文章信息
- 郭恺, 顾建锋, 王江岭, 胡加付
- Guo Kai, Gu Jianfeng, Wang Jiangling, Hu Jiafu
- Devibursaphelenchus hunanensis的重新描述
- Redescription of Devibursaphelenchus hunanensis (Yin et al., 1988) (Nematoda: Ektaphelenchinae) from Pinus massoniana in China with the Synonymy of D. eproctatus (Sriwati et al., 2008)
- 林业科学, 2014, 50(7): 82-89
- Scientia Silvae Sinicae, 2014, 50(7): 82-89.
- DOI: 10.11707/j.1001-7488.20140712
-
文章历史
- 收稿日期:2013-02-03
- 修回日期:2014-02-13
-
作者相关文章
2. 宁波出入境检验检疫局技术中心 宁波 315012
2. Technical Centre, Ningbo Entry-Exit Inspection and Quarantine Bureau Ningbo 315012
Bursaphelenchus hunanensis(Yin et al., 1988)was described from dead wood of Pinus massoniana in Hunan Province,China. Braasch(2009)re-established genus Devibursaphelenchus Kakuliya, 1967, and transferred B. hunanensis to genus Devibursaphelenchus.
Bursaphelenchus eproctatus(Sriwati et al ., 2008 was first isolated from dead Japanese black pine in Japan, and it was also replaced to Devibursaphelenchus by Braasch(2009). Because a few specimens were available then,the measurements were based only on 6 males and females, and molecular methods were not applied.
During a survey of Bursaphelenchus spp. in dead or declining Pinus massoniana in Ningbo,Zhejiang,China since 2005,one nematode species were repeatedly detected. With further morphological and molecular study, and compared with type specimens of D. hunanensis and D. eproctatus,our specimens were identified as D. hunanensis, and D. eproctatus is regarded as synonymy of D. hunanensis.
1 Materials and methods 1.1 Nematode culturing and morphological observationsSawn samples taken from dead or declining pine wood(from Yuyao and Cicheng in Ningbo,China)were cut into small pieces less than 1 cm wide. Nematodes were extracted by a modified Baermann funnel technique for 24 h. The feeding habit of the collected nematodes was observed on a slide in water and recorded. Multiplication on agar-fungi plates(Botryotinia fuckeliana)failed. Measurements were made on specimens fixed in TAF and processed to glycerin following the method of Seinhorst(1959). Light micrographs were made using a Zeiss Imager Z1 microscope equipped with a Zeiss AxioCam MRm CCD camera. Paratypes of D. hunanensis(slide 86c/8/1)were obtained on loan from the Rothamsted Nematode Collection at Fera,UK. Paratypes of D. eproctatus(slides E-02 and E-03)were obtained on loan from USDA Nematode Collection,USA.
1.2 Molecular analysesDNA samples of D. hunanensis were prepared according to Li et al.(2008). Four sets of primers(synthesised by Invitrogen,Shanghai,China)were used in the PCR analyses to amplify the partial SSU region,the ITS1/2 region,the D2D3 LSU region of rDNA and partial mtCOI gene,respectively. Primers for amplification of SSU were forward primer K4f(5′-ATG CAT GTC TAA GTG GAG TAT TA -3′) and reverse primer K1r(5′- TTC ACC TAC GGC TAC CTT GTT ACG ACT -3′)(Penas et al., 2006). Primers for amplification of ITS1/2 were forward primer F194(5′- CGT AAC AAG GTA GCT GTA G -3′)(Ferris et al., 1993) and reverse primer 5368r(5′- TTT CAC TCG CCG TTA CTA AGG -3′)(Vrain,1993). Primers for amplification of D2/D3 LSU were forward primer D2A(5′-ACA AGT ACC GTG AGG GAA AGT TG-3′) and reverse primer D3Br(5′-TCG GAA GGA ACC AGC TAC TA-3′)(De Ley et al., 1999). Primers for amplification of mtCOI were forward primer COI-F1(5′-CCT ACT ATG ATT GGT GGT TTT GGT AAT TG-3′) and COI-R2(5′-GTA GCA GCA GTA AAA TAA GCA CG-3′)(Kanzaki et al., 2005). PCR conditions were as described by Ye et al.(2007) and Li et al.(2008). PCR products were separated on 1% agarose gels and visualised by staining with ethidium bromide. PCR products of sufficiently high quality were purified for cloning and sequencing by Invitrogen,Shanghai,China. For ITS-RFLP profiles,suitable aliquots of the amplified ITS rDNA were digested for at least 3 h at 37 ℃ using 10 U of each of the five restriction endonucleases RsaI,HaeIII,MspI,HinfI and AluI(Takara,Japan)following the manufacturer’s instructions. Fragments were resolved by electrophoresis in a 2.5% agarose gel and stained with ethidium bromide.
