文章信息
- 陈亮, 孙庚午, 王洪凯, 吴树敬, 林福呈, 刘会香
- Chen Liang, Sun Gengwu, Wang Hongkai, Wu Shujing, Lin Fucheng, Liu Huixiang
- 葡萄座腔菌原生质体的制备及gfp的转化
- Protoplast Preparation and gfp Transformation of Botryosphaeria dothidea
- 林业科学, 2014, 50(6): 131-137
- Scientia Silvae Sinicae, 2014, 50(6): 131-137.
- DOI: 10.11707/j.1001-7488.20140617
-
文章历史
- 收稿日期:2014-02-17
- 修回日期:2014-04-22
-
作者相关文章
2. 浙江大学生物技术研究所 水稻生物学国家重点实验室 杭州 310058;
3. 山东农业大学园艺科学与工程学院作物生物学国家重点实验室国家苹果工程技术研究中心 泰安 271018
2. State Key Laboratory for Rice Biology Biotechnology Institute, Zhejiang University Hangzhou 310058;
3. National Research Center for Apple Engineering and Technology State Key Laboratory of Crop Biology College of Horticulture Science and Engineering, Shandong Agricultural University Tai'an 271018
Botryosphaeria dothidea is an economically important pathogen infecting many woody plant hosts and causing fruit rot, frogeye leaf spot, stem and branch canker, die-back, gummosis, and in some cases tree death(Slippers et al., 2007; Liu et al., 2009). It has been reported in Asia, Europe, America, Oceania and Africa with its infectivity to poplar, apple, pear, peach, eucalyptus and olive trees(Phillips et al., 2005; Pitt et al., 2010; Rodas et al., 2009; Yu et al., 2009; Slippers et al., 2007; Tang et al., 2012). Attention has been devoted to chemical and biological control of B. dothidea in China(Ji et al., 2008; Yang et al., 2002; Guo et al., 2009), but longterm prevention effect should be improved. Underst and ing infection and pathogenic process are critical for efficient control of this pathogen.
Genetic transformation approaches including electroporation(Chakraborty et al., 1990), restriction enzyme-mediated integration(REMI)(Sanchez et al., 1998) and Agrobacterium tumefaciens-mediated transformation(de Groot et al., 1998), PEG-CaCl2-mediated transformation(Kao et al., 1974)have been widely used to transfer fungi for genetic modification. Protoplasts of high quantity and quality st and for an essential premise for genetic transformation, however no efficient method has been developed so far for protoplast preparation of B. dothidea. PEG could cause the protoplasts to clump together(Fincham, 1989), which facilitates the trapping of DNA, thus foreign gene can be easily transformed to the genome of fungi mediated by this method. Green fluorescent protein as a reporter gene, has been widely applied in molecular analysis of fungi, including gene expression, protein subcellular localization fungal-host interaction(Lu et al., 2004; Rajasekaran et al., 2008).The desirable traits of GFP such as convenient detection, stable fluorescent, real-time observation, nonhazardous and heterologous cells versatility(Li et al., 1997)provide a convenient approach to study filamentous fungi in the molecular level(Lorang et al., 2001).
In this study, optimization of the preparation procedure for the protoplast from mycelia of B. dothidea was conducted and a foreign gene gfp was transformed and expressed in B. dothidea successfully. This protocol provides an essential step for establishing a protoplast-mediated transformation system and a better approach for further studying the infection and pathogenic mechanism and functional genomics of B. dothidea.
1 Materials and methods 1.1 Strain and culture conditionsBotryosphaeria dothidea strain SDAU11-76 with high virulence was isolated from the infected apple tree branches in Qixia, Yantai, Sh and ong province, China(120°44.121′E, 37°21.220′N), and it was saved by this laboratory, stored at 4 ℃ with 30% sterile glycerin. Plasmid pKO1-HPH containing a positive selective gene hygromycin phosphotransferase(hph) and a reporter gene gfp conferring green fluorescent protein was stored at -80 ℃ with 30% sterile glycerin.
