文章信息
- 张晓文, 邢世岩, 吴岐奎, 刘晓静
- Zhang Xiaowen, Xing Shiyan, Wu Qikui, Liu Xiaojing
- 氮添加对银杏幼林土壤有机碳化学组成及土壤微生物群落的影响
- Effects of Nitrogen Addition on Chemical Composition of Soil Organic Carbon and Soil Microbial Community in a Young Ginkgo biloba Plantation
- 林业科学, 2014, 50(6): 115-124
- Scientia Silvae Sinicae, 2014, 50(6): 115-124.
- DOI: 10.11707/j.1001-7488.20140615
-
文章历史
- 收稿日期:2014-01-04
- 修回日期:2014-04-21
-
作者相关文章
Nitrogen(N)inputs from anthropogenic sources are currently estimated to be 30% to 50% greater than those from natural terrestrial sources and tenfold greater than anthropogenic inputs from 100 years ago(Fang et al.,2011). In Asia,emissions of reactive N have increased dramatically,leading to deposition of 30-73 kg·hm-2a-1N in some subtropical forests of southern China(Fang et al.,2011). Increased nitrogen(N)deposition caused by human activities has altered ecosystem functioning and biodiversity. Soil microbial communities sustain many vital ecosystem processes,such as nutrient cycling,decomposition of organic matter and waste,nutrient availability,degradation of pesticides and contaminants,soil structure,and plant growth and health(van Elsas et al.,2006). Assessing the effect of nitrogen deposition on microbial community structure,diversity and activity in soil is therefore critical to advancing the underst and ing of the functionality,stability,and resilience of managed and natural ecosystems(Kennedy et al.,1995). So better underst and ing of microbial compositional and physiological acclimation mechanisms is critical for predicting terrestrial ecosystem responses to global change(Bi et al.,2012).
In recent years,numerous studies on the effects of N addition or simulated N deposition on soil microbes have been conducted both in the field and laboratory across different forest or plantation type,however,most of the related studies focused on the mature plantation or forest(Wallenstein et al.,2006; Cusack et al.,2011; Wu et al.,2013; Hu et al.,2010),whereas few studies focused on the young plantations. In addition,these studies have not shown consistent effects of N addition on microbial biomass and community structure,with positive,negative,and neutral effects being reported. For example,across temperate forests,Nilsson et al.(2007)reported that N input had no effect on total fungal biomass in the soils of oak forests along a natural N deposition gradient,but Demoling et al.(2008) and Fraterrigo et al.(2006)found that fungal biomarkers were decreased in soils of N-fertilized plots. In contrast,Gallo et al.(2004) reported that fungal biomarkers increased under N addition in north temperate forest soils. In tropical forest,Cusack et al.(2011)found that soil microbial biomass increased in response to N fertilization in both distinct tropical rain forests,but Liu et al.(2013)found that nitrogen additions decreased soil microbial biomass in the short term but returned to pre-treatment levels over the long term after four years N addition in a tropical forest of subtropical China. A common element to these studies is that they were carried out on mature forests or plantation. Nitrogen deposition is increasing in non-agricultural area including forest ecosystem and many previous studies have proven that nitrogen deposition significantly affects soil and plants. In addition,amounts of newly-built plantations were widely distributed after clear-cutting. Less is known,however,about how N addition alters on chemical composition of soil organic carbon(SOC) and microbial community characteristics in a young subtropical plantation.
Ginkgo biloba(Ginkgoaceae),a famous and widely cultivated plant,is often referred to as a living fossil(Yan et al.,2009). In addition,G. biloba which is a deciduous gymnosperm species,is the only remaining species of the once large order Ginkgoales,with geological records indicating this plant has been growing on the Earth for 150-200 million years,and it is widely used for street and l and scape trees(He et al.,2009; Ling et al.,2003). However,few studies focused on the effect of global change such as nitrogen deposition on G. biloba plantation,especially on soil microbes. In the present study,to acquire an insight into the mechanisms of soil microbial response to nitrogen deposition in subtropical young plantation of G. biloba,a one-year long field simulated nitrogen deposition experiment was established. Based on relevant expectations arising from previously reported studies on other subtropical plantations,we hypothesized that: 1)N addition would affect chemical composition of SOC. 2)High-N addition would significantly decrease soil microbial biomass; 3)N addition would significantly influence soil microbial community composition regardless of the amount of N loaded.
1 Materials and methods 1.1 Study siteThe study site was situated in a young G. biloba (6-year-old)plantation(5 hm2)at Xing’an county,Guangxi province(25°55′N,110°24′E),China,and has a subtropical monsoon and humid climate. The mean annual precipitation is 1 842 mm,about 65% of which occurs from March to September. The annual average relative humidity is 76% and the mean annual temperature is 18.6℃ with the lowest and highest monthly average temperature of 14.5 ℃ and 28.5 ℃. The main ground cover species include Cibotium barometz, Blechnum orientale,Lophatherum gracile,Miscanthus floridulu and Ottochloa nodosa. The soil is classified as Red soil,which predominantly derived from the granite weathering.