For Cicheng isolate,sequences of the partial SSU region,the ITS1/2 region,the D2D3 LSU region of rDNA and partial mtCOI gene were sequenced, and ITS-RFLP profiles following the methods described by Wang et al. (2012). For Yuyao isolate,only the ITS1/2 region was sequenced.
The ITS and partial LSUsequences were analysed and aligned using the program ClustalW implemented in MEGA version 4.0(Tamura et al., 2007).Phylogenetic trees were generated with the Neighbor Joining(NJ)method using the Tajima-Nei distance option. Bootstrapping analysis was performed with 1 000 repicates.
2 Results 2.1 MeasurementsMorphometric values of the original description and two isolates from Ningbo are compared in Tab.1.
|
|
1)Female Body slender,cylindrical,annulated,ventrally curved when killed by gentle heat. Cuticle marked by fine trans-verse striae. Lateral fields with three incisures. Lip region offset,6.5-9.7 μm diam.,2.9-4.2 μm high(Fig. 1A). Stylet 18-22 μm long,lacking basal knobs. Median bulb elongate-oval,median bulb valve well developed; plate located slightly posterior to middle of bulb. Pharyngeal gl and lobe well developed,ca six body diam. long,overlapping intestine dorsally. Nerve ring located posterior to median bulb. Excretory pore ca 10-15 μm posterior to median bulb,hemizonid conspicuous,located just posterior to excretory pore(Fig. 1B,C). Ovary single,outstretched,oocytes arranged in multiple rows from anterior to middle part of ovary. Spermatheca and quadricolumella obscure. Uterine region roundish. Vulva not protuberant,vulval flap absent,vagina sllightly inclined anteriorly(Fig. 1I-J). Postuterine sac short,less than one body diam. long. Rectum and anus very difficult to discern and probably non-functional. Intestine terminating as blind sac. Tail tapering to conoid with bluntly rounded or pointed terminus(Fig. 1L-O).

|
Fig.1 D.hunanensis A: Anterior body; B,C: Median bulb region(show excretory pore position); D: Feeding on Aphelenchoides sp.; E-H: Male tail; I,J: Vulva region; K: Male tail(ventral view); L-O:Female tail. Scale bars=10 μm. |
2)Male Anterior body region and cuticle similar to female,body strongly curved ventrally when killed by gentle heat. Testis outstretched,anterior half with sperm cells,spermatocytes arranged in two rows. Sperm spherical. Spicule mitten-shaped,condylus rounded,slightly elongated,lamina smoothly and symmetrically curved,rostrum conical with bluntly pointed tip,junction of rostrum and calomus smoothly curved,cucullus small. Tail strongly recurved with pointed tip,bursa spade-shaped(Fig. 1E-H). Two pairs of caudal papillae present: one pair located slightly precloacal and the second subventral pair located just anterior to beginning of bursal flap(Fig. 1K).
2.3 Comparison with original descriptionThe measurements of the two isolates from Ningbo and the original measurements of D. hunanensis and D. eproctatus are very similar. Only that in D. hunanensis,the stylet length is slightly longer(19-21 μm and 20-26 μm vs 16-20 μm and 18-22 μm for males and females,respectively).
Sriwati et al.(2008)stated that D. eproctatus is distinguished from D. hunanensis by the presence of three lateral lines vs four,rostrum squared vs rounded and absence vs presence of a functional rectum and anus. Yin et al.(1988)described that the lateral as having “2 longitudinal,refractive lines on either side on a narrow refractive b and ”. In our observation of the paratypes,there’s only three lateral lines, and which is accord with Sriwati et al.(2008). Also in our observation of paratypes of D. hunanensis,only indistinct anus remaining sometimes exist(Fig. 2A-B),so no clear rectum and anus was shown in D. hunanensis. And as to the rostrum,we didn’t see any difference from paratypes of D. hunanensis and D. eproctatus(Fig. 2C,D). Yin et al.(1988)described that the distal end of spicules obtuse,without cucullus,but we can see that the small culullus in paratypes of D. hunanensis. So D. eproctatus is regarded as being synonymous with D. hunanensis.
