均最高气温39.7 ℃,年平均最低气温-19.6 ℃,≥10 ℃有效积温4 200 ℃左右。年均降雨量644 mm,其中7—9月的降水量占全年降水量的70%以上。年平均日照时数2 769 h,植物生长期222天,无霜期180天。试验地土壤属山地淤积母质淋溶褐土,pH约7.6,土壤有机质含量1.66%~1.96%,全N、速效P、速效K含量分别为770,5.4,180 mg·kg-1。
1.2 试验材料试验材料采用1年生天然更新苗分别是北京西山(BJ):苗高(19.16±1.93)cm,地径(4.26±0.17)mm;山东泰山(SD):苗高(20.22±2.05)cm,地径(3.12±0.16)mm);山西绛县(SX): 苗高(22.62±2.11)cm,地径(4.41±0.24)mm。2011年5月20日—6月11日将个体大小相对一致的天然更新苗木移植到普昭院苗圃地中,试验区面积360 m2(20 m×18 m),幼苗按2 m× 2 m株行距栽植,共栽植90株。
1.3 干旱处理土壤水分梯度的设置: 采用田间试验,于2011年9月15日起对3个产地的幼苗进行干旱胁迫处理,设置对照(CK,土壤田间持水量的75%~80%)、中度胁迫(MS,土壤田间持水量的55%~65%)、重度胁迫(SS,土壤田间持水量的35%~45%)3个水分梯度,每个水分处理面积约108 m2,每处理30株。试验期间,每隔7天采用烘干称重法对不同处理下的土壤含水量进行测定。
干旱胁迫处理: 干旱胁迫处理前充足浇水3天,此后每天傍晚根据预试验土壤水分损失情况进行补水,使土壤含水量维持在3个水分梯度水平。
1.4 气体交换参数的测定2011年9月初(胁迫前)、9月下旬(胁迫中期)和10月中旬(胁迫后期),随机选取3个产地幼苗接近平均水平的健康苗木各3~5株,利用LI-6400便携式光合测定系统(LI-COR Inc.,USA),在8:30—11:00期间,采用焦念元等(2013)的方法测定样株中上部成熟叶片的光响应曲线(Pn-PAR)及CO2响应 曲线 (Pn-Ci)。测定Pn-PAR曲线时,将叶室内的CO2浓度控制在400 μmol·mol-1; 测定Pn-Ci曲线时,将光照强度稳定在 1 700 μmol·m-2s-1。开启仪器自动控温系统将叶室温度和相对湿度分别维持在 (25±1)℃和 (35±5)%,待叶片在每个光强或CO2浓度平衡2~3 min后开始记录数据。
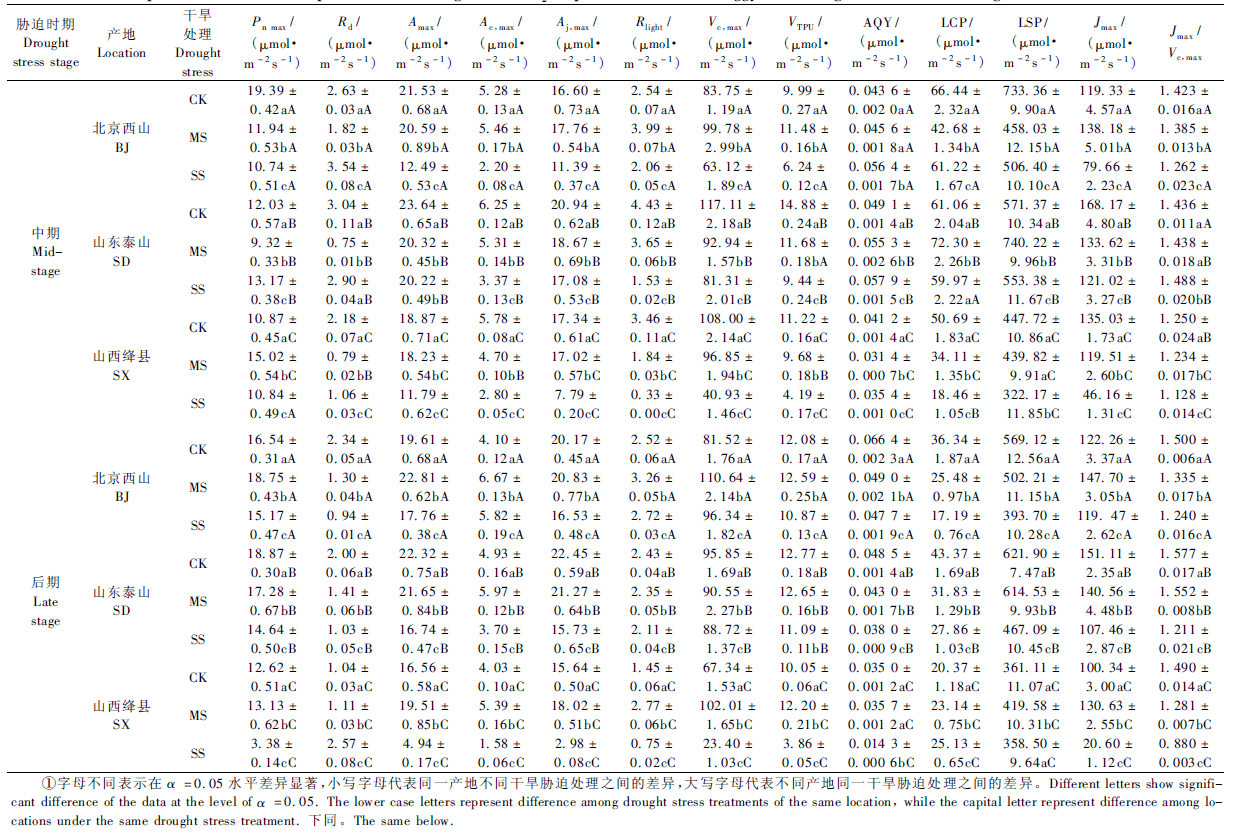
1.5 气体交换参数的计算 1.5.1 光响应参数采用非直角双曲线模型(Thornley,1976; 王荣荣等,2013)计算幼苗的Pn,max (μmol·m-2s-1)、LCP(μmol·m-2s-1)、LSP(μmol·m-2s-1)和Rd (μmol·m-2s-1),并通过对弱光范围(PAR≤ 200 μmol·m-2s-1)内的Pn-PAR曲线进行线性回归拟合计算AQY(μmol·m-2s-1)(表 1)。