Mycelia of 0.1 g obtained from pure culture(5 d)on PDA were added into a complete medium(CM, D-glucose 10 g·L-1, peptone 2 g·L-1, yeast extract 1 g·L-1, casamino acid 1g·L-1, 20× nitrate salts 50 mL·L-1, trace elements 1 mL·L-1, vitamine solution 1 mL·L-1)broth at 28 ℃ with shaking of 120 r·min-1. Mycelia were collected by 4 layers sterilized gauze for analysis.
1.2 Protoplast preparationDifferent enzymes for the cell wall digestion were driselase from Basidiomycetes sp.(Sigma Chemical), lysing enzymes from Trichoderma harizanum(Sigma Chemical) and glucanase(Grindsted Products AS), and which were dissolved in osmotic stabilizes agent solution, and filter-sterilized through 0.25 μm membrane filter and stored at 4 ℃.
A total of 1 g of mycelia was incubated with 10 mL of enzyme solutions at the designed temperatures and incubation times with a shaking speed of 100 r·min-1. Protoplasts were obtained after filtration with 2-layer sterilized lens paper and harvested by centrifugation(3 000 r·min-1)for 10 min. Then the protoplasts were washed with STC(Sorbitol 1.2 mol·L-1, Tris-HCl pH 7.5 10 mmol·L-1, CaCl2 50 mmol·L-1)for 2 times, quantified and maintained with STC and then stored the protoplasts at -80 ℃.
To establish an efficient B. dothidea protoplast isolation method, 7 kinds enzyme conditions (Tab. 1), variable enzyme concentrations(1.0%, 1.5%, 2.0%, 2.5%, 3.0%, 3.5%, 4.0%), mycelial age(18, 24, 30, 36, 42, 48, 54, 60 h), enzymolysis time(1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5 h), temperature(22, 25, 28, 31, 34, 37 ℃) and osmotic stabilizes agent(NaCl, KCl, MgSO4·7H2O, sorbitol, mannitol and sucrose)were analyzed respectively. All the experimental procedures were triplicated.
1.3 Protoplast regenerationThe obtained protoplast suspensions were separately diluted into a concentration of 1×103 cell·mL-1 with 0.7 mol·L-1 NaCl and sterile water was used as a control. PDA, Czapek-Dox agar(CD, NaNO3 6.0 g·L-1, KCl 0.52 g·L-1, KH2PO4 1.52 g·L-1, glucose 10.0 g·L-1, 1 mol·L-1 MgSO4·7H2O 2 mL·L-1, trace elements solution 1 mL·L-1, agar 15 g·L-1, final pH was 6.5), yeast extract peptone dextrose(YPD, yeast extract 3.5 g·L-1, peptone 5 g·L-1, glucose 10 g·L-1, agar 15 g·L-1) and YPS(yeast extract 3.5 g·L-1, peptone 5 g·L-1, 1 mol·L-1 sucrose, agar 15 g·L-1)media were prepared for protoplast regeneration. A hundred microliters of suspension from two kinds of dilution above were spread on the surface of those plates respectively and incubated at 28 ℃ for 2 d. Regeneration rate(R, %)of protoplasts was calculated as the formula followed:
$$ R = \frac{{{N_R} - {N_C}}}{{{N_T}}} \times 100 $$Here NR is the number of colonies grown on the regeneration plate, NC is the number of colonies on control plate and NT is the total number of protoplasts inspected.
