文章信息
- 王传宏, 刘超, 刘静, 向伟, 黄先智, 徐立
- Wang Chuanhong, Liu Chao, Liu Jing, Xiang Wei, Huang Xianzhi, Xu Li
- 新疆药用植物黑桑的抗氧化性
- Antioxidant Activity of Medicine Mulberry (Morus nigra) in Xinjiang
- 林业科学, 2014, 50(8): 53-59
- Scientia Silvae Sinicae, 2014, 50(8): 53-59.
- DOI: 10.11707/j.1001-7488.20140808
-
文章历史
- 收稿日期:2013-10-14
- 修回日期:2014-06-05
-
作者相关文章
Reactive oxygen species(ROS), kinds of active chemical materials which were generated by the biological oxidation, includes O2-, H2O2, ·OH and so on(Sies, 1986). With the process of oxidation in vivo, ROS will result in the damage to all kinds of biological macromolecules, such as protein, fat, and DNA(David et al., 1997; Shukla et al., 2009). Furthermore, this damage is also likely to contribute to the decline and death of cells, coronary heart diseases, atheromas, cancers, diabetes, arthritis, neurological disorders etc(Germanas et al., 2007; Devasagayam et al., 2004; Finkel et al., 2000).
Many synthetic antioxidants such as butylated hydroxyanisole(BHA) and butylated hydroxytoluene(BHT), which have been proved to possess potential health risk to human beings(Botterweck et al., 2000), are hoped to be replaced by some natural antioxidants. Recent studies suggest that blueberry and apple extracts could prolong the life of fruit flies(Peng et al., 2012; 2011), and daily consumption of blueberry juice would improve elderly people’s memory function(Krikorian et al., 2010). All of these functions result from the great antioxidant capacity of natural products. Furthermore, plants’ substances with great antioxidant capacities and health benefits have been extracted from persimmon skin, orange peel, tea, grape seeds and skins, even some of which are of great interest in the industry after they are put into practice(Wolfe et al., 2003; Bocco et al., 1998; Tang et al., 2002; Yusuf et al., 2004).
Medicine mulberry(Morus nigra)which is rarely seen in nature is the only black mulberry in China. Medicine mulberry is regarded as folk medicine by Uygur people, ethanol extracts of whose fruits have been drinking to keep fit for a long time, and its fruits are often used as anti-pharyngitis drugs. However, reports on the antioxidant activity of extracts from each part of it are still unknown. Therefore, this investigation is going to explore the antioxidant activity of the extracts of Medicine mulberry’s fruits, branches, leaves, and roots, in terms of total polyphenols content, total flavonoids content, free radical scavenging capacity and Fe2+-chelating activity, aiming to find a new natural green food antioxidant and lay a foundation for the future reasonable development of Medicine mulberry.
1 Materials and methods 1.1 MaterialsAscorbic acid(Vc), gallic acid, rutin, 1,1-diphenyl-2-picrylhydrazyl(DPPH) and lecithin were purchased from Sigma(St. Louis, Mo, USA); potassium ferricyanide, ferrozine, thiobarbituric acid(TBA), phosphoric acid, ethanol, sodium hydroxide, trichloroacetic acid(TDA), sodium carbonate were purchased from Kelong(Chengdu, Sichuan, China); iMark microplate reader(Japan), FA2004A electronic balance(Shanghai, China), B.U Chi R-210 rotavapor(Flawil, Switzerl and ).
The samples of Medicine mulberry(Morus nigra)were collected from Xinjiang Hetian Sericulture Science Institute in November 2012. The leaves, branches and roots were dried at 50 ℃ and the fruits stored in -80 ℃ refrigerator.
1.2 Sample preparationEach sample(20 g)was powdered at low temperature and extracted with 200 mL 75%(V/V)ethanol, for 2 h, 3 times, and the filtrate was evaporated to dryness until the weight would never change. The dried extracts of each sample were named as EEML, EEMB, EEMF and EEMR(ethanolic extracts of mulberry leaves, branches, fruits and roots, respectively). The volume was adjusted with 75%(V/V)ethanol to 500 mL. Percentage yields of EEML, EEMB, EEMF and EEMR of dry weight were 20.16%, 7.7%, 86.76% and 17.65%(w/w), respectively.