1.2 Field experimental treatment and samplingTwelve plots(20 m × 20 m)were r and omly staked out with an at least 15 m buffer in the G. biloba nursery garden. The experiment included four treatments with 3 replicates for each treatment: 1)Control(without any N addition applied,C); 2)50 kg·hm-2a-1 N(Low-N); 3)100 kg·hm-2a-1 N(Medium-N); 4)150 kg·hm-2a-1 N(High-N). The rates of N addition referred to the relevant studies also conducted in plantations of subtropical China,especially in Dinghu Mountain Biosphere Reserve(DHSBR)(Mo et al.,2007; 2005; Zhang et al.,2008),which was relatively near to the studied site in this paper. N additions(applied as NH4NO3)were initiated in May 2012 and applied bimonthly over the course of one year at the rate of 0 g(Control),952.38 g(Low-N),1 904.77 g(Medium-N) and 2 857.15 g(High-N)for each N application(Liu et al.,2013). Before N addition,NH4NO3 was weighed,dissolved in 20 L distilled water and applied to each plot below the canopy using a backpack sprayer(Mo et al.,2007). Each control plot received 20 L of water without N addition as well. During the experiment,nurse and management were not conducted in order to exclude the anthropogenic disturbance.
Soil samplings were conducted in May 2013. From each plot,10 soil cores(3.5 cm inner diameter)were collected r and omly from a 10 cm soil depth and compounded to one composite sample. After removing impurities,soils were sieved to 2 mm mesh size and divided into two parts,one part was retained for measuring soil chemical properties and the other was frozen at -20 ℃ as soon as possible for analysis of soil microbial biomass and community structure.
1.3 MeasurementsSoil moisture content was measured by oven-drying method using 20 g of sampled soil oven-dried at 105 ℃ for 24 h. Soil pH was measured in a 1:2.5 soil / water suspension. SOC was measured by dichromate oxidation and titration with ferrous ammonium sulfate(Lu,2000). Total N,NH4+-N and NO3--N in filtered 2 mol·L-1 KCl - extracts of fresh soil sample were measured with a flow injection auto analyzer(FIA)(Lachat Quik-Chem 8000,Lachat Instruments,USA). Available P concentration was analyzed colorimetrically after acidified ammonium persulfate digestion(Anderson et al.,1993). Chemical composition of SOC was determined using solid-state 13C nuclear magnetic resonance(NMR)spectroscopy(Bruker BioSpin Gmbh,Karlsruhe,Germany).
Soil respiration was measured using the static chamber and gas chromatography techniques,gas samples were taken with a 100 mL plastic syringe at 0,10,20,and 30 min after chamber closure(Mo et al.,2007). CO2 concentrations were determined using an Agilent 7890A gas chromatograph.
Soil microbial biomass carbon(MBC) and microbial biomass N(MBN)were estimated by chloroform fumigation-extraction. MBC and MBN(mg·kg-1)were calculated according to Wu et al.(1990) and Joergensen et al.,1990),respectively.
Soil microbial biomass and community structure were determined using phospholipid fatty acid(PLFA)analysis as described by Bossio et al.(1998). Concentrations of each PLFA were calculated based on the 19:0 internal st and ard concentrations. The relative abundance of individual fatty acid was expressed as the proportion(mol%)of the sum of all fatty acids. Gram-positive bacteria were identified by the PLFAs: i14: 0,i15: 0,i16: 0,i17: 0,a15: 0,a17: 0,Gram-negative bacteria were identified by the PLFAs: cy17:0,15:0 3OH,16:1 2OH(Liu et al.,2013; Frostegård et al.,1996). The fungi were identified by the PLFAs: 18:1ω9c(Myers et al.,2001),and PLFAs 16:1ω5c were used as a marker for arbuscular mycorrhizal fungi(AMF)(Olsson,1999). The ratio of fungal-to-bacterial PLFAs(18:1ω9c / i14:0,i15:0,a15:0,i16:0,i17:0,a17:0,cy17:0)was used as an indicator of changes in the relative abundance of these two microbial groups(Cao et al.,2010). The actinomycetes were identified by the PLFAs 10Me 17: 0 and 10Me 18: 0(Zak et al.,1996).