1.4 PEG-CaCl2-mediated transformation of protoplastsProtoplasts of 100 μL(1×106protoplast·mL-1)mixed with plasmid pKO1-HPH of 5 μg were kept on ice for 25 min, and then gentle mixed with 1 mL transformation solution [40%(W/V)PEG 4000, 50 mmol·L-1 CaCl2, 50 mmol·L-1 Tris-HCl pH 8.0] incubated for 15 min at 25 ℃, followed 5 mL of STC was added with gentle mixing. Transformed protoplasts were centrifuged for 10 min with 3 000 r·min-1 at 4 ℃, resuspended with STC of 100 μL and mixed with overlay agar(YPS containing 0.5%, 20 μg·mL-1 hygromycin B)plated on underlay agar(YPS containing 1.5% agar, 20 μg·mL-1 hygromycin B). The plates were incubated at 28 ℃ for 7 d and the colonies were transferred to PDA plates containing 20 μg·mL-1 hygromycin B(Data of sensitivity of B. dothidea to hygromycin B were not shown).
1.5 GFP detection of transformantsFive r and omly selected transformants grown on PDA plate for five successive generations were used to determine the existence and stability of the inserted gfp gene by PCR analysis(Primers: gfp1, 5′-ATGGTGAGCAAGGGCGAGGAG-3′, gfp2, 5′-CTTGTACAGCTCGTCCATGCCG-3′). Green fluorescence of mycelia were analyzed and imaged under fluorescent microscope [Eclipse 80i microscope(Nikon)equipped with Plan APO VC 50X/1.40 objective] with the wild-type strain SDAU11-76 as a control.
2 Results 2.1 Influence of six parameters on protoplast preparationTotally 7 enzyme conditions were analyzed for protoplast release, the final concentration of enzyme solutions were all 3%(enzyme mixture was consisted of two or three different enzyme all of which were in the same proportion). The results showed the treatments with each of the three individual enzymes produced protoplasts the driselase turned out to be the most efficient [(2.80±0.29)×108 cell·mL-1] while the lysing enzyme mixture was the weakest one with a yield of (1.39±0.23)×108 cell·mL-1. In comparison with the reactions containing multiple enzymes, the combination containing 1.5% driselase and 1.5% glucanase was able to produce the protoplasts of (6.13±0.32)×108 cell·mL-1, which was the highest among the combinations(Tab. 1).
|
|
The concentration of enzyme mixture(driselase∶glucanase=1∶1)were further analyzed ranged from 1.0% to 4.0%. The result showed that the protoplast release efficiency increased as the enzyme concentration increased from 1% to 3%, and it reached the plateau at 3%, hereafter it had a slight decline at 4%(Fig. 1A) The production efficiency of protoplasts varied greatly with different growth ages. It increased from the 18 h and reached a peak at the growth age of 42 h with a yield of (6.15±0.03)×108 cell·mL-1, and sharply decreased when the growth age exceeded 48 h(Fig. 1B).
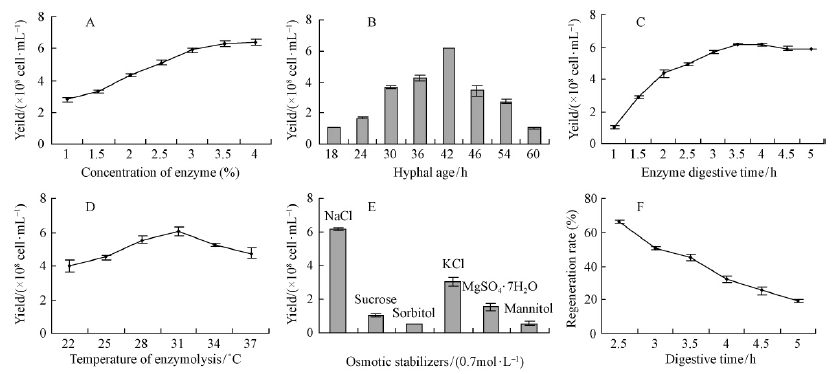 |
Fig. 1 Protoplast releasing from B. dothidea strain SDAU 11-76 on different condition A. Effect of enzyme concentration on protoplasts yield; B. Influence of hyphal growth stage on protoplasts yield; C.Time course of protoplast isolation; D.Influence of temperature on protoplasts release; E.Influence of osmotic stabilizer on protoplasts yield; F. Effect of digestive time on protoplast regeneration. Results are calculated based on three replicates experiments, and standard error of the mean (SEM) is shown. |
As shown in Fig. 1C, protoplasts released when treated with enzymes after 1 hour and the yield reached its plateau of (6.17±0.04)×108 cell·mL-1 after 3.5 h incubation, and it showed a slight decline with extended incubation in the experiments(Fig. 1C).