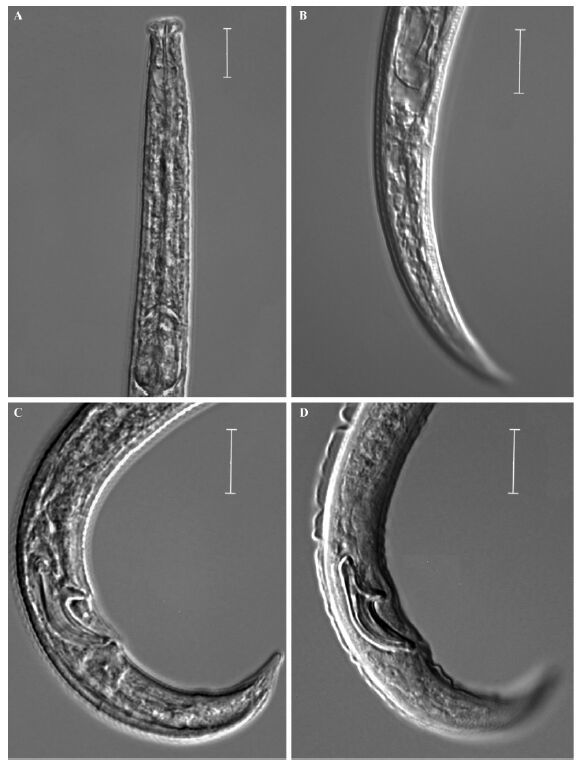
|
Fig.2 Photograph of D.hunanensis paratypes A: Anterior body of D. eproctatus; B: Female tail of D. hunanensis; C: Male tail of D. eproctatus; D: Male tail of D. hunanensis. Scale bars=10 μm. |
The samples of both isolates from Ningbo are very similar to D. hunanensis. Sriwati et al.(2008)described the female tail terminus as “pointed”,but Yin et al.(1988)described it as “finely rounded”. While in isolated from Ningbo,both types of female tail terminus is found,so we conclude that this is due to interspecies variation. The spicules shape are very similar. The spicules length along arc is 18.8-20.8 μm in the original description(Sriwati et al., 2008),which is not accord with our measurements(Tab. 1),so the three paratype males loaned from USDA Nematode Collection,USA were remesured. It’s spicule length in chord is 17.1,17.3 and 17.6 μm,in arc is 14.3,14.3,14.4 μm. So it is now clear that when measuring the spicule length in arc,Sriwati et al.(2008)measured it from the condylus. According to Ryss et al.(2005),the spicule length along arc should start from the middle of the capitulum.
So both isolates from Ningbo is identified as D. hunanensis.
2.4 Molecular profiles and phylogenetic statusThe partial SSU region(JN122012,924 bp),the ITS1/2 region(JN122010 for Cicheng isolate and JN122011 for Yuyao isolate,both 918 bp),the D2D3 LSU region of rDNA(JN122009,807 bp) and partial mtCOI gene sequences(JN122013,675 bp)are deposited in the GenBank database. The ITS region sequences of the two isolates of D. hunanensis (from Cicheng and Yuyao in Ningbo)only differs at 2 base positions,but they are shorter than another isolate “NB” also from Ningbo(EU400449),a 29 bp sequence is lost(Tab.2). The sequence of the D2D3 LSU region of Cicheng isolate and isolate “NB” differs only at 1 base pair.
|
|
The molecular phylogenetic status of D. hunanensis has already done in Gu et al.(2010). The ITS-RFLP pattern of Cicheng isolate is shown in Fig. 3,which is identical with the pattern shown in Burgermeister et al.(2009)as B. hunanensis(Fig. 3).
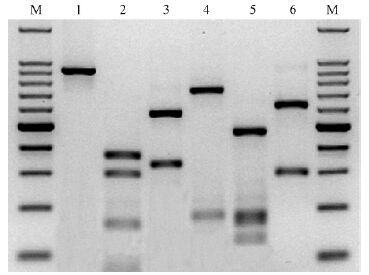
|
Fig.3 ITS-RFLP pattern of D. hunanensis M:Molecular size marker(100 bp ladder); Lane 1: rDNA amplification product; Lanes 2-6: Digestion products obtained with RsaⅠ,HaeⅢ,MspⅠ,HinfⅠ and AluⅠ. Sizes of PCR product and its restriction fragments are shown in Tab.2. |
In Fig. 4 and 5,Devibursaphelenchus,Ektaphelenchoides and Ektaphelenchus forms a well-supported clade,D. wangi and D. hunanensis are very close,though D. lini is more close to Ektaphelenchoides pini.