1.3 Total polyphenols contentTotal polyphenols content was estimated by using the Folin-Ciocalteu colorimetric method described previously but with a slight modification(Bae et al., 2007). 0.1 mL extract was mixed with 0.1 mL Folin-Ciocalteu reagent(50%, V/V), shaken vigorously, and after 5 min reaction, 3 mL Na2CO3(0.2 g·mL-1)was added into each mixture. The reaction mixture was incubated at 20 ℃ for 2 h. The absorbance was determined at 765 nm. Results were compared with a gallic acid calibration curve and expressed as milligram of gallic acid equivalent per one mL of extracts.
1.4 Total flavonoids contentThe total flavonoids content assay was carried out by the previously described way with a few modifications and used rutin as a st and ard flavonoid(Hairi et al., 1991). 0.5 mL(50 mg·mL-1)NaNO2 was added into 0.1 mL extract and shaken vigorously, leaving the mixture at room temperature for 6 min, and then, 0.5 mL Al(NO3)3 was added into reaction mixture. After 6 min st and ing, 2.5 mL NaOH was mixed together with mixture and left at room temperature for 15 min. The absorbance was determined at 510 nm. Results were compared with a rutin calibration curve and expressed as milligram of rutin equivalent per one mL of extracts.
1.5 Reducing activityThe reducing power assay was in accordance with the previous reference(Yen et al., 1995). 80 μL extracts were added to 1 mL phosphate buffer(pH6.6), 1 mL potassium ferricyanide(10 mg·mL-1)was mixed with the buffer and incubated at 50 ℃ for 20 min before having a ice-water bath. Soon afterwards, 1 mL trichloroacetic acid(100 mg·mL-1) and 1 mL ferric chloride(1 mg·mL-1)were added to the mixture in turn, shaken vigorously. The absorbance was determined at 700 nm after 10 min incubation and compared with an ascorbic acid calibration curve.
1.6 DPPH radical scavenging capacityThe assay was carried out following the previously described approach with a few modifications(Chu et al., 2000). Different volumes of extracts(20, 50, 80, 100, 200, 300, 400 μL)were reacted with 250 μL DPPH respectively, and then the total volume was adjusted with 70%(V/V)ethanol to 4 mL and shaken vigorously. After incubated at 25 ℃ for 10 min, the absorbance was determined with a Microplate reader at 517 nm. A lower level of absorbance indicated a stronger scavenging activity. The scavenging ability was calculated according to following formula: Scavenging effect(%)=[1-(As/Ac)] × 100. Where Ac is the absorbance of the control groups and As is the absorbance in the presence of extracts.
1.7 Hydroxyl radical scavengingAccording to the previous reference with a few modifications(Jeong et al., 2009), 1.5 mL FeSO4(1.5 mmol·L-1) and 1 mL H2O2(6 mmol·L-1)were mixed together, then water bath in 37 ℃. After 30 min, hydroxyl radical was generated from Fenton reaction. 0.5 mL salicylic acid(20 mmol·L-1)as well as various volume extracts were added into the mixture and the total volume was adjusted with 70%(V/V)ethanol to 4 mL, allowed to react for 1 h at 37 ℃. The absorbance was determined at 562 nm. A lower level of absorbance indicated a stronger scavenging ability.
The percentage of hydroxyl radical was calculated by using the formula given below: Scavenging effect(%)=[1-(As/Ac)] × 100. Where Ac is the absorbance of the control and As is the absorbance in the presence of extracts or other scavenger.
1.8 Fe2+-chelating activityAccording to a literature procedure, the assay was measured but with a few modifications(Chang et al., 2011). Fe2+-chelating ability of extracts was monitored by the absorbance of the ferrous iron-ferrozine complex at 562 nm. Different volumes of extracts(100-700 μL)were added into 1 mL FeCl2 (0.2 mmol·L-1). The reaction was initiated by the addition of 0.5 mmol·L-1 ferrozine(0.1 mL)dissolved in filled water, then the total volume was adjusted with 70%(V/V)ethanol to 4 mL. The absorbance of the Fe2+-ferrozine complex was measured at 562 nm against a blank group. A lower level of absorbance indicated a stronger chelating activity.