Analysis of six different temperatures showed that as the incubation temperature rose from 22 ℃, protoplast release efficiency increased, it reached a peak at 31 ℃ and then decreased sharply(Fig. 1D).
Six substances NaCl, KCl, MgSO4·7H2O, sorbitol, mannitol and sucrose(each in 0.7 mol·L-1)were used to dissolve enzymes and wash protoplasts respectively. The results showed that clear variations and NaCl was the most efficient osmotic stabilizer with maximum protoplast production of (6.14±0.08)×108 cell·mL-1 compared with others(Fig. 1E).
2.2 Regeneration of protoplastsDifferent media were used in the assays and the regeneration rates were calculated by colony counting after incubated at 28 ℃ for 48 h. The results showed that YPS was the most suitable regeneration medium with the highest protoplast viability of 48.33%(Tab. 2).
|
|
The released protoplasts were round, size ranged from 6.72 μm to 32.35 μm in diameter with an average of 21.71 μm(Fig. 2). Analysis of the growth ability in liquid YPS medium showed that the regeneration started from 2 h incubation and the mycelia were well produced at 18 h incubation at 28 ℃(Fig. 3).
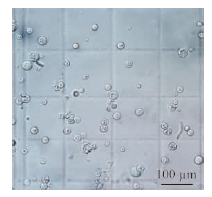 |
Fig. 2 Protoplast examination of B. dothidea strain SDAU |
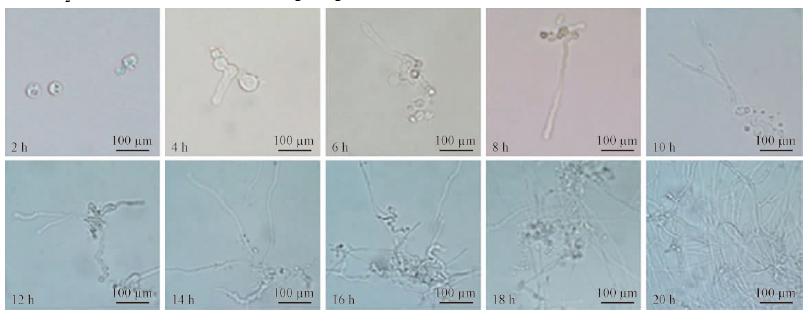 |
Fig. 3 Regeneration morphology of protoplasts releasing from B. dothidea strain SDAU11-76 following 20 h incubation in culture broths |
Further analysis of regeneration of the protoplasts isolated with different digesting times showed that the regenerate efficiency was higher for a short digestion time(2.5 h) and it decreased as the incubation extended. Combined with yield and acceptable regeneration efficiency were obtained with enzyme digestion period for 3.5 h(Fig. 1F).
2.3 PEG-CaCl2-mediated transformation of protoplastsHygromycin B resistant colonies appeared on the selective YPS regeneration media after approximately 7 days’ culture, and the transformation efficiency about 3 transformants per 1 μg.
After successive growth of 5 generations, all of the transformants had no apparent change in morphology and pathogenicity. PCR analysis showed the 0.72 kb of gfp was amplified in all 5 transformants, but absence with the non-transformed recipient DNA template(Fig. 4). Green fluorescence detection showed the high expression of gfp gene in the mycelia of the transformants, but not in the wild-type(Fig. 5). This result indicates the stability of transformants and the successful transformation of B. dothidea using A. tumefaciens with plasmid pKO1-HPH.