1.4 Statistical analysesOne-way ANOVAs and Duncan’s test were used to determine statistical significant differences(P< 0.05)in soil chemical characteristics,soil microbial variables among different N addition treatments,which was performed using SPSS software package 19.0 for Windows. Additionally,correlations between soil chemical properties and microbial variables were determined using the Pearson’s correlation coefficients. The composition of soil microbial community was summarized using a principle component analysis(PCA)on the relative abundances(mol%)of 23 PLFAs in each sample. Furthermore,the relationship between soil microbial community composition and soil chemical properties was determined using Redundancy analysis(RDA). In addition,soil chemical properties were tested for significant contribution to the explanation of the variation in soil microbial community composition with the Monte Carlo permutation test(P< 0.05). PCA and RDA were conducted using CANOCO software for Windows 4.5(Microcomputer Power,Inc.,Ithaca,NY). The figures were plotted by Origin Pro 9.0(Origin Lab Corporation,Northampton,MA,USA).
2 Results 2.1 Soil chemical properties and chemical compositions of SOC with N additionsBy the time of sampling in May 2013,Medium-N and High-N addition significantly altered soil chemical properties(Tab. 1). High-N treatment significantly decreased soil pH by 12.5%,SOC concentration in High-N addition plots was significantly lower(18.1%)than that in the Control plots(P< 0.05). Moreover,concentrations of soil total N,NH4+-N and NO3--N significantly increased in Medium-N and High-N treatment plots in comparison with the Control plots(P<0.05). Soil available P and total P concentrations were not significantly influenced by N additions(P> 0.05)(Tab. 1).
|
|
SOC of all treatments included four carbon functional groups: Alkyl-C [(0-47)×10-6],O-alkyl C[(47-112)×10-6],Aromatic-C[(112-165)×10-6] and Carbonyl-C[(165-215)×10-6].High-N treatment led a significant decline in the proportion of O-alkyl C by 6.1% and increase in the ratio of Alkyl-C and O-alkyl C by 6.1%(P<0.05)(Tab. 2).Other variables including Alkyl-C,Aromatic-C,Hydrophobic-C,Hydrophilic-C and Hydrophobic-C/Hydrophilic-C were not significantly influenced by N addition(P>0.05)(Tab. 2).
|
|
Medium-N and high-N treatments significantly decreased MBC concentration and increased MBN concentration relative to the Control treatment(P<0.05)(Fig. 1a,b).Soil respiration in High-N treatment plot [(43.2±1.2)mg·m-2 h-1CO2-C] was 10.3% lower compared with the Control plots [(48.1±2.7)mg·m-2 h-1CO2-C](Fig. 2).In addtion total microbial biomass,bacterial biomass and fungal biomass in Medium-N and High-N treatment plots decreased compared to the Control plot(P<0.05)(Fig. 3a,b,c).
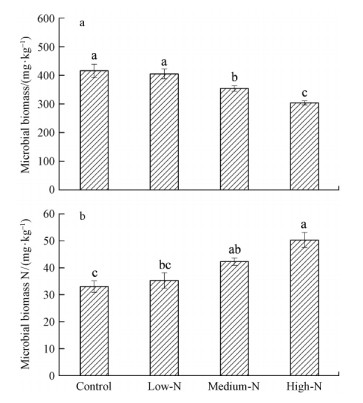 |
Fig. 1 Microbial biomass C (a) and Microbial biomass N (b) in soil samples after one-year N additions Significant differences (P<0.05, Duncan’s test) among treatments are indicated by different letters. Error bars show standard errors (n=3). |
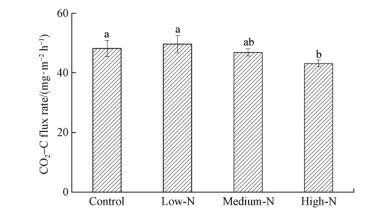 |
Fig. 2 Comparisons of soil respiration among treatments Values are means for three months (March 2013 to May 2013). Significant differences (P<0.05, Duncan’s test) among treatments are indicated by different letters. Error bars show standard errors (n=9). |
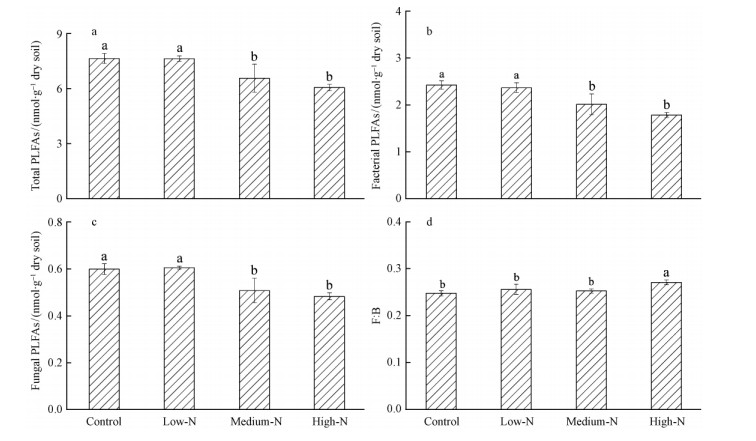 |
Fig. 3 Comparisons of soil total PLFAs (a), bacterial PLFAs (b), fungal PLFAs (c), and F:B (d) among treatments after one-year N addition F:B: the ratio of fungal to bacterial PLFAs. Significant differences (P<0.05, Duncan’s test) among treatments are indicated by different letters. Error bars show standard errors (n=3). |
The ratio of fungi to bacterial PLFAs was significantly increased by high-N treatment(9.5%)compared to the control plots(P<0.05)(Fig. 3d). Futhermore,Medium-N and High-N treatments significantly decreased the relative abundance of gram-positive bacterial PLFAs by an average of 4.7% and 8.5%,but significantly increased the relative abundance of actinomycetes PLFAs by an average of 8.0% and 7.0% relative to the control plots(P<0.05)(Fig. 4).