1.9 Inhibiting lipid oxidation activityAccording to the reference described previously with a few modifications(Tamura et al., 1991), lecithin(300 mg)was dissolved in 30 mL phosphate buffer(10 mmol·L-1, pH7.4)by using an ultrasonic cleaner, which was carried out in ice-water. Different volumes of extracts(0.2-1.2 mL)were mixed with 1 mL sonicated solution(10 mg·mL-1), 1 mL FeCl3 (0.4 mmol·L-1) and 1 mL ascorbic acid(0.4 mmol·L-1), then the total volume was adjusted with 70%(V/V)ethanol to 4.5 mL and shaken vigorously. The mixture was added into 2 mL TCA-TBA-HCl after incubated at 37 ℃ for 60 min in a dark place, the centrifuge tubes were put into boiling water for 15 min, which was then in ice-cold water bath for 5 min and centrifuged at 5 000 r·min-1 for 5 min. The absorbance of supernatant was measured at 532 nm. The blank group contained all reagents except lecithin. A lower level of absorbance indicated a stronger protective activity.
Inhibiting lipid oxidation capacity of each sample was calculated via the formula: Inhibition(%)=[1-(As/Ac)] × 100. Where Ac is the absorbance of the control and As is the absorbance in the presence of extracts.
1.10 Statistical analysisEach experiment was carried out in triplicate. The data were analyzed by SPSS(version 18.0) and expressed as meanst and ard deviation(SD). Results were considered significantly at P < 0.05.
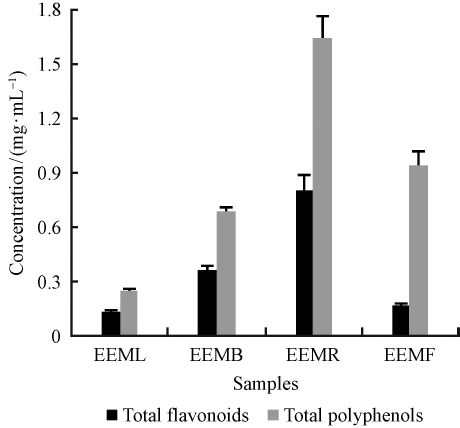
2 Results and discussion 2.1 Total polyphenols content and total flavonoids contentAccording to the regression equations of gallic y=1.212 3x + 0.033 5(R2=0.999 9), the concentration of the total polyphenols was calculated. As is shown in Fig. 1, EEMR has the highest concentration of polyphenols, reaching1.643 4 mg·mL-1, and EEML contains the lowest polyphenols, only 0.247 9 mg·mL-1. In other words, each gram of dry extract contains 30.74 mg(EEML), 222.32 mg(EEMB), 232.84 mg(EEMR) and 27.20 mg(EEMF)of polyphenols separately.
|
Fig.1 Total polyphenols content and total flavonoids content of each sample |
On the basis of the regression equations of rutin y=0.419 5x-0.009 3(R2 =0.999 7), the concentration of the flavonoids content were calculated. Just as the content of total polyphenols, EEMR also has highest content of flavonoids. Fig. 1 shows EEMR contains the highest total flavonoids content, reaching 0.801 7 mg·mL-1. The total flavonoids content in these extracts are in the order of EEMR>EEMB>EEMF>EEML. The high content of polyphenols and flavonoids is far more than those in other tested mulberry(Chang et al., 2011). It has been widely recognized that due to the free radical scavenging capacity and strong reducing power of polyphenols in vivo(Birt et al., 2001), it has the function of inhibiting atherosclerosis and tumor cell proliferation(Cheung et al., 2003). Therefore, the main reason why the mulberry branches and roots have been regarded as an herbal medicine is the abundant polyphenols in them. Besides, the difference of polyphenols content and flavonoids content indicated that the four samples of polyphenols not only exist as flavonoids.
2.2 Reducing powerThere are linear regressions and a significant relationship between total flavonoids and reducing power in all extracts. It can be obviously found in Tab.1 that EEMF has strongest reducing power, which was about 1.17-fold, 2.82-fold, 7.37-fold, greater than that of EEMR, EEMB and EEML respectively. Except EEMF, the polyphenols and flavonoids content of EEMR, EEMB and EEML were significantly correlated with reducing power, the linear correlation coefficient of total polyphenols and reducing power is r=0.998 0, and the total flavonoids content correlated extraordinarily well(Fig. 2). Furthermore, as is shown in Tab.2, the reducing power of different concentration of EEMF, EEMR, EEMB and EEML were noticeably correlated with total flavonoids. In many vitro studies, polyphenols and flavonoids compounds were demonstrated the main material of antioxidant activities(Chew et al., 2009; Socha et al., 2009; Proestos et al., 2006). Our data are consistent with the previous studies.