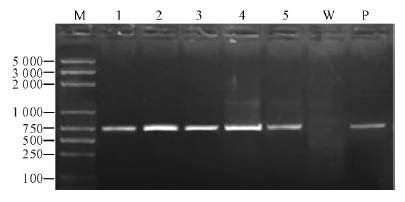 |
Fig. 4 PCR analysis of the gfp gene M:DNA molecular size markers (in base pair). Lanes 1-5: Transformants PLG1-5 respectively. W:Negative control with wild-type SDAU11-75. P: Positive control with pKO1-HPH. |
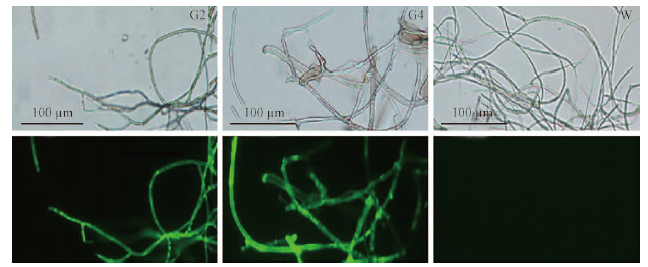 |
Fig. 5 Microscopic observations of mycelium samples exposed to ultraviolet and white light Samples were collected from the transformants G2,G4 and the wild-type SDAU11-76, respectively. |
Protoplast isolation has been well studied in fungi(Stasz et al., 1988; Li et al., 2011; Feng et al., 2012), while for a specific fungus the optimal method is required for high quality protoplast. Though spores always are used as the source in some fungi(Cheng et al., 2000; Zhang et al., 2013), it is experimental difficult to gather enough spores of B. dothidea for processing, the method established in this study provides a simple and efficient way to isolate protoplasts using mycelia of B. dothidea for genetic transformation.
In general, the process of protoplast isolation is to remove cell wall while keeping integrity of other components, especially the cytoplasm membrane. Enzymatic digestion of cell walls and osmotic stabilizers represent two major factors affecting protoplast isolation efficiency. The former removes the cell wall while the later keeps the cell stable after its cell wall is removed. In this study, driselase showed a high efficiency in protoplast production of B.dothidea in comparison with other two enzymes. It is worth noting that in this study we considered the driselase as one unit, however, it actually consisted of multiple enzymes including laminarinase, xylanase, cellulase and protease, which could effectively digest cell walls. Combination of enzymes often acts synergistically to enhance cell wall degradation and increases protoplast yield(Tilburn et al., 1983; Solis et al., 1996), the similar results were obtained when driselase mixed with lysing enzymes or mixed with glucanase in the present study.
Osmotic stabilizer is another important factor during protoplast generation. It was proved that 0.7 mol·L-1 NaCl acted as a good osmotic stabilizer for a large amount of protoplast isolation of B. dothidea. Li et al.(2011)also used 0.7 mol·L-1 NaCl as osmotic stabilizer for protoplast preparations. MgSO4, sorbitol, mannitol and sucrose were used as stabilizers in protoplast isolations for various reasons and these substances had showed important influence on protoplast productions of many different organisms. However, they were not as good as 0.7 mol·L-1 NaCl for B. dothidea in this study due to unknown reasons.
The protoplasts isolated in the present study were regenerated well especially in YPS medium, and regeneration rate is much higher of the protoplasts obtained with a short period of digestion than these with a longer digestion(Fig. 1F), which is similar to that reported previously(Feng et al., 2012). The exact reason is unclear, and it is possible that the decrease is likely caused by the damage of cytoplasm membrane proteins as the cell-wall digesting enzyme mix usually contains proteases, which remains to be investigated.