| $${P_{\rm{a}}} = \frac{{{\rm{AQY}} \times {\rm{PAR}} + {P_{{\rm{n}},\max }} - \sqrt {{{\left( {{\rm{AQY}} \times {\rm{PAR}} + {P_{{\rm{n}},\max }}} \right)}^2} - 4 \times {\rm{AQY}} \times {\rm{PAR}} + {P_{{\rm{n}},\max }}} }}{{2\theta }} - {R_{\rm{d}}}。$$ | (1) |
式中: PAR为光合有效辐射(μmol·m-2s-1);θ为曲线曲角。
|
|
根据经典FvCB光合生化模型(Farquhar et al.,1980; Bernacchi et al.,2003; Sharkey et al.,2007):
| $${A_{\rm{n}}} = \min \left\{ {{w_{\rm{c}}},{w_{\rm{j}}},{w_{\rm{p}}}} \right\}\left( {1 - \frac{{{\Gamma ^*}}}{{{C_{\rm{i}}}}}} \right) - {R_{{\rm{light}}}},$$ | (2) |
当胞间CO2浓度较低,光合速率受Rubisco数量、活性及其动力学特性限制时:
| $${w_{\rm{c}}} = \frac{{{V_{{\rm{c}},\max }}{C_{\rm{i}}}}}{{{C_{\rm{i}}} + {K_{\rm{c}}}\left( {1 + O/{K_{\rm{o}}}} \right)}};$$ | (3) |
当胞间CO2浓度较高,RuBP再生能力成为限制叶绿体羧化速率的主要因素,而RuBP的再生速率取决于电子在PSⅡ电子传递链的传递速率时:
| $${w_{\rm{j}}} = \frac{{J{C_{\rm{i}}}}}{{4.5{C_{\rm{i}}} = 10.5{\Gamma ^ * }}};$$ | (4) |
当叶片羧化速率仅受磷酸丙糖利用速率限制时:
| $${w_{\rm{p}}} = \frac{{3{V_{{\rm{TPU}}}}}}{{1 - {\Gamma ^ * }/{C_{\rm{i}}}}}。$$ | (5) |
式中: wc,wj,wp分别为由Rubisco活性、RuBP及无机磷酸再生所限制的CO2潜在同化速率(μmol·m-2s-1); Γ*为不包括暗呼吸的CO2补偿点(μmol·mol-1); J为用于RuBP再生的电子传递速率(μmol·m-2s-1); Kc(μmol·mol-1)和Ko(mmol·mol-1)分别为羧化和加氧作用的Michaelis-Menten常数; O为叶绿体羧化部位氧气分压(通常默认为210 mmol·mol-1)(Farquhar et al.,1980)。
