文章信息
- 白林利, 李昌晓
- Bai Linli, Li Changxiao
- 水淹对水杉苗木耐旱性的影响
- Effects of Submersion on Susceptibility of Metasequoia glyptostroboides Seedlings to Drought
- 林业科学, 2014, 50(11): 166-174
- Scientia Silvae Sinicae, 2014, 50(11): 166-174.
- DOI: 10.11707/j.1001-7488.20141122
-
文章历史
- 收稿日期:2014-01-20
- 修回日期:2014-04-21
-
作者相关文章
三峡水库“冬蓄夏排”的反季节水位调度管理方式,使库区水位(在海拔145~175 m之间)呈现周期性消涨变化,这导致消落带岸生树种处于水淹与陆生的周期性交替变化环境之中,受到相应的水分胁迫(Li et al.,2006; Jiang et al.,2006)。研究岸生树种对消落带多种水分逆境条件的生长与生理响应,对于建设和保护消落带生态植被具有重要意义。然而,目前有关三峡库区岸生树种对消落带多种水分胁迫的响应的研究报道还较少。
目前国内外针对周期性水分胁迫的研究主要集中于枫杨(Pterocarya stenoptera)(王朝英等,2013; 王振夏等,2013)、湿地松(Pinus elliottii)(张晔等,2011; 王振夏等,2012)、黑柳(Salix nigra)(Li et al.,2005; Carpenter et al.,2008)、细柱柳(Salix gracilistyla)(Nakai et al.,2011)、落羽杉(Taxodium distichum)(Anderson et al.,2000; Anderson et al.,2001; Elcan et al.,2002)、纳塔栎(Quercus nuttallii)与沼生栗栎(Quercus michauxii)(Anderson et al.,2000; Anderson et al.,2001)等树种的光合与生长特性,以及水稻(郭相平等,2013)、香蒲(Typha latifolia)(Li et al.,2004)等植物的生长特性等方面。有关水杉(Metasequoia glyptostroboides)对周期性水分胁迫的响应的研究报道较少。
水杉是三峡库区库岸带典型的乡土树种,属杉科(Taxodiaceae)水杉属,中国特有孑遗珍贵树种; 其根系发达,耐寒与耐受多种水分逆境的能力较强,具有极其重要的经济价值和观赏价值(白祯等,2011)。目前,关于水杉的研究主要集中在基因结构(杨星宇等,2011; Chen et al.,2003; Ahuja,2009; Cui et al.,2010; Zhao et al.,2013)、遗传变异(Du et al.,2013)、无性繁殖(Kolasinski,2012)、化学组成及其特性(Bajpai et al.,2007; Mou et al.,2007; Bajpai et al.,2010; Dong et al.,2011; Zeng et al.,2012)和种群变化(Tang et al.,2011)等方面。本研究模拟三峡库区消落带土壤水分变化格局,旨在从生理生态学的角度深入了解水杉树苗对水淹-干旱逆境胁迫的响应机制,以便为三峡库区消落带植被保护与建设提供理论支撑。
1 材料与方法 1.1 材料考虑到三峡库区库岸防护林体系建设多采用2年生苗木,本试验以2年生水杉树苗为试验材料。2012年11月20日将生长基本一致的90株树苗带土盆栽(土壤性质见表 1),每盆1株(盆中央内径20 cm,盆高17 cm),置于西南大学三峡库区生态环境教育部重点试验室实验基地大棚(海拔249 m)进行培养。2013年1月18日正式开始试验(处理开始时,苗高97.05±1.53 cm)。
|
|
结合三峡库区消落带水位变化实际情况,试验分为3个阶段。第1阶段(phase I)为淹水处理期,共设3个处理组,分别为对照组(control,C)、半淹组(half-submersion,HS)和全淹组(full-submersion,FS)3组,每组试验苗木30株,共90株。其中,C为常规供水组,保持土壤含水量为田间持水量的75%~80%(李昌晓等,2010); HS组苗盆放入水池中,池水保持淹没 植物中段; FS组苗盆也放入水池中,但池水保持淹没至植物顶端20 cm。60天后,每组各取6株水杉用于光合和生物量测定,其余24株用于后续试验。