|
Fig.2 Linear regressions of reducing power and flavonoids concentration |
|
|
|
|
The capability of scavenging radical is regarded as one of the regular indexes of evaluating the ability of antioxidant activity. Among the ROS, hydroxyl radical is the most reactive and induces severe damage to bio-molecules(Sakanaka et al., 2005). Because of the hydroxyl radical’s high reactivity which enables itself to react with a wide range of molecules found in living cells such as amino acids, sugars, lipids and nucleotides, hydroxyl radical scavenging is an important antioxidant activity(Stohs et al., 1995). From the Fig. 3, it can be easily found EEMF has the strongest capacity of scavenging ·OH radical, which may as a result of the high content of anthocyanins, which had the functions of excellently resisting oxidation, getting rid of hydroxyl radicals(Kang et al., 2006).
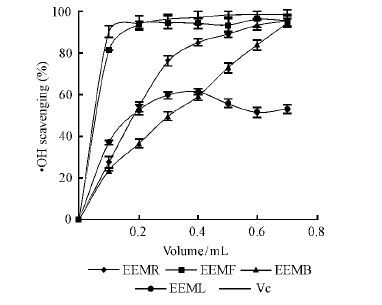
|
Fig.3 Scavenging hydroxyl radical activity of the samples |
Besides, the four extracts have similar ability of scavenging radical. As is shown in Fig. 4, four extracts all possess the ability of scavenging DPPH radical. Although EEMR, EEMB and EEML have the similar trend in the reaction system, EEMF has the strongest scavenging capacity obviously, it can be demonstrated IC50 in the Tab.1.
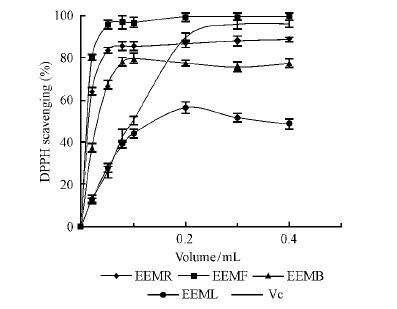
|
Fig.4 Scavenging DPPH effects of the samples |
Fig. 5 implies that the abilities of inhabiting lipid oxidation of the four extracts are remarkably different. With the increasing concentration, the capacities of inhabiting lipid oxidation were on the increasing trend, and EEMR and EEMF were stronger than EEML and EEMB at the same concentration. Moreover, anti-lipid oxidation capacity of EEMF was proportional to the concentration, and EEMR exhibited the strongest capability by 37.78%, while EEMF was 71.14%, revealing that EEMF is the most effective in preventing lipid oxidation in vitro. Lipid oxidation is a harmful process occurring in a cell’s physiological metabolism. Induced by ROS, lipid oxidation will lead to cellular damage and promote the pathological progression of carcinogenesis, atherosclerosis and diabetes(Wang et al., 2007). Medicine mulberry fruit wine is seen as high-end nutritional nourishment by Uygur. In the experiment, EEMF’s ability to inhibit the oxidation of lipid is stronger than that of the others, which will contribute to the cell membrane antioxidant, aging prevention and pigmentation.

|
Fig.5 Liposome protection of the samples |
Compared with the control group, it was easily found all of the extracts have Fe2+-chelating activity from Fig. 6. Obviously, EEMR and EEMB have chelating activities, meanwhile EEMF and EEML even have stronger chelating activities than theirs. Although as the essential metal element for the body, iron contains an unpaired electron, so that they are able to participate in the single-electron transfer reactions, which is harmful to human body(Lloyd et al., 1997). It can be figured out from this experiment that despite the same chelating Fe2+ function of the four extracts, chelating ability of EEMF is much stronger. So EEMF can replace deferoxamine which is the only iron chelator in clinical used for the treatment of iron overload disease.
