文章信息
- 杨明秀, 宋瑞清
- Yang Mingxiu, Song Ruiqing
- 中国10省金黄壳囊孢菌的菌落特征和遗传多样性
- Colony Characteristics and Genetic Diversity of Cytospora chrysosperma Strains from Ten Provinces in China
- 林业科学, 2014, 50(6): 160-166
- Scientia Silvae Sinicae, 2014, 50(6): 160-166.
- DOI: 10.11707/j.1001-7488.20140621
-
文章历史
- 收稿日期:2013-10-09
- 修回日期:2014-04-23
-
作者相关文章
2. 东北农业大学农学院 哈尔滨 150030
2. Agricultural School, Northeast Agricultural University Harbin 150030
Poplar canker is common in many countries of the world(Adams et al., 2005), affecting the growth of trees and causing the death of infected trees. In China 17 kinds of pathogens of poplar canker have been recorded(Zhang et al., 2003). Poplar canker disease is mostly caused by three pathogens, Cytospora chrysosperma, Phomopsis macrospora and Fusicoccum aesculi(Ren et al., 2013; Wang et al., 2008). We tested the colony characteristics of 30 C. chrysosperma strains and analyzed their genetic diversity using r and om amplification polymorphic DNA(RAPD)method. Although the cultural conditions can cause changes of pathogenic fungus in some colony characteristics, the differences in genetic, physiological and pathological characteristics can be reflected from the colony characteristics. Previous research on colonial characters of C. chrysosperma on various culture media has been carried out(Jiang et al., 2012). Zang et al.(2007)investigated the diversity of 7 isolates with different colony characteristics on PDA among 61 isolates of C. spp. obtained from different apple producing areas of Shaanxi province. The results indicated that C. spp. isolated from apple trees had diversity and different virulence in Shaanxi. Zhang et al.(2007)investigated the genetic change and diversity of 30 isolates of C. chrysosperma from 9 provinces in China, the results showed that there were significant differences of colony characteristics among these isolates. Xu(2010)tested the relationship between RAPD genetic diversity and geographical distribution of 32 strains of C. chrysosperma from 10 provinces in China.
The study of the colony characteristics, genetic diversity and the relationship between geographical distribution of C. chrysosperma has important theoretical and practical significance in poplar disease-resistant breeding and the control of the disease.
1 Materials and methods 1.1 Source of strainsThirty of C. chrysosperma strains were provided by the China Forestry Microbial Strains Preserved Management Center(Research Institute of Forest Ecological Environment and Protection, Chinese Academy of Forestry). The host plants and geographical positions of isolated strains are shown below in Tab. 1.
|
|
C. chrysosperma strains were cultured on potato dextrose agar(PDA)in Petri dishes at 25 ℃ in the dark for four days. Three 9 mm diameter mycelial plugs were cut r and omly from the margin of each colony and placed in PDA medium. The colony shape, color, and production of fructification of each strain were observed after seven days of incubation. The fruiting bodies were investigated after 30 days(Zhang et al., 2007; Fang, 2007). Observations were made from three replicates of each strain.
Cluster analysis of the strains was carried out using NTSYS(Version 2.10 software).
1.3 Analysis of genetic diversity1)DNA extraction The strains were cultured in PDA medium at 25 ℃ for 7 days for mycelia collection. Before extracting the DNA, the mycelia(100-150 mg)were frozen at -80 ℃ and then ground to a fine powder in liquid nitrogen with a mortar and pestle. DNA was extracted with the CTAB method [2%(W/V)CTAB; 100 mmol·L-1 Tris-HCl, pH8.0; 20 mmol·L-1 EDTA, pH8.0; 1.4 mol·L-1 NaCl](Duraisamy et al., 2012; Jiang et al., 2008; Zhang et al., 2004; Gu, 1998).
2)Screening RAPD primers 60 r and om 10-base primers(Operon Technology Inc.) and 10 r and om 10-base primers(Xu, 2010)were independently used for the polymerase chain reaction of RAPD analysis. The DNA extracted from 10 strains that were isolated from 10 provinces(Inner Mongolia, Heilongjiang, Sichuan, Xinjiang, Shaanxi, Liaoning, Jilin, Gansu, Qinghai, Sh and ong).