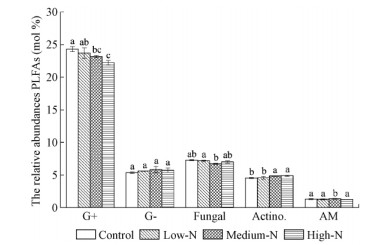 |
Fig. 4 Relative abundances of the individual PLFAs (mol%) in soil samples G+: The proportion of gram-positive bacterial PLFAs. G-: The proportion of gram-negative bacterial PLFAs; Fungal: The proportion of fungal PLFAs. Actino.: The proportion of actinomycetes PLFAs. AM: The proportion of AM fungal PLFAs. Significant differences (P<0.05, Duncan’s test) among treatments are indicated by different letters. Error bars show standard errors (n=3). |
Principal Components Analysis(PCA)of the microbial community composition,defined by the PLFA profile using 23 individual PLFAs,demonstrated that the first two axes explained 36.6% and 19.6% of the total variation in microbial communities(Fig. 5a,5b),respectively. According to the patterns in the PCA plot,the Control treatment samples were so close to the Low-N treatment samples,showing that Low-N treatment did not significantly affect soil microbial community compositions relative to the Control treatment(Fig. 5a). In addition,Medium-N and High-N treatment samples were located in different quadrants and clearly separated from the Control and Low-N treatment samples by PC2,indicating that Medium-N and High-N treatments significantly altered the microbial community structure,the main reason might be their lower relative abundance of gram-positive bacterial PLFAs and higher relative abundance of actinomycetes PLFAs under N addition compared to the Control samples(Fig. 5a,Fig. 4).
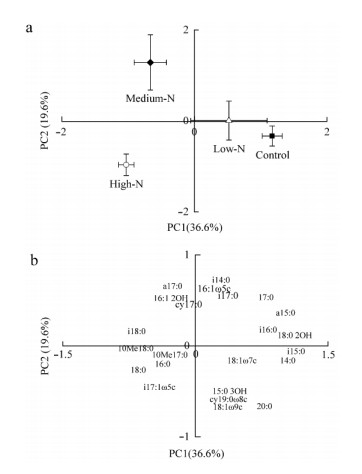 |
Fig. 5 Phospholipid fatty acid (PLFA) pattern in soil samples from different N addition plots (a) and PC loadings of the individual PLFAs (b) Values are means (n=3) with bidirectional error bars of axis 1 and 2. For both the PCA plots, values on the PC1 and PC2 axes represent the percent variation explained by PC1 and PC2, respectiv |
For individual PLFAs,Gram-positive bacterial PLFAs such as a15: 0,i15: 0 and i16: 0 got higher scores on PC1,other PLFAs such as 18: 0 2OH also got higher scores on PC1. While on PC2,Gram-negative bacterial PLFAs such as cy17: 0 and 16: 1 2OH got higher scores. Additionally,other PLFAs such as Gram-positive bacterial PLFAs(i14: 0,i17: 0 and a17: 0) and AMF PLFAs biomarker 16: 1ω5c got higher scores on PC2 as well(Fig. 5b).
2.4 Soil microbial community structure and soil chemical propertiesThe correlations between soil microbial community and soil chemical properties were analyzed by RDA and the significance of environmental variables present in the ordination was determined by Monte Carlo permutation tests(P<0.05). The results showed that seven soil variables,including soil pH,SOC,NH4+,NO3-,TN,available P and total P,explained 48.5% of the variation in soil microbial community composition(Fig. 6). Soil microbial community composition was significantly related to NO3-(F=4.09,P=0.002) and total P(F=2.03,P=0.028),with RDA axis1 explaining 33.6% of the variance and RDA axis 2 explaining 14.9%,respectively(Fig. 6). The Control plot samples were almost clearly separated from Medium-N and High-N treatment samples by RDA axis 2. In addition,NO3- and total P showed a negative association with RDA axis 1(Fig. 6).