利用式(3)、(4)计算Ac,max (μmol·m-2s-1)和Aj,max (μmol·m-2s-1),Jmax (μmol·m-2s-1)和Amax (μmol·m-2s-1)则分别根据式(6)、(7)计算(McMurtrie et al.,1993; Ögren et al.,1993; Bernacchi et al.,2003; Ethier et al.,2004; Sharkey et al.,2007)(表 1)。
| $${\theta _{{\rm{PSII}}}}{J^2} - \left( {{Q_2} + {J_{\max }}} \right)J + {Q_2}{J_{\max }} = 0,$$ | (6) |
| $${A_{\max }} = \frac{{{J_{\max }}\left( {{C_{\rm{i}}} - {\Gamma ^ * }} \right)}}{{4.5{C_i} + 10.5{\Gamma ^ * }}} - {R_{{\rm{light}}}}。$$ | (7) |
式中: Q2为叶片吸收的光量子中用于电子传递的潜在最大数量(μmol·m-2s-1); θPSII 为曲线曲角。
按照Farquhar等(1980)、Bernacchi等(2001)和Long等(2003)方法校正依赖于温度的参数Kc,Ko和Γ*,参照Harley等(1992)、McMurtrie等(1993)、Medlyn等(2002)和Lenz等(2010)的温度函数计算Vc,max和Jmax(表 1)。
本研究中,Vc,max,Jmax,VTPU分别于Ci≤200 μmol·mol-1,200 μmol·mol-1<Ci≤ 900 μmol·mol-1和Ci> 900 μmol·mol-1计算。
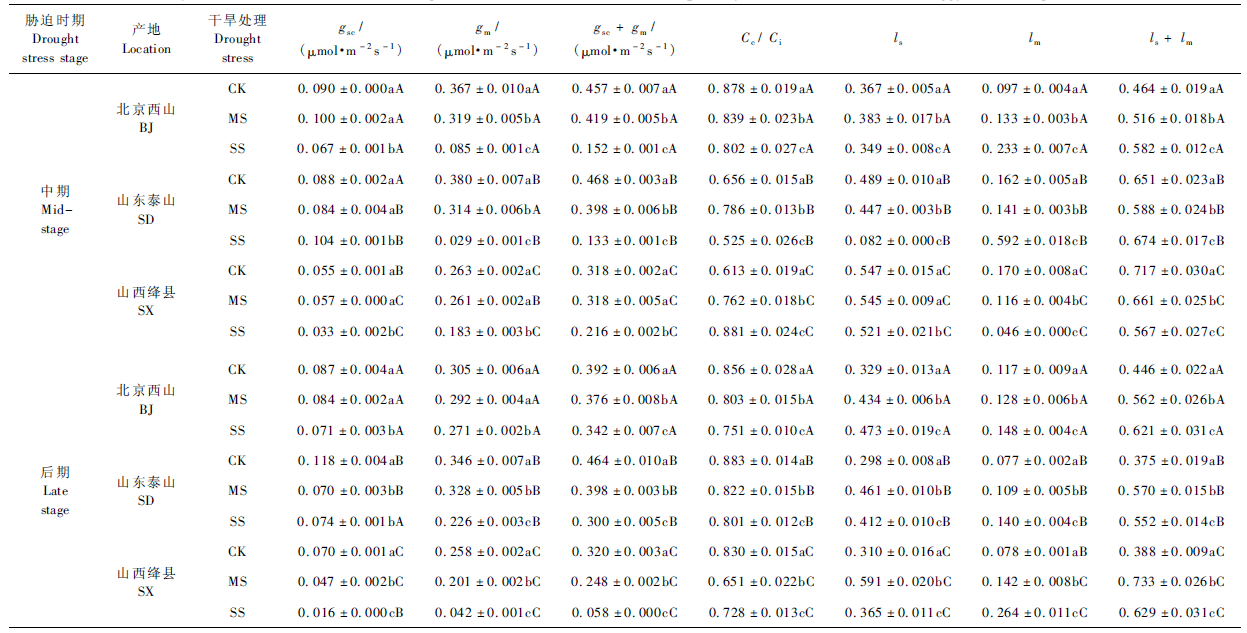
1.5.3 气体交换限制的定量分析由式(2)及(8)~(11)计算gm(μmol·m-2s-1)及 Cc (μmol·m-2s-1)(Wilson et al.,2000; Ethier et al., 2004; Grassi et al., 2005; Galmés et al.,2007; Sharkey et al.,2007; Niinemets et al.,2009)(表 1)。
| $${g_{{\rm{tot}}}} = \frac{{{A_{\rm{n}}}}}{{{C_{\rm{a}}} - {C_{\rm{i}}}}},$$ | (8) |
| $${g_{{\rm{sc}}}} = \frac{{{g_{{\rm{sw}}}}}}{{1.6}},$$ | (9) |
| $$\frac{1}{{{g_{{\rm{tot}}}}}} = \frac{1}{{{g_{{\rm{sc}}}}}} + \frac{1}{{{g_{\rm{m}}}}},$$ | (10) |
| $${C_{\rm{c}}} = {C_{\rm{i}}} - \frac{{{A_{\rm{n}}}}}{{{g_{\rm{m}}}}}。$$ | (11) |