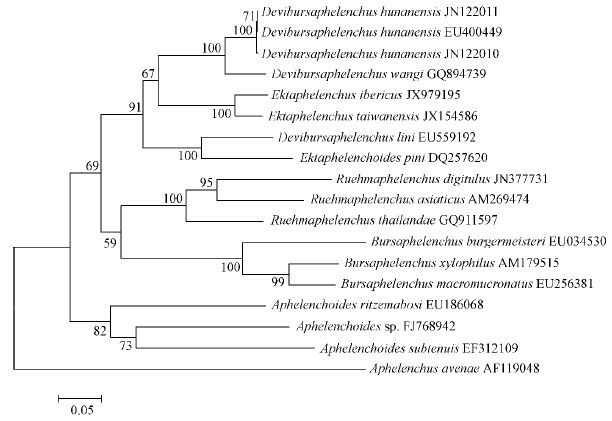
|
Fig.4 Molecular phylogenetic status of D.hunanensis based on ITS sequences Aphelenchus avenae served as the outgroup species. Numbers at branching points are bootstrap values obtained using 1 000 repetitions. Scale bar: Substitutions/site. |

|
Fig.5 Molecular phylogenetic status of D.hunanensis based on partial LSU sequences A.avenae served as the outgroup species. Numbers at branching points are bootstrap values obtained using 1 000 repetitions. Scale bar: substitutions/site. |
D. hunanensis is characterized by three incisures in the lateral field; relatively long stylet(about 15-20 μm and 18-22 μm for males and females)with wide lumen and lacking basal knobs; vulva flap absent,very short postuterine sac; female rectum and anus indistinct or disappear; the spicules are of medium size,16-19 μm long in chord, and arcuate with a terminal cucullus; presence of a distinct bursa flap in ventral view; and a predatory lifestyle.
Braasch(2009)re-established the genus Devibursaphelenchus Kakuliya,1967 belonging to Ektaphelenchinae which contains six species: D. typographi Kakuliya,1967; D. eproctatus(Sriwati et al., 2008)Braasch,2009; D. hunanensis(Yin et al., 1988)Braasch,2009; D. lini(Braasch,2004)Braasch,2009; D. teratospicularis (Kakuliya et al., 1965)Braasch,2009 and D. wangi Gu et al., 2010. D. eproctatus is now regarded as synonymous with D. hunanensis.
D. hunanensis is distinguished from D. wangi Gu et al., 2010 by the size of spicules(16.5-19.4 μm vs 14.2-15.6 μm long measured along chord); different size of stylet(15-20 μm and 18-22 μm for males and females,respectively vs 12.4-16.6 μm and 16.7-17.4 μm); different c ratio of males(c=13.3-16.3 vs c=16.2-18.8). Their RFLP pattern are different(Fig. 3 and Tab.2). The range of ITS sequence divergence between different isolates of D. hunanensis is 0.2%,but it’s 17.9%-18.2% between D. hunanensis and D. wangi. Also the range of 28S sequence divergence between different isolates of D. hunanensis is only 0.1%,but it’s 9.0%-9.2% between D. hunanensis and D. wangi.
2.6 Feeding habitatTens of D. hunanensis,including males,females and juveniles were found feeding on B. mucronatus,B. rainulfi and Aphelenchoides sp.(Fig. 1D).
3 DiscussionDespite the clear rectum and anus of females,Braasch(2009)temporaty transferred B.hunanensis to Devibursaphelenchus. Now with the re-study of the paratypes,we have come to a conclusion that really those rectum and anus don’t exist,only sometimes indistinct anus remainings in several samples. Also a predatory behaviour supports the placement within a genus of Ektaphelenchinae.
With the re-study of paratypes of D. epcrotatus,we also decided that it is as synonymous with D. hunanensis.
The wide-lumened stylet,indistinct or nonfunctional rectum and anus suggests a predatory or ecto-parasitic lifestyle,it’s possible that all Devibursaphelenchus species are predatory on other nematodes. It will be very interesting and of practical interest to investgate the potential efficiency for controling B. xylophilus in the future.