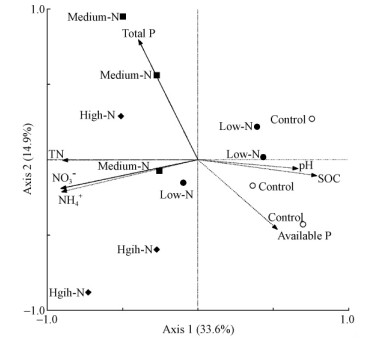 |
Fig. 6 Redundancy Analysis (RDA) results of soil microbial community composition and soil chemical properties In RDA plots, values on the x and y axes represent the percent variation explained by RDA axis 1 and RDA axis 2, respectively (P<0.05). SOC and TN refer to soil organic carbon and soil total nitrogen, respectively. |
The proportional abundance of gram-positive bacterial PLFAs was positively correlated with pH,SOC and C/N,and negatively correlated with Total N,NH4+-N and NO3--N(P<0.01)(Tab. 3). For the proportional abundance of gram-negative bacterial PLFAs,it was negatively correlated with pH,SOC,Avail. P and C/N(P<0.05)(Tab. 3). In addition,the proportional abundance of fungal PLFAs was positively correlated with C/N(P<0.05) and negatively correlated with Total P,Total N,NH4+-N and NO3--N(P<0.05)(Tab. 3).Moreover,the proportional abundance of actinomycetes PLFAs was positively correlated with Total N,NH4+-N and NO3--N(P<0.01),but negatively correlated with pH,SOC and C/N(Tab. 3). Furthermore,the proportional abundance of AMF PLFAs was not significantly correlated with soil chemical properties(P>0.05)(Tab. 3).
|
|
In this study,High-N addition significantly decreased soil organic carbon content(Tab. 1). N fertilization has been observed to stimulate ecosystem C loss(Liu et al.(2009)) and such a decline was consistent with a recent report by Wei et al.(2011). A possible mechanism is that N addition stimulated SOC decomposition more than plant production,leading to a net loss of ecosystem C(Mack et al.,2004),and another one is that higher atmospheric N deposition resulted in higher C loss by increasing heterotrophic respiration and dissolved organic carbon leaching(Bragazza et al.,2006). In addition,N addition have significant effects on C inputs processes from plants,such as amount of litterfall and litter decomposition(Knorr et al.,2005),fine root biomass(Wallenstein et al.,2006) and root exudation(Bowden et al.,2004),which were closely related to soil organic carbon content dynamics.
High-N treatment significantly decreased soil pH(Tab. 1),such a finding was consistent with the result from a N addition study on larch plantation(Hu et al.,2010). A decline in soil pH may be resulted from NH4+ uptake by plants,nitrification of NH4+ in soils,and NO3- leaching(Matthew et al.,2006). In a mature subtropical forest of China,Lu et al.(2009)reported that the ecosystem was sensitive to high N addition,and soil acidification was significantly enhanced after continuous two-year N additions(50-150 kg·hm-2a-1 N). Furthermore,Fan et al.(2007)found that exchangeable base cations(e.g. Ca2+ and Mg2+)reduced with increasing N addition in a subtropical Chinese fir plantation after three years of N addition(60-240 kg·hm-2a-1N),the nitrate leached out was accompanied by positively charged “counter-ions”,the base cations K+,Ca2+ and Mg2+,resulting in the further acidification of the leached soil,or hydrogen and aluminum ions,which may cause the acidification of receiving ecosystems.
3.2 Effects of N addition on soil microbial biomass and community compositionMedium-N and High-N additions significantly decreased MBC concentration in this study(Fig. 1a). Decreased soil organic carbon and pH may be the main reason of reduced MBC,according to the related studies by Sarathchandra et al.(2001) and Wallensteina et al.(2006). Additionally,Medium-N and High-N application significantly increased microbial biomass N in the present study(Fig. 1b),mainly because soil microbial biomass serves as both an important source and sink for plant available nutrients,and N availability was increased after N addition and consequently immobilized by microbes,which led to an increase in soil microbial biomass N and a decline in nutrient loss(Garcia et al.,1994; Wang et al.,2008).
Medium-N and High-N additions significantly decreased total microbial biomass,bacterial biomass and fungal biomass,these findings are corresponding with the results obtained in field studies(Fig. 3),where N fertilization decreased soil microbial biomass by an average of 11% to 35%(Ramirez et al.,2012). One explanation is the decrease in soil acidity,soil pH is a major factor influencing the structure of the soil microbial community and individual microbial PLFAs,the strongest influence of soil pH was on the fungal and bacterial growth(Bååth et al.,2003; Rousk et al.,2010),so the declines of bacterial biomass and fungal biomass in this study may be due to the higher growth inhibition of Medium-N and High-N additions to bacterial and fungal communities,consequently leading a decline in soil total biomass. In addition,soil C availability and plant belowground C allocation play dominant roles in regulating soil microbial population size and community composition. The influences of N addition on soil microbial biomass and community structure are strongly linked to changes of plant production and belowground C supply(Hu et al.,2010),so another possible mechanism to explain the reduction of soil microbial biomass is the changes of belowground C under N additions. In the present study,High-N treatment significantly increased the ratio of fungal to bacterial,such a result was consistent with a recent report from a comparable pH gradient in an arable soil,where the fungal:bacterial growth ratio also increased 50-fold between pH 8.3 and 4.0(Rousk et al.,2009). In addition,Liu et al.(2013)reported that F:B ratios were significant higher in the N-addition plots comparing to the control plots after four years N addition in a tropical forest of subtropical China.