PEG-CaCl2-mediated transformation as an efficient tool is commonly used for transformation of different species of fungi(Robinson et al., 2001; Lin et al., 2008), In this study, a foreign gene gfp was transformed and expressed successfully in B. dothidea strain SDAU11-76 mediated by PEG-CaCl2, and the obtained transformants has no apparent change in morphology and pathogenicity, further more the transformants could express GFP stably. The efficiency of transformation can be influenced by many reasons such as the status of protoplasts and conditions of transformation, and that still need to be investigated in the future study. The transformants with high expressed GFP can be used as a vital tool for invasion, colonization, localization and interaction study.
In conclusion, this paper provides an efficient protocol for protoplast preparation and gfp transformation of B. dothidea as the first time. All the results in this research will help for further exploring the infection and pathogenic mechanism and functional genomics of B. dothidea.
| [1] |
Chakraborty B, Kapoor M. 1990. Transformation of filamentous fungi by electroporation. Nucleic Acids Research, 18 (22): 6737.( 1) 1)
|
| [2] |
Cheng Y, Belanger R R. 2000. Protoplast preparation and regeneration from spores of the biocontrol fungus Pseudozyma flocculosa. Fems Microbiology Letters, 190 (2 ): 287-291.( 1) 1)
|
| [3] |
De Groot M J, Bundock P, Hooykaas P J, et al. 1998. Agrobacterium tumefaciens-mediated transformation of lamentous fungi. Nature Biotechnology,16:839-842.( 1) 1)
|
| [4] |
Feng H T, Sun Z G, Li H J, et al. 2012. Preparation, purification and regeneration optimizing research of protoplasts from Rhizoctonia solani. African Journal of Microbiology Research, 6 (13 ): 3222-3230.( 2) 2)
|
| [5] |
Fincham J R. 1989.Transformation in fungi. Microbiological Reviews, 53 (1): 148-170.( 1) 1)
|
| [6] |
Guo L Y(国立耘), Li J Y(李金云),Li B H(李保华), et al. 2009. Investigations on the occurrence and chemical control of Botryosphaeria canker of apple in China. Plant Protection(植物保护), 35(4): 120-123.( 1) 1)
|
| [7] |
Ji Z L(纪兆林), Ling Z(凌筝), Zhang Q X(张清霞), et al. 2008. Study on the inhibition of Bacillus licheniformis on Botryosphaeria berengeriana f. sp. piricola and Glomerella cingulata and biocontrol efficacy on postharvest apple diseases. Journal of Fruit Science (果树学报), 25(2): 209-214.( 1) 1)
|
| [8] |
Kao K, Michayluk M. 1974. A method for high-frequency intergeneric fusion of plant protoplasts. Planta, 115 (4): 355-367.( 1) 1)
|
| [9] |
Li L L(李伶俐), Yan H(严红), Li X H(李兴红), et al. 2011. Optimizing method for protoplast preparation and regeneration in Fusarium oxysporum f. sp. conglutinans. Chinese Agricultural Science Bulletin (中国农学通报), 27 (10 ): 203-207.( 2) 2)
|
| [10] |
Li S D(李寿东), Qi Y P(齐义鹏),Hu J H(胡建红), et al. 1997. Construction of a novel cloning vector and screening the recombinants by green and white colonies. Chinese Journal of Biotechnology (生物工程学报),13(3): 323-325.( 1) 1)
|
| [11] |
Lin J F, Zheng M Y, Wang J, et al. 2008. Efficient transformation and expression of gfp gene in the edible mushroom Pleurotus nebrodensis. Progress in Natural Science, 18 (7): 819-824.( 1) 1)
|
| [12] |
Liu H X(刘会香), Li X D(李向东), Zhu X P(竺晓平), et al. 2009. First report of pomegranate stem scab caused by Botryosphaeria dothidea in China. Plant Pathology(植物病理学报),58 (2 ): 400.( 1) 1)