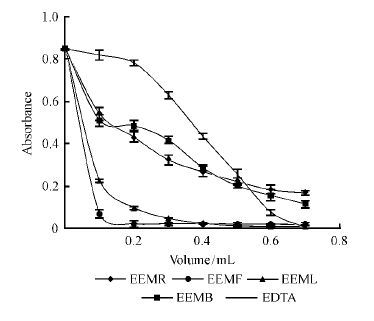
|
Fig.6 Fe2+-chelating activity of the samples |
Although the content of total flavonoids in EEMF is lower than that in EEMR, EEMB and EEML, EEMF’s reducing power is more powerful than that of others. According to some reports, per 100 grams of fruits extract liquid have the content of 418 mg of VC and 6.2 g of reducing sugar(Maimaiti et al., 2007). Apart from that, it was found that Medicine mulberry fruits possess higher content of antioxidants, such as β-carotene, VB and VE(Maimaiti et al., 2002). Besides, anthocyanins content in Medicine mulberry fruits reached 0.02%(w/w)(Jiang, 2010). The main antioxidant physiological function of anthocyanins is scavenging free radicals, studies have shown that the antioxidant capacity of anthocyanins is 20 times higher than the VE, 50 times higher than VC, and even stronger than that of rutin(Jiang, 2010; Tsuda et al., 2003; Wang et al., 2000). What's more, anthocyanins could protect cells from damage, and prevent lung cancer cell proliferation(Chen et al., 2006).
A large number of Medicine mulberry(Morus nigra)are planted in Xinjiang to fix s and s. However, because of the heavy drug smell of their leaves, the leaves can not be used to feed silkworms. A great quantity of branches were cut down every year, but they only to use as firewood, this is not only a waste lots of resources, but also produces environmental pollution. From this investigation, Medicine mulberry could be developed into a kind of antioxidant additives which are new and green.
| [1] |
Bae S H, Suh H J. 2007. Antioxidant activities of five different mulberry cultivars in Korea. LWT-Food Sci Tech, 40(6): 955-962.( 1) 1)
|
| [2] |
Birt D F, Hendrich S, Wang W, et al. 2001. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacology and Therapeutics, 90(2/3): 157-177.( 1) 1)
|
| [3] |
Bocco A, Cuvelier M E, Richard H, et al. 1998. Antioxidant activity and phenolic composition of citrus peel and seed extracts. Journal of Agricultural and Food Chemistry, 46(6): 2123-2129. ( 1) 1)
|
| [4] |
Botterweck A A M, Verhagen H, Goldbohm R A. 2000. Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study. Food and Chemical Toxicology, 38(7): 599-605. ( 1) 1)
|
| [5] |
Chang L W, Juang L J, Wang B S. 2011. Antioxidant and antityrosinase activity of mulberry (Morus alba L.) twigs and root bark. Food and Chemical Toxicology, 49(4): 785-790.( 2) 2)
|
| [6] |
Cheung L M, Cheung P C K, Ooi V E C. 2003. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chemistry, 81(2): 249-255. ( 1) 1)
|
| [7] |
Chen P N, Chu S C, Chiou H L. 2006. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Letters, 235(2): 248-259.( 1) 1)
|
| [8] |
Chew Y L, Goh J K, Lim Y Y. 2009. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem, 116(1): 13-18. ( 1) 1)
|
| [9] |
Chu Y H, Chang C L, Hsu H F. 2000. Flavonoid content of several vegetables and their antioxidant activity. Journal of the Science of Food and Agriculture, 80(5): 561-566.( 1) 1)
|
| [10] |
David P T, Gerard M J. 1997. The use of endogenous antioxidants to improve photoprotection. Photochem Photobiol, 41 (1/2): 1-10.( 1) 1)
|
| [11] |
Devasagayam T, Tilak J C, Boloor K K. 2004. Free radicals and antioxidants in human health: current status and future prospects. Assoc Physicians India, 52: 794-804.( 1) 1)
|
| [12] |
Finkel T, Holbrook N J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature, 408: 239-247.( 1) 1)
|
| [13] |
Germanas J P, Wang S, Miner A, et al. 2007. Discovery of small-molecule inhibitors of tyrosinase. Bioorg Med Chem Lett, 24(17): 6871-6875.( 1) 1)
|
| [14] |
Hairi B, Salle G, Andary C. 1991. Involvement of flavonoids in the resistance of two popular cultivars to mistletoe (Viscum album L.). Protoplasma, 162: 20-26.( 1) 1)