The PCR reaction mixtures contained 10 ng of template DNA, 10 μmol·L-1 of primers, 10 μL of Premix Taq Version 2.0(TaKaRa) and sterile water up to 20 μL. The amplifications were performed in a Biometra DNA thermal cycle programmed for the following conditions: an initial denaturation at 94 ℃ for 5 min, 35 cycles of 94 ℃ for 1 min, 44 ℃ for 1 min, and 72 ℃ for 2 min and a final extension step of 72 ℃ for 10 min. The amplification products were separated by electrophoresis at 110 V on ethidium-bromide-stained(0.5 mg·mm-1), 1.5% agarose gels run in 0.5×TBE buffer and visualized under UV light(Duraisamy et al., 2012; Jain et al., 2007; Abreu et al., 2012; Denoyes-Rothan et al., 2003). Each strain was analyzed twice by PCR reaction.
3)RAPD and data analysis PCR amplification of DNA was performed with each of the primers that were screened in 1.3.2. The method of PCR amplification of DNA is same to 1.3.2. Each strain was scored for the presence or absence of each amplicon. When a primer revealed a polymorphism, all amplified DNA fragments were used for analysis and treated as binary characters. The character of each RAPD b and was scored for each individual strain of C. chrysosperma and the genetic similarities were computed between all pairs of strains. Dissimilarities were computed as a similarity coefficient and the data was used to construct a phenogram using the unweighted pair group method with arithmetic average(UPGMA)(Zhang et al., 2006; Belabid et al., 2004; Bayraktar et al., 2009).
2 Results 2.1 Colony characteristics of C. chrysosperma strainsThe colonial traits of C. chrysosperma strains were shown in Fig. 1 and Tab. 2. The clustering analysis was performed and a dendrogram was obtained using the UPGMA. The 30 strains were grouped into two clusters. The first cluster included all of the strains from Beijing, Xinjiang, Liaoning, Jilin, Sh and ong, Heilongjiang and one strain from each of Sichuan, Shaanxi and Inner Mongolia, and was further separated into the geographic groups of Xinjiang, Beijing-Northeast(Heilongjiang, Jilin, Liaoning) and Sh and ong at a similarity coefficient of 0.71. The second cluster included the five of the strains of Inner Mongolia, four strains of Gansu, two strains of Sichuan and one strain each of Qinghai and Shaanxi, and was further separated into the geographic groups of Inner Mongolia and the geographic group including Sichuan, Gansu, Shaanxi, Qinghai at a similarity coefficient of 0.62. Five strains(S01, S09, S11, S15, S20)were not classified into the geographic group of sources(Fig. 2).
 |
Fig. 1 The colony characteristics of C. chrysosperma strains in PDA medium Colonial color: A.White; B.Light gray; C. Gray; D.Dark gray; E.Beige; F.Orange. Colonial shape: A, C.Sparse and equatorial; B, D.Dense and bulged. Colonial edge: A, C .Regular; B,D.Irregular. Fruiting bodies: G.No fruiting bodies; H.Produced fruiting bodies. |
|
|
 |
Fig. 2 Clustering analysis of the colony characteristics of 30 strains of C.chrysosperma from different geographic origins |
DNA of 10 strains that isolated from 10 provinces were amplified by using 70 r and om primers, 9 of them were shown to yield strong and clear b and s with good reproducibility and specificity(Tab. 3).
|
|
RAPD analysis of 30 strains demonstrated that the length of the amplified DNA fragments ranged from 120 to 2 200 bp(Tab. 3). By re-sequencing using the 9 primers, 58 polymorphisms were identified. The number of polymorphic b and s ranged from 5 to 9 by using single primer. Cy-6 primer that amplified polymorphic b and s up to 9(Fig. 3), all of the RAPD b and s detected were polymorphic. These results showed that the C. chrysosperma strains from ten provinces in China had rich genetic diversity.