In the present study,Medium-N and High-N additions significantly altered the soil microbial community structure(Fig. 5a). Allison et al.(2008)summarized the results of studies previously reported on the soil microbial community composition response to the mineral fertilization(N/P/K),and the majority of these studies demonstrate that composition is sensitive to disturbance. More than 80% of the mineral fertilization(N/P/K)studies found significant effects of disturbance on microbial composition. And this study proved that soil microbial community composition in a young plantation of Ginkgo biloba was significantly sensitive to Medium-N and High-N additions.
3.3 Effect of N addition on soil respirationHigh-N treatment led a significant reduction in soil respiration after 12 months N addition in this study(Fig. 2),which was also found in other forest ecosystems(Mo et al.,2007; Burton et al.,2004; Chen et al.,2002). Ramirez et al.(2012)found that decreases in soil respiration by N addition ranged between 8% and 15%. The reductions in soil respiration after nitrogen additions may be possibly related to the following mechanisms: 1)N additions decreased autotrophic respiration from plant roots,decrease in soil respiration was related to the reduction in st and ing root biomass and root exudation in N-fertilized plots(Bowden et al.,2004); 2)Heterotrophic respiration from the microbial community may be reduced by N addition,which was indicated by that the decomposition rates of litter and soil organic matter were suppressed(Mo et al.,2007; Jiang et al.,2010).In the present study,soil respiration as an indices of soil microbial activity was measured for three months only to determine the difference of soil microbial activity among treatments rather than the dynamics of CO2 emission,such a experiment design was also reported in other N addition studies(Liu et al.,2013),so the mean soil respiration for three months as an indicator of soil microbial activity was appropriate. In contrast,several neutral effects of N addition on soil respiration have also been reported across different ecosystems. For example,Jiang et al.(2010)found that nitrogen deposition(during growing season)tended to decrease CO2 emission,but the differences caused by nitrogen deposition were not significant in a short-term simulated nitrogen deposition experiment in an alpine meadow on the Qinghai-Tibetan Plateau. Similarly,Liu et al.(2013)reported that soil respiration did not significantly change after four years N addition in a tropical forest. So it is,therefore,that effects of N addition on soil respiration were different across ecosystems and need a better underst and ing.
3.4 ConclusionThe effects of N additions on soil microbial biomass and community structure in a young plantation of G.biloba were concluded that: 1)Low-N treatment did not significantly affect soil microbial biomass; 2)Medium-N and High-N treatments significantly decreased MBC(C)concentration but increased microbial biomass nitrogen(N)concentration; 3)Medium-N and High-N treatments significantly decreased soil total microbial biomass,fungal biomass,and bacterial biomass; 4)Medium-N and High-N treatments significantly decreased the relative abundance of gram-positive bacterial PLFAs,and increased fungal:bacterial ratio and the relative abundance of actinomycetes PLFAs; 5)High-N treatment significantly decreased soil pH,respiration,SOC concentration and the proportion of O-alkyl but increased Alkyl:O-alkyl. Our conclusion indicated that the responses of soil microbial biomass and community structure in a young plantation of G.biloba varied due to the amount of N loaded.