ls和lm则根据式(12)~(14)得出(Grassi et al.,2005; Flexas et al.,2012; Muir et al.,2014)(表 1)。
| $${l_{\rm{s}}} = \frac{{{g_{{\rm{tot}}}}k}}{{{g_{{\rm{sc}}}}\left( {{g_{{\rm{tot}}}} + k} \right)}},$$ | (12) |
| $${l_{\rm{m}}} = \frac{{{g_{{\rm{tot}}}}k}}{{{g_{\rm{m}}}\left( {{g_{{\rm{tot}}}} + k} \right)}},$$ | (13) |
| (14) |
利用Origin 9.1软件绘制图表,SPSS 20.0软件拟合光合模型并进行差异显著性检验(显著性α =0.05),根据非线性最小二乘法的Levenberg-Marquardt迭代原理计算光合参数。
干旱胁迫中、后期光响应及 CO2响应曲线各特征参数相对变化量的计算为:(干旱胁迫后各参数值-干旱胁迫前各参数值)/ 干旱胁迫前各参数值。
2 结果与分析 2.1 持续干旱胁迫对黄栌幼苗叶片CO2同化能力的影响黄栌幼苗叶片的CO2同化能力随干旱胁迫程度的加剧和胁迫时间的延长显著下降(P <0.05)(表 2)。中度胁迫环境中幼苗的Pn,max,Amax,Ac,max,Aj,max,Vc,max,VTPU,Rlight在胁迫中期分别比对照低14.2%,7.7%,2.1%,2.6%,6.2%,9.0%,9.1%,而在重度胁迫环境中则分别比对照低17.8%,30.5%,42.9%,33.9%,40.0%,44.9%,62.4%; 受重度胁迫处理幼苗的Pn,max,Amax,Aj,max在胁迫后期分别比对照低30.9%,32.6%,39.5%。
|
|
在土壤水分胁迫环境中,不同产地黄栌幼苗叶片的CO2同化能力差异显著(P <0.05),其中,山东泰山幼苗叶片的CO2同化能力在重度胁迫环境中显著高于其他产地,而北京产地幼苗叶片的CO2同化能力显著高于山西产地(表 2,图 1)。总体上, 受重度胁迫处理山东泰山幼苗的Pn,max,Amax,Ac,max,Aj,max,Vc,max,VTPU,Rd,Rlight分别是山西幼苗的1.96,2.21,2.51,3.05,2.64,2.55,1.08,3.37倍,而重度胁迫环境中北京幼苗叶片的Pn,max,Amax,Ac,max,Aj,max,Vc,max,VTPU在胁迫后期分别是山西幼苗的4.49,3.60,4.82,5.55,4.12,2.82倍。重度胁迫环境中山西幼苗Pn,max,Amax,Ac,max,Aj,max,Vc,max,VTPU的降幅(相对胁迫处理前)分别比北京产地和山东产地的幼苗高38.0%和50.3%、40.7%和46.3%、45.7%和51.2%、52.4%和54.2%、47.3%和55.8%、55.5%和82.6%。
 |