 |
Fig. 3 RAPD patterns of C. chrysosperma strains amplified by primer Cy-6 |
Clustering analysis was performed and a dendrogram was constructed using the UPGMA method. The 30 strains of C. chrysosperma were classified into two clusters. The first cluster included all of the strains from Beijing, Xinjiang, Liaoning, Shaanxi, Jilin, Qinghai, Gansu, Heilongjiang and Sh and ong; and one strain from Inner Mongolia. The first cluster was further separated into the geographic groups of Beijing, Xinjiang, Liaoning, Gansu-Qinghai and the geographic groups include Sh and ong, Heilongjiang, Jilin and Shaanxi at a similarity coefficient of 0.66. The second cluster included all of the strains from Sichuan and five strains from Inner Mongolia, and was further separated into the geographic groups of Inner Mongolia and Sichuan at the same similarity level. Four strains(S12, S25, S23, S30)were not separated into either of the geographic groups(Fig. 4).
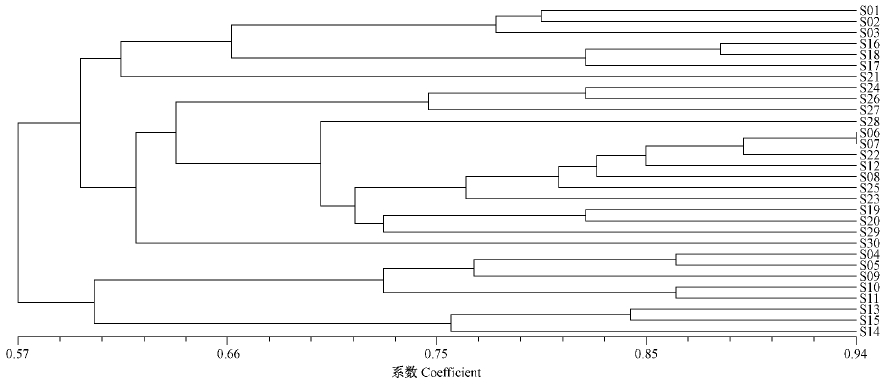 |
Fig. 4 Dendrogram derived from UPGMA cluster analysis based on the similarity coefficient of RAPD markers of 30 C.chrysosperma strains |
The results also showed that strains from the same province had a high genetic similarity, and most of these values were greater than 0.7. The genetic similarity coefficients were 0.780 between the S01(Beijing) and S03(Pinggu, Beijing), 0.720 between the S09(Hohhot, Inner Mongolia) and S12(Mangui, Inner Mongolia), 0.840 between the S13(Hongya, Sichuan) and S15(Kangding, Sichuan) and 0.880 between the S6(Daqing, Heilongjiang) and S8(Mohe, Heilongjiang), respectively(Fig. 4). Thus, C. chrysosperma strains from ten provinces in China showed abundant genetic variation and low genetic differentiation in geographically similar strains of same province.
2.4 The colony characteristics and RAPD genetic diversity analysisThe colony characteristics and genetic diversity of most C. chrysosperma strains were related to the geographical origin and environments of the strains. The geographical environment of Inner Mongolia is predominantly grassl and , but although Beijing is close to Inner Mongolia, the strains from these two regions were not in the same cluster in the genetic diversity analyses. Most of the strains were grouped into the same cluster in the colony characteristics and genetic diversity analyses except the strains from Gansu and Qinghai Provinces. It is shown in Tab. 4.
|
|
In this research, the colony characteristics and genetic diversity of C. chrysosperma strains from ten provinces in China were tested. The results showed that the C. chrysosperma had abundant diversity in morphological characters, and the 30 strains could be classified into two geographic groups. The first group mainly included the strains from Xinjiang, Beijing-Northeast(Heilongjiang, Jilin, Liaoning) and Sh and ong, and the second group mainly included the strains from Inner Mongolia, Sichuan and Gansu-Qinghai. The results are similar to the results found by Zhang et al.(2007)in which the 30 strains of Cytospora chrysosperma isolated from poplar in China were grouped into Northeast and Northwest geographic groups based on colony characteristics.