|
| [15] |
Jeong J B, Seo E W, Jeong H J. 2009. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food and Chemical Toxicology, 47(8): 2135-2141.( 1) 1)
|
| [16] |
Jiang Y(江 岩). 2010. Extraction and determination of anthocyanins from Morus nigra L. growing in Xinjiang. Food Science(食品科学), 31(14): 93-96.( 2) 2)
|
| [17] |
Kang T H, Hur J Y, Kim H B. 2006. Neuroprotective effects of the cyaniding-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neuroscience Letters, 391: 122-126. ( 1) 1)
|
| [18] |
Krikorian R, Shidler M D, Nash T A, et al. 2010. Blueberry supplementation improves memory in older adults. J Agric Food Chem, 58(7): 3996-4000.( 1) 1)
|
| [19] |
Lloyd R V, Hanna P M, Mason R P. 1997. The origin of the hydroxyl radical oxygen in the Fenton reaction. Free Radic Biol Med, 22(5): 885-888.( 1) 1)
|
| [20] |
Maimaiti Yiming(买买提依明). 2007. Study on Xinjiang medicine mulberry. North Sericulture(北方蚕业), 28(1): 1-3.( 1) 1)
|
| [21] |
Maimaiti Yiming(买买提依明), Guo H X(郭洪荣), Wu L L(吴丽莉). 2002. Xinjiang medicine mulberry fruits' pigment separation and nutrition analysis. China Sericulture(中国蚕业), 23(3): 15-16.( 1) 1)
|
| [22] |
Peng C, Chan H Y E, Huang Y, et al. 2011. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J Agric Food Chem, 59(5): 2097-2106.( 1) 1)
|
| [23] |
Peng C, Zuo Y Y, Kwan K M, et al. 2012. Blueberry extract prolongs lifespan of Drosophila melanogaster. Experimental Gerontology, 47(2): 170-178.( 1) 1)
|
| [24] |
Proestos C, Boziaris I S, Nychas G J E, et al. 2006. Analysis of flavonoids and phenolic acids in Greek aromatic plants. Investigation of their antioxidant capacity and antimicrobial activity. Food Chem, 95(4): 664-671.( 1) 1)
|
| [25] |
Sakanaka S, Tachibana Y, Okada Y. 2005. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem, 89(4): 569-575.( 1) 1)
|
| [26] |
Shukla S, Mehta A, Bajpai V K, et al. 2009. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food and Chemical Toxicology, 47 (9): 2338-2343.( 1) 1)
|
| [27] |
Sies H. 1986. Biochemistry of oxidative stress. Angewandte Chemie International Edition in English, 25 (12): 1058-1071.( 1) 1)
|
| [28] |
Socha R, Juszczak L, Pietrzyk S, et al. 2009. Antioxidant activity and phenolic composition of herbhoneys. Food Chem, 113(2): 568-574.( 1) 1)
|
| [29] |
Stohs S J, Bagchi D. 1995.Oxidative mechanism in the toxicity of metal ions. Free Radic Biol Med, 18(2): 321-336.( 1) 1)
|
| [30] |
Tamura H, Shibamoto T. 1991. Antioxidantive activity measurement and 4-hydroxy nonenal. Am Oil Chem Soc, 68: 941-943. ( 1) 1)
|
| [31] |
Tang S Z, Kerry J P, Sheehan D, et al. 2002. Antioxidative mechanisms of tea catechins in chicken meat systems. Food Chemistry, 76(1): 45-51.( 1) 1)
|
| [32] |
Tsuda T, Horio F, Uchida K, et al. 2003. Dietary cyaniding 3-O-beta-D-glucoside rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J Nutr, 133: 2125-2130.( 1) 1)
|
| [33] |
Wang B S, Chang L W, Yen W J, et al. 2007. Antioxidative effect of sesame coat on LDL oxidation and oxidative stress in macrophages. Food Chem, 102(1): 351-360. ( 1) 1)
|
| [34] |
Wang S Y, Jiao H. 2000. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J Agric Food Chem, 48:5677-5684.( 1) 1)
|
| [35] |
Wolfe K, Wu X, Liu R H. 2003. Antioxidant activity of apple peels. Journal of Agricultural and Food Chemistry, 53(3): 609-614.( 1) 1)
|
| [36] |
Yen G C, Chen H Y. 1995. Antioxidant activity of various tea extracts in relation to their antimutagenicity. Agric Food Chem, 43(1): 27-32.( 1) 1)
|
| [37] |
Yusuf Y, Romeo T, Toledo J. 2004. Major flavonoids in grape seeds and skins: Antioxidant capacity of catechin, epicatechin, and gallic acid. Agric Food Chem, 52(2): 255-260.( 1) 1)
|
 2014, Vol. 50
2014, Vol. 50