| [1] |
Braasch H. 2009. Re-establishment of Devibursaphelenchus Kakuliya, 1967 (Nematoda, Aphelenchoididae) and proposal for a new combination of several Bursaphelenchus species. Journal of Nematode Morphology and Systematics, 12(1):1-5.( 6) 6)
|
| [2] |
Braasch H. 2004. A new Bursaphelenchus species (Nematoda: Parasitaphelenchidae) sharing characters with Ektaphelenchidae from the People’s Republic of China. Zootaxa, 624:1-10.( 1) 1)
|
| [3] |
Burgermeiser W, Braasch H, Metge K, et al. 2009. ITS-RFLP analysis, an effcient tool for differentiation of Bursaphelenchus species. Nematology, 11(5): 649-668.( 2) 2)
|
| [4] |
De Ley P, Félix M A, Frisse L M, et al. 1999. Molecular and morphological characterisation of two reproductively isolated species with mirror-image anatomy (Nematoda: Cephalobidae). Nematology, 1(6): 591-612.( 1) 1)
|
| [5] |
Ferris V R, Ferris J M, Faghihi J. 1993. Variation in spacer ribosomal DNA in some cyst-forming species of plant parasitic nematodes. Fundamental and Applied Nematology, 16(2): 177-184.( 1) 1)
|
| [6] |
Gu J, Wang J, Zheng J. 2010. Devibursaphelenchus wangi sp. n. (Nematoda: Ektaphelenchinae) feeding on Aphelenchoides sp. Russian Journal of Nematology, 18(1): 49-57.( 2) 2)
|
| [7] |
Kakuliya G A. 1967. New nematode genus Devibursaphelenchus gen.n.(Nematoda: Aphelenchoididae). Bulletin of the Academy of Sciences of the Georgian SSR, 47: 439-443.( 2) 2)
|
| [8] |
Kakuliya G A, Devdariani T G. 1965. A new nematode species Bursaphelenchus teratospicularis Kakuliya et Devdariani, sp. nov. (Nematoda, Aphelenchoididae). Bulletin of the Academy of Sciences of the Georgian SSR, 38: 187-191.( 1) 1)
|
| [9] |
Kanzaki N, Futai K. 2005. Description of Bursaphelenchus parvispicularis n. sp. (Nematoda: Parasitaphelenchidae) isolated from a dead oak tree, Quercus mongolica var. grosseserrata. Nematology, 7(5): 751-759.( 1) 1)
|
| [10] |
Li H, Trinh P Q, Waeyenberge L, et al. 2008. Bursaphelenchus chengi sp. n. (Nematoda: Parasitaphelenchidae) isolated at Nanjing, China, in packaging wood from Taiwan. Nematology, 10(3): 335-346.( 2) 2)
|
| [11] |
Penas A C, Metge K, Mota M, et al. 2006. Bursaphelenchus antoniae sp. n. (Nematoda: Parasitaphelenchidae) associated with Hylobius sp. from Pinus pinaster in Portugal. Nematology, 8(5): 659-669.( 1) 1)
|
| [12] |
Ryss A, Vieira P, Mota M, et al. 2005. A synopsis of the genus Bursaphelenchus Fuchs, 1937 (Aphelenchida:Parasitaphelenchidae)with keys to species.Nematology, 7:394-458.( 1) 1)
|
| [13] |
Seinhorst J W. 1959. A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica,4(1): 67-69.( 1) 1)
|
| [14] |
Sriwati R, Kantaki N, Phan L K, et al. 2008. Bursaphelenchus eproctatus n. sp. (Nematoda: Parasitaphelenchidae) isolated from dead Japanese black pine, Pinus thunbergii Pars. Nematology, 10(1): 1-7.( 7) 7)
|
| [15] |
Tamura K, Dudley J, Nei M, et al. 2007. MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24(8):1596-1599.( 1) 1)
|
| [16] |
Vrain T C. 1993. Restriction fragment length polymorphism separates species of the Xiphinema americanum group. Journal of Nematology, 25(3): 361-364.( 1) 1)
|
| [17] |
Wang J, Gu J. 2012. Bursaphelenchus paraburgeri sp. n. (Nematoda: Parasitaphelenchidae) in packaging wood from Malaysia. Nematology, 14(1):39-50.( 1) 1)
|
| [18] |
Ye W, Giblin-Davis R M, Braasch H, et al. 2007. Phylogenetic relationships among Bursaphelenchus species (Nematoda: Parasitaphelenchidae) inferred from nuclear ribosomal and mitochondrial DNA sequence data. Molecular Phylogenetics and Evolution, 43(3): 1185-1197.( 1) 1)
|
| [19] |
Yin K, Fang Y, Tarjan A C. 1988. A key to species in the genus Bursaphelenchus with a description of Bursaphelenchus hunanensis sp. n. (Nematoda: Aphelenchoididae) found in pine wood in Hunan Province, China. Proceedings of Helmithological Society of Washington, 55(1): 1-11.( 5) 5)
|
 2014, Vol. 50
2014, Vol. 50