The results of the RAPD clustering showed that the C. chrysosperma strains were also classified into two geographic groups. The first group includes Beijing, Xinjiang, Northeast(Heilongjiang, Jilin, Liaoning), Qinghai, Gansu and Shaanxi. The second group mainly included the strains from Inner Mongolia and Sichuan. The results are similar to some views of Xu(2010).
The clustering analysis of the colony characteristics and the RAPD genetic diversity of the strains reflected that there was a certain relationship between the colony characteristics and the genetic diversity of C. chrysosperma. For example, the strains S16, S17 and S18 from Xinjiang were clustered into the same group in both cluster analyses. Five strains in the study of colony characteristics and four strains in research of RAPD analysis were not separated into the geographic group of sources. Most of the 30 strains were in the same cluster in both cluster analyses, indicated that it is nearly reasonable to classify strains based on colony characteristics and RAPD genetic traits. The results showed that the colony characteristics and genetic diversity of C. chrysosperma were related to geographical origin of the pathogenic fungus. Strains from Heilongjiang and Jilin had the closest genetic traits and the Inner Mongolia strains had the least genetic traits to the other province strains.
In summary, this study showed that C. chrysosperma from China has abundant genetic diversity, and there are more differences in population structure and genetic variation of C. chrysosperma from different geographic origins. Therefore, in order to better underst and the occurrence and epidemic, and to be able to predict the development of this disease, we must have a timely and accurate underst and ing of the population’s genetic structure and variation laws of C. chrysosperma. So as to provide a scientific basis for formulating effective control measures to poplar canker.
Comparing to the results of colony characteristics and genetic diversity, some strains which are from the same province were not in the same geographic group what is the reason needs to be further researched. Because RAPD technology is based on genomic DNA as a template, so the results may have been affected by the differences of genome. At present, five molecular marker technologies(RFLP, RAPD, AFLP, ISSR, SSR)(Liao et al., 2008; Belabid et al., 2004; Bayraktar et al., 2009; Duraisamy et al., 2012; Cao et al., 2012) and most commonly used to analyze the population’s genetic diversity of pathogens. RAPD molecular marker technology was adopted in this study. We aim to carry out further research on the relationship of the genes of C. chrysosperma using other molecular marker technologies, combined with morphological characteristics, pathogenicity and geographical environment.
| [1] |
Abreu L M, Costa S S, Pfenning L H, et al. 2012. Chemical and molecular characterization of Phomopsis and Cytospora-like endophytes from different host plants in Brazil. Fungal Biol, 116 (2): 249-260.( 1) 1)
|
| [2] |
Adams G C, Wingfield M J, Common R, et al. 2005. Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol, 52: l-144.( 1) 1)
|
| [3] |
Bayraktar H, Dolar F S. 2009. Genetic diversity of wilt and root rot pathogens of chickpea, as assessed by RAPD and ISSR. Turk J Agric, 33: 1-10.( 2) 2)
|
| [4] |
Belabid L, Baum M, Fortas Z, et al. 2004. Pathogenic and genetic characterization of algerian isolates of Fusarium oxysporum f. sp. lentis by RAPD and AFLP analysis. Afr J Biotechno, 3 (1): 25-31.( 2) 2)