| [1] |
Anderson J M, Ingram J S I. 1993. Tropical Soil Biology and Fertility: A Handbook of Methods. 2nd ed. Wallingford, UK: CAB International.( 1) 1)
|
| [2] |
Allisonand S D, Martiny J B H. 2008. Resistance, resilience, and redundancy in microbial communities. Proceedings of the National Academy of Sciences of the United States of America, 105: 11512-11519.( 1) 1)
|
| [3] |
Bååth E, Anderson T H. 2003. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biology & Biochemistry, 35 (7): 955-963.( 1) 1)
|
| [4] |
Bi J, Zhang N L, Liang Y, et al. 2012. Interactive effects of water and nitrogen addition on soil microbial communities in a semiarid steppe. Journal of Plant Ecology, 5 (3): 320-329.( 1) 1)
|
| [5] |
Bossio D A, Scow K M. 1998. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecology, 35 (3/4): 265-278.( 1) 1)
|
| [6] |
Bowden R D, Davidson E, Savage K, et al. 2004. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. Forest Ecology and Management, 196 (1): 43-56.( 2) 2)
|
| [7] |
Bragazza L, Freeman C, Jones T, et al. 2006. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proceedings of the National Academy of Sciences of the United States of America, 103 (51): 19386-19389.( 1) 1)
|
| [8] |
Burton A J, Pregitzer K S, Crawford J N, et al. 2004. Simulated chronic NO3- deposition reduces soil respiration in northern hardwood forests. Global Change Biology, 10 (7): 1080-1091.( 1) 1)
|
| [9] |
Cao Y S, Fu S G, Zou X M, et al. 2010. Soil microbial community composition under Eucalyptus plantations of different age in subtropical China. European Journal of Soil Biology, 46 (2): 128-135.( 1) 1)
|
| [10] |
Chen C R, Xu Z H, Hughes J M. 2002. Effects of nitrogen fertilization on soil nitrogen pools and microbial properties in a hoop pine (Araucaria cunninghamii) plantation in southeast Queensland, Australia. Biology and Fertility of Soils, 36 (4): 276-283.( 1) 1)
|
| [11] |
Cusack D F, Silver W L, Torn M S. 2011.Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology, 92 (3): 621-632.( 2) 2)
|
| [12] |
Demoling F, Nilsson L O, Bååth E. 2008. Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biology & Biochemistry, 40 (2): 370-379.( 1) 1)
|
| [13] |
Fan H B (樊后保), Liu W F (刘文飞), Li Y Y (李燕燕), et al. 2007. Tree growth and soil nutrients in response to nitrogen deposition in a subtropical Chinese fir plantation. Acta Ecologica Sinica (生态学报), 27 (11): 4630-4642.( 1) 1)
|
| [14] |
Fang Y T, Yoh M, Koba K, et al. 2011. Nitrogen deposition and forest nitrogen cycling along an urban-rural transect in southern China. Global Change Biology, 17 (2): 872-885.( 1) 1)
|
| [15] |
Fraterrigo J M, Balser T C, Turner M G. 2006. Microbial community variation and its relationship with nitrogen mineralization in historically altered forests. Ecology, 87 (3): 570-579.( 1) 1)
|
| [16] |
Frostegård A, Bååth E. 1996. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biology and Fertility of Soils, 22n (1/2): 59-65.( 1) 1)
|
| [17] |
Gallo M, Amonette R, Lauber C, et al. 2004. Microbial community structure and oxidative enzyme activity in nitrogen-amended north temperate forest soils. Microbial Ecology, 48 (2): 218-229.( 1) 1)
|
| [18] |
Garcia F O, Rice C W. 1994. Microbial biomass dynamics in tall grass prairie. Soil Science Society of America Journal, 58 (3): 816-823.( 1) 1)
|
| [19] |
He X Y, Huang W, Chen W, et al. 2009. Changes of main secondary metabolites in leaves of Ginkgo biloba in response to ozone fumigation. Journal of Environmental Sciences, 21 (2): 199-203.( 1) 1)
|
| [20] |
Hu Y L, Zeng D H, Liu Y X, et al. 2010. Responses of soil chemical and biological properties to nitrogen addition in a Dahurian larch plantation in Northeast China. Plant and Soil, 333(1/2): 81-92.( 3) 3)
|
| [21] |
Jiang C M, Yu G R, Fang H J, et al. 2010. Short-term effect of increasing nitrogen deposition on CO2, CH4 and N2O fluxes in an alpine meadow on the Qinghai-Tibetan Plateau, China. Atmospheric Environment, 44: 2920-2926.( 1) 1)
|
| [22] |
Joergensen R G, Brookes P C. 1990. Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 m K2SO4 soil extracts. Soil Biology and Biochemistry, 22 (8): 1023-1027.( 1) 1)
|
| [23] |
Kennedy A C, Smith K L. 1995. Soil microbial diversity and the sustainability of agricultural soil. Plant Soil, 170 (1): 75-86.( 1) 1)
|
| [24] |
Knorr M, Frey S D, Curtis P S. 2005. Nitrogen additions and litter decomposition: a meta-analysis. Ecology, 86 (12): 252-257.( 1) 1)
|
| [25] |