图 1 持续干旱胁迫环境中黄栌幼苗CO2同化能力参数的相对变化 Fig. 1 Relative characteristic parameters of CO2 assimilation capacity of C. coggyria seedlings under continuous drought stress |
干旱胁迫显著降低黄栌幼苗的叶片导度(P <0.05),受严重干旱胁迫处理幼苗的叶肉导度对其CO2同化作用的影响大于气孔导度(表 3),主要表现为中度和重度胁迫环境中幼苗叶片的gsc,gm,gsc+ gm,Cc/Ci分别比对照低13.0%和28.1%、10.6%和56.4%、11.1%和50.5%、1.1%和4.8%。干旱胁迫环境中幼苗的gsc,gm在胁迫后期比在胁迫中期分别低18.7%,81.0%。
气孔限制和叶肉扩散限制分别是适度干旱和重度干旱胁迫环境中导致黄栌幼苗净光合速率下降的主要原因,叶肉扩散限制对幼苗净光合速率的影响随胁迫程度的加剧及胁迫时间的延长而更加显著(表 3)。受中度胁迫处理苗木的ls,lm分别比对照高36.9%和25.3%; 受重度胁迫处理幼苗的ls,lm分别比对照高9.7%和103.0%。持续干旱胁迫环境中幼苗的平均ls及平均lm在胁迫中期分别比对照高17.7%和46.0%,在胁迫后期则分别比对照高47.0%和71.1%。
在持续干旱胁迫环境中,叶片导度在不同产地幼苗间具有显著差异(P <0.05)(表 3),尤其在胁迫后期,山西绛县幼苗的平均gm分别比北京和山东产地幼苗低56.8%和56.1%,而平均lm分别比北京和山东产地幼苗高47.1%和63.1%,平均CO2扩散限制值(ls + lm)则分别高15.1%和21.4%。
|
严重干旱胁迫环境显著加剧了黄栌幼苗光合能力的下降程度(P <0.05)(图 1),主要是因为幼苗的CO2传导及光合电子传递受阻、光能利用率下降显著抑制了叶片的光合潜力(表 2,3)。受重度胁迫处理幼苗的gsc + gm,AQY,LSP-LCP,Jmax,Jmax/Vc,max分别比对照低50.5%,12.0%,21.0%,37.9%,16.9%。幼苗的gsc,gm,AQY,LSP-LCP,Jmax/Vc,max在胁迫后期分别比在胁迫中期低18.7%,81.0%,19.3%,4.6%,5.5%。重度胁迫环境中山西幼苗的gsc + gm,AQY,Jmax,Jmax / Vc,max的降幅(相对对照)分别比北京产地和山东产地幼苗高和15.8%和4.0%、29.4%和33.0%、54.1%和43.2%、12.3%和16.3%,说明山西产地幼苗对严重干旱胁迫环境的反应比北京产地和山东产地更加强烈。 受持续干旱胁迫处理幼苗的AQY,LCP,LSP,Rd,Vc,max,Jmax,Jmax/Vc,max分别是胁迫处理前的1.04,0.63,0.91,0.83,1.05,0.74,0.67倍(图 1,2),因此,加强对弱光的转化利用、提高叶片Rubisco活性是黄栌苗木光合代谢适应干旱逆境的重要策略。