|
| [13] |
Lorang J M, Tuori R P, Martinez J P, et al. 2001. Green fluorescent protein is lighting up fungal biology. Applied and Environmental Microbiology, 67 (5 ): 1987-1994.( 1) 1)
|
| [14] |
Lu Z, Tombolini R, Woo S, et al. 2004. In vivo study of Trichoderma-pathogen-plant interactions, using constitutive and inducible green fluorescent protein reporter systems. Applied and Environmental Microbiology, 70 (5 ): 3073-3081.( 1) 1)
|
| [15] |
Phillips A, Rumbos I, Alves A, et al. 2005. Morphology and phylogeny of Botryosphaeria dothidea causing fruit rot of olives. Mycopathologia, 159 (3): 433-439.( 1) 1)
|
| [16] |
Pitt W, Huang R, Steel C C, et al. 2010. Identification, distribution and current taxonomy of Botryosphaeriaceae species associated with grapevine decline in New South Wales and South Australia. Australian Journal of Grape and Wine Research, 16 (1): 258-271.( 1) 1)
|
| [17] |
Rajasekaran K, Cary J W, Cotty P J, et al. 2008 Development of a GFP-expressing Aspergillus flavus strain to study fungal invasion, colonization, and resistance in cottonseed. Mycopathologia, 165 (2):89-97.( 1) 1)
|
| [18] |
Robinson H L, Deacon J W. 2001. Protoplast preparation and transient transformation of Rhizoctonia solani. Mycological Research, 105(11): 1295-1303.( 1) 1)
|
| [19] |
Rodas C, Slippers B, Gryzenhout M, et al. 2009. Botryosphaeriaceae associated with Eucalyptus canker diseases in Colombia. Forest Pathology, 39 (2): 110-123.( 1) 1)
|
| [20] |
Sanchez O, Navarro R, Aguirre J, et al. 1998. Increased transformation frequency and tagging of developmental genes in Aspergillus nidulans by restriction enzyme-mediated integration (REMI). Molecular and General Genetics, 258 (1/2): 89-94.( 1) 1)
|
| [21] |
Slippers B, Wingfield M J, Smit W A, et al. 2007. Taxonomy, phylogeny and identification of Botryosphaeriaceae associated with pome and stone fruit trees in South Africa and other regions of the world. Plant Pathology, 56 (1): 128-139.( 2) 2)
|
| [22] |
Solis S, Flores M E, Huitron C, et al. 1996. Protoplasts from pectinolytic fungi: isolation, regeneration and pectinolytic enzyme production. Letters in Applied Microbiology, 23(1): 36-42.( 1) 1)
|
| [23] |
Stasz T, Harman G, Weeden N F, et al. 1988. Protoplast preparation and fusion in two biocontrol strains of Trichoderma harzianum. Mycologia, 80: 141-150.( 1) 1)
|
| [24] |
Tang W, Ding Z, Zhou Z Q, et al. 2012. Phylogenetic and pathogenic analyses show that the causal agent of apple ring rot in China is Botryosphaeria dothidea. Plant Disease, 96 (4): 486-496.( 1) 1)
|
| [25] |
Tilburn J, Scazzocchio C, Taylor G G, et al. 1983. Transformation by integration in Aspergillus nidulans. Gene, 26: 205-221.( 1) 1)
|
| [26] |
Yang W H(杨炜华), Liu K Q(刘开启). 2002. Resistance detection of Botryosphaeria berengeriana f. sp. piricola to carbendazim and thiophanate-methyl. Journal of Plant Protection (植物保护学报), 29(2):191-192.( 1) 1)
|
| [27] |
Yu L, Chen X L, Gao L L, et al. 2009. First report of Botryosphaeria dothidea causing canker and shoot blight of Eucalyptus in China. Plant Disease, 93 (7):764.( 1) 1)
|
| [28] |
Zhang Z L(张振鲁), Du Q(杜茜), Zhou X(周幸), et al. 2013. A preliminary study on protoplast preparation conditions of Streptomyces gongzhulingensis 769. Chinese Agricultural Science Bulletin (中国农学通报), 29 (9):155-158.( 1) 1)
|
 2014, Vol. 50
2014, Vol. 50