|
| [5] |
Denoyes-Rothan B, Guérin G, Délye C, et al. 2003. Genetic diversity and pathogenic variability among isolates of Colletotrichum species from strawberry. Phytopathology, 93: 219-228.( 1) 1)
|
| [6] |
Duraisamy K, Choi S H, Choi T K, et al. 2012. Assessment of genetic diversity among varieties of mulberry using RAPD and ISSR fingerprinting. Sci Hortic, 134: 79-87.( 3) 3)
|
| [7] |
Cao Z M(曹支敏), Du L(杜林), Wang Q H(王秦虎), et al. 2012. Genetic diversity of poplar rust fungus Melampsora larici-populina in China. Mycosystema(菌物学报), 31(4): 510-522( 1) 1)
|
| [8] |
Fang Z D(方仲达). 2007. The Research Method of Plant Pathology. Beijing:China Agriculture Press (in Chinese)(中国农业出版社).( 1) 1)
|
| [9] |
Gu H Y(顾红雅). 1998. The plant molecular biology laboratory manual. Beijing: Higher Education Press (in Chinese)(高等教育出版社).( 1) 1)
|
| [10] |
Jain P K, Saini M L, Pathak H, et al. 2007. Analysis of genetic variation different banana (Musa species) variety using random amplified polymorphic DNAs (RAPDs). Afri J Biotechnol, 6(17): 1987-1989.( 1) 1)
|
| [11] |
Jiang Z H(姜自红), Ji K S(季孔庶), Huang Y(黄焱). 2008. Analysis of genetic diversity of two populations of Buxus sinica var. parvifoliaby RAPD. Journal of Nanjing Forestry University:Natural Sciences Edition(南京林业大学学报:自然科学版), 32 (1): 11-14. ( 1) 1)
|
| [12] |
Jiang Z R(姜自如), Zhang G L(张刚龙), Cao Z M(曹支敏), et al. 2012. Geographical species of poplar canker pathogen in Shaanxi Province. Journal of Northwest A & F University :Natural Science Edition(西北农林科技大学学报:自然科学版), 27 (2):102-108.( 1) 1)
|
| [13] |
Liao T L(廖太林), Ye J R(叶建仁), Cheng J D(陈建东). 2008. AFLP analysis of Fusarium circinatum and relative species. Scientia Silvae Sinicae(林业科学), 44(9): 82-86( 1) 1)
|
| [14] |
Ren H J, Li H, Wang F Y, et al. 2013. Biocontrol potential of an endophytic Bacillus pumilus JK-SX001 against poplar canker. Biological Control, 67 (3): 421-430.( 1) 1)
|
| [15] |
Wang Y(王勇), Wu X Q(吴小芹). 2008. Study on several kinds of poplar canker disease and the pathogenicity of the pathogens in north of Jiangsu. Journal of Nanjing Forestry University: Natural Sciences Edition(南京林业大学学报: 自然科学版), 32 (5): 47-50( 1) 1)
|
| [16] |
Xu M(徐明). 2010. Study on the occurrence and control technology of Cytospora canker of poplar in Jiangsu Province. Master Degree Thesis of Nanjing Forestry University(南京林业大学硕士学位论文).( 3) 3)
|
| [17] |
Zang R(臧睿), Huang L L(黄丽丽), Kang Z S(康振生), et al. 2007. Biological characteristics and pathogenicity of different isolates of Cytospora spp. isolated from apple trees in Shaanxi Province. Acta Phytopathologica Sinica(植物病理学报), 37(4): 343-351( 1) 1)
|
| [18] |
Zhang B(张博), Zhang L(张露), Zhu G Q(诸葛强), et al. 2004. A rapid and simple method of total DNA extraction from tree. Journal of Nanjing Forestry University: Natural Sciences Edition(南京林业大学学报: 自然科学版), 28 (1): 13-16.( 1) 1)
|
| [19] |
Zhang X Z, Hwa-yeong K, Byung-sup K. 2006. Analysis of genetic diversity of Phytophthora infestans in Korea by using molecular markers. J Microbiol Biotechnol, 16 (3): 423-430( 1) 1)
|
| [20] |
Zhang X Y(张星耀), Luo Y Q(骆有庆). 2003. Major Forest Diseases and Insect Pests in China. Beijing: Chinese Forestry Press(中国林业出版社), 93-94.( 1) 1)
|
| [21] |
Zhang X Y(张星耀), Chen H Y(陈海燕), Liang J(梁军), et al. 2007. Cultural morphology and vegetative compatibility of Cytospora chrysosperma isolates. Journal of Northwest A & F University: Natural Science Edition(西北农林科技大学学报: 自然科学版), 35 (3): 99-105.( 3) 3)
|
 2014, Vol. 50
2014, Vol. 50