山西受持续干旱胁迫处理幼苗的 AQY,LCP,LSP,Rd,Vc,max,Jmax分别比北京产地和山东产地低41.2%和39.9%、31.2%和47.5%、17.2%和35.2%、27.2%和9.2%、28.8%和25.6%、34.7%和37.0%,说明持续干旱胁迫环境中山西黄栌幼苗可通过降低光补偿点、减少呼吸损耗而弥补由水分亏缺导致的碳损失。 3 结论与讨论持续干旱胁迫环境中3个产地黄栌幼苗叶片的CO2同化能力具有显著差异。光合作用变化是植物在干旱环境中调节生长发育与碳供给平衡的重要反应之一(Yordanov et al.,2000; Flexas et al.,2009),土壤水分含量的降低极大地限制了植物生长和产量(Martin-StPaul et al.,2012; Ramalho et al.,2014),而植物光合作用的自我调节适应能力是长期进化的结果(张亚黎等,2008; Pinheiro et al.,2011; 苏华等,2012; Ramalho et al.,2014),与植物种类、种源(或产地)和品种,干旱胁迫程度和胁迫时间等具有密切关系(Chaves et al.,2009; Hommel et al.,2014)。持续干旱胁迫环境中3个产地黄栌幼苗叶片光能利用参数变化规律相对一致,但干旱环境中各产地幼苗光合能力受抑制程度存在明显差异(图 1,2),说明除干旱胁迫程度及胁迫时间外,产地也是影响黄栌苗木光合作用对水分亏缺反应的重要因素。 严重干旱胁迫环境中,黄栌幼苗CO2传导及光合电子传递受阻、光能利用率下降,叶片光合同化能力因而显著下降。干旱环境中植物叶片气孔保卫细胞组织水势下降(Yordanov et al.,2000; 夏江宝等,2007),叶片气孔部分关闭,气孔导度及叶肉细胞导度显著下降(马富举等,2012),叶绿体羧化部位CO2浓度降低(张彦敏等,2012),ATP合成及RuBP再生受阻(Flexas et al.,2002),导致Rubisco光合作用限速酶蛋白含量及活化水平下降(Chave et al.,2009; 崔晓阳等,2004)、叶片碳水化合物输出减缓(曾伟等,2008)。严重干旱胁迫环境中叶片光合机构受损加剧了光化学代谢的受抑制程度(Flexas et al.,2002; Martin-StPaul et al.,2012),光合产物积累大幅降低(Yordanov et al.,2000; 季杨等,2013)。重度胁迫环境中黄栌幼苗的gsc + gm,AQY,LSP-LCP和Jmax明显下降,幼苗的Pn,max,Amax,Ac,max,Aj,max,Vc,max和VTPU由此显著降低(表 2,3)。暗呼吸及光呼吸速率反映了植物的生理活性及代谢水平(苏华等,2012; 王荣荣等,2013),此外,光呼吸可通过加速植物体内无机磷的周转利用而减轻光抑制、防止光破坏(苏华等,2012),暗呼吸对光合机构也具有一定的保护作用(许大全,2002)。重度胁迫环境中幼苗的Rd及Rlight均显著低于对照(表 2),说明水分亏缺导致黄栌苗木光合作用自我保护能力降低,而幼苗可通过减少光合产物的消耗而维持体内碳收支平衡。 黄栌幼苗通过增强弱光利用能力、提高叶片Rubisco活性而应对干旱逆境。受持续干旱胁迫处理幼苗的AQY和Vc,max均高于胁迫处理前,而LCP,LSP,Rd,Jmax则较胁迫处理前低(图 2),说明干旱环境中黄栌幼苗可在一定程度上向利于其正常光合代谢的方向进行物质及能量的再分配。