Ling J, Lin Y R, Xue L M, et al. 2003. Identification of a sex-associated RAPD marker in Ginkgo biloba. Acta Botanica Sinica, 45: 742-747.( 1) 1)
|
| [26] |
Liu L, Zhang T, Frank S, et al. 2013. Interactive effects of nitrogen and phosphorus on soil microbial communities in a tropical forest. PLOS ONE, 8 (4): e61188.( 5) 5)
|
| [27] |
Liu L L, Greave T L. 2009. A review of nitrogen enrichment effects on three biogenic GHGs: the CO2 sink may be largely offset by stimulated N2O and CH4 emission. Ecology Letters, 10 (12): 1103-1117.( 1) 1)
|
| [28] |
Lu R K (鲁如坤). 2000. Soil Agro-Chemical Analyses. Beijing: Agricultural Technical Press of China (中国农业科技出版社).( 1) 1)
|
| [29] |
Lu X K, Mo J M, Gundersen P, et al. 2009. Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere, 19 (2): 189-198.( 1) 1)
|
| [30] |
Mack M C, Schuur E A G, Bret-Harte M S, et al. 2004. Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature, 431: 440-443.( 1) 1)
|
| [31] |
Mo J M (莫江明), Fang Y T (方运霆), Xu G L (徐国良), et al. 2005. The short-term responses of soil CO2 emission and CH4 uptake to simulated N deposition in nursery and forests of Dinghushan in subtropical China. Acta Ecologica Sinica (生态学报), 25 (4): 682-690.( 1) 1)
|
| [32] |
Mo J M, Zhang W, Zhu W X, et al. 2007. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Global Change Biology, 14 (2): 403-412.( 4) 4)
|
| [33] |
Myers R T, Zak D R, White D C, et al. 2001. Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Science Society of America Journal, 65 (2): 359-367.( 1) 1)
|
| [34] |
Nilsson L O, Bååth E, Falkengren-Grerup U, et al. 2007. Growth of ectomycorrhizal mycelia and composition of soil microbial communities in oak forest soils along a nitrogen deposition gradient. Oecologia, 153 (2): 375-384.( 1) 1)
|
| [35] |
Olsson P A. 1999. Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. Fems Microbiology Ecology, 29 (4): 303-310.( 1) 1)
|
| [36] |
Ramirez K S, Craine J M, Fierer N. 2012. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biology, 18 (6): 1918-1927.( 1) 1)
|
| [37] |
Rousk J, Brookes P C, Bååth E. 2009. Contrasting soil pH effects on fungal and bacterial growth suggests functional redundancy in carbon mineralization. Applied and Environmental Microbiology, 75 (6): 1589-1596.( 1) 1)
|
| [38] |
Rousk J, Brookes P C, Bååth E. 2010. The microbial PLFA composition as affected by pH in an arable soil. Soil Biology & Biochemistry, 42(3): 516-520.( 1) 1)
|
| [39] |
Sarathchandra S U, Ghani A, Yeates G W, et al. 2001. Effect of nitrogen and phosphate fertilizers on microbial and nematode diversity in pasture soils. Soil Biology & Biochemistry, 33 (7/8): 953-964.( 1) 1)
|
| [40] |
van Elsas J D, Trevors J T, Wellington E M H. 2006. Modern Soil Microbiology. 2nd ed. New York: CRC Press.( 1) 1)
|
| [41] |
Wallenstein M D, McNulty S, Fernandez I J, et al. 2006. Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. Forest Ecology and Management, 222 (1/3): 459-468.( 2) 2)
|
| [42] |
Wang Q K, Wang S L, Liu Y X. 2008. Responses to N and P fertilization in a young Eucalyptus dunnii plantation: Microbial properties, enzyme activities and dissolved organic matter. Applied Soil Ecology, 40 (3): 484-490.( 1) 1)
|
| [43] |
Wei Y Y (卫云燕), Yin H J(尹华军), Liu Q (刘庆), et al. 2011. Responses on rhizosphere effect of two subalpine coniferous species to night-time warming and nitrogen fertilization in western Sichuan, China. Acta Ecologica Sinica (生态学报), 31 (3): 698-708.( 1) 1)
|
| [44] |
Wu J, Joergensen R G, Pommerening B, et al. 1990. Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biology and Biochemistry, 22: 1167-1169.( 1) 1)
|
| [45] |
Wu J P, Liu W F, Fan H B, et al. 2013. Asynchronous responses of soil microbial community and understory plant community to simulated nitrogen deposition in a subtropical forest. Ecology and Evolution, 3(11): 3895-3905.( 1) 1)
|
| [46] |
Yan X L, Chen Y Y, Guan B C, et al. 2009. Eleven novel microsatellite markers developed from the living fossil Ginkgo biloba (Ginkgoaceae). Conservation Genetics, 10: 1277-1279.( 1) 1)
|
| [47] |
Zak D R, Ringelberg D B, Pregitzer K S, et al. 1996. Soil microbial communities beneath Populus grandidentata crown under elevated atmospheric CO2. Ecological Applications, 6 (1): 257-262.( 1) 1)
|
| [48] |
Zhang W, Mo J M, Zhou G Y, et al. 2008. Methane uptake responses to nitrogen deposition in three tropical forests in southern China. Journal of Geophysical Research, 113 (D11), Doi:10.1029/2007JD009195.( 1) 1)
|
 2014, Vol. 50
2014, Vol. 50