适度干旱胁迫环境中,黄栌苗木净光合速率主要受气孔限制因素影响,而叶肉扩散限制为严重干旱胁迫环境中导致叶片净光合速率下降的主要原因。适度干旱胁迫环境中植物叶肉细胞膨压降低、根系向地上部释放ABA等化学信号(Chaves et al.,2009; Ramalho et al.,2014),叶片气孔部分关闭,气孔导度的降低有利于植株维持体内水分含量,从而保证水分利用效率(Pinheiro et al.,2011; Hommel et al.,2014; Ramalho et al.,2014)。叶肉导度是限制苗木光合生产力的重要因素(Galmés et al.,2007; Flexas et al.,2012; Muir et al.,2014; Tomás et al.,2013),受叶片物理特性(如CO2溶解度、叶绿体外膜表面积、叶片单位面积干质量)和代谢过程(如水通道蛋白运输、碳酸酐酶催化HCO3-和CO2的可逆反应)等因素影响(Flexas et al.,2009; Pinheiro et al.,2011; Tomás et al.,2013; Hommel et al.,2014),当干旱胁迫程度加剧或胁迫时间延长时,植物叶肉细胞渗透性降低、叶片失水收缩(Chaves et al.,2009; Flexas et al.,2008; 2012),光合生化代谢机构受损(崔晓阳等,2004; 季杨等,2013),叶肉导度显著下降,叶肉扩散限制逐渐取代气孔限制而占据主导地位(夏江宝等,2007; Flexas et al.,2008)。黄栌幼苗叶片气孔限制及叶肉扩散限制作用在持续中度和重度环境中均有所增加,但叶肉扩散限制随胁迫程度的加剧及胁迫时间的延长而愈加显著,土壤干旱环境中黄栌苗木光合作用限制表现出明显的产地差异(表 3)。 综上所述,北京西山、山东泰山和山西绛县3产地黄栌幼苗对持续干旱胁迫环境反应的敏感性具有显著差异。总体而言,重度胁迫环境中山东泰山幼苗叶片的碳同化能力显著高于北京产地和山西产地,而北京产地幼苗叶片的碳同化能力显著高于山西产地。持续干旱胁迫环境中山西产地幼苗的CO2扩散阻力及光合电子传递阻力显著高于北京产地和山东产地,而该产地的 LCP及Rd在3产地中均最低,由此可见,山西产地黄栌幼苗对水分亏缺的反应较北京产地和山东产地剧烈,但该产地幼苗通过降低光补偿点及暗呼吸代谢水平以保证光合同化产物积累的能力相对较强。
参考文献(References)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 2015, Vol. 51
2015, Vol. 51


 1)
1)