第2阶段(phase Ⅱ)为干旱处理期,将第1阶段每组剩余的24株苗木再随机均分为2组,其中一组进行轻度干旱胁迫处理,另一组则继续保持第1阶段的水分处理。故第2阶段共有对照-干旱组(control followed by drought,CD)、半淹-干旱组(half-submersion followed by drought,HSD)、全淹-干旱组(full-submersion followed by drought,FSD),以及对照组(C)、半淹组(HS)、全淹组(FS)6个处理组,每组12株,共72株。轻度干旱处理组土壤含水量为田间持水量的47%~50%(Li et al.,2010)。为监测轻度干旱处理的效果,清晨(6:00—7:00)测定水杉树苗上部成熟叶水势(以美国Wescor公司生产的露点水势仪Psypro测定),以小于-0.5 MPa为标准(图 1)。60天后,每组各取6株水杉用于光合和生物量测定,其余6株用于后续试验。
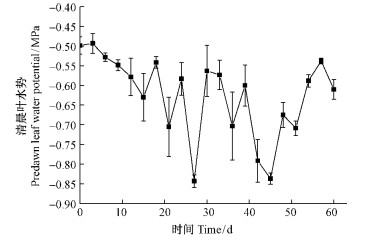 |
图 1 轻度干旱阶段水杉清晨叶水势 Fig. 1 Changes of predawn leaf water potential of M. glyptostroboides during drought stress(phase Ⅱ) |
第3阶段(Phase Ⅲ)为恢复处理阶段,将HS与FS处理组苗盆从水池中取出,所有处理组均参照对照组C进行常规供水,时间为21天。
1.3 数据测定1)光合测定 采用Li-Cor 6400便携式光合分析系统(Li-Cor Inc.,USA)测定叶片光合参数。经预备试验,采用晴天9:00—12:00测定,CO2浓度400 μmol·mol-1,饱和光强1 200 μmol·m-2s-1,以红蓝光为光源,叶室6 cm2。参考Anderson等(2001)的测定方法,选取苗木从上往下数的第3至第4片叶,每个处理测定6株。测定指标包括净光合速率(net photosynthetic rate,Pn)、气孔导度(stomatal conductance,Sc)、蒸腾速率(transpiration rate,Tr)与胞间CO2浓度(intercellular CO2 concentration,Ci)。
2)生物量测定 将试验盆钵中的苗木小心挖出,用自来水冲净根系,然后将根(收集断根,并用吸水纸吸干根表面水分)、茎、叶分别放置在80 ℃烘箱中烘干至恒质量,用分析天平称量,计算根冠比。
1.4 统计分析利用SPSS16.0软件,进行单因素方差分析(One-way ANOVA)揭示水分处理对水杉光合与生长的影响,并用Tukey检验法对各个光合与生长指标进行多重比较,检验每个指标在同一阶段不同处理间(α=0.05)的差异显著性。
2 结果与分析 2.1 水杉对水淹的光合与生长响应(阶段1)第1阶段末,与C组相比,HS组的Pn显著增加,而Sc,Tr,Ci则显著降低(图 2)。因FS组叶呈未展开的叶芽形式,无法测定其光合参数,该组叶生物量显著低于C与HS组,但茎、根生物量以及总生物量却均显著高于C与HS组(表 2)。同时,FS组的根冠比显著高于C组,但与HS组之间并无显著差异。此外,各个处理组水杉树苗存活率都达100%,即水淹未影响水杉树苗存活率。
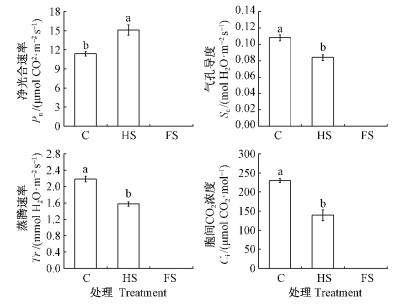 |
图 2 第1阶段末各处理组光合指标比较
Fig. 2 Comparison of photosynthetic indexes among
treatment groups at the end of phase I
图中数据为6株水杉叶片的光合平均值和标准误; 不同字母表示各指标在P =0.05水平差异显著。下同。 Data shown are means±standard error (n=6), and different letters show significant differences at P =0.05. The same below. |
|
|
干旱阶段结束时,与C组相比,CD,HS,HSD组的Pn,Sc,Tr均显著降低。就Pn而言,CD与HSD组之间无显著性差异,这与FSD显著高于CD与HSD组形成鲜明对照,而FSD与C组间则差异不显著。CD,HS,HSD,FSD各组与C组间的Ci均无显著性差异; 但HSD组的Ci则显著高于CD与HS组,而与FSD组无显著差异(图 3)。
 |
图 3 第2阶段末各处理组光合指标比较
Fig. 3 Comparison of photosynthetic indexes among
treatment groups at the end of phase Ⅱ
FS组叶片未完全展开,未能获得光合数据。 Photosynthetic parameter of the FS group was unavailable due to the unopened leaves of the seedlings. |
与C组相比,FS,FSD组的叶、茎、根生物量、总生物量显著降低,并且叶生物量、总生物量还均显著低于CD,HS,HSD 处理组。不同的是,CD,HS,HSD组叶、茎生物量与C组间均无显著性差异,但其根生物量、总生物量均显著低于C组。对于根冠比而言,HS组显著低于其余各个处理组,而且各处理组(HS组除外)相互之间均无显著性差异(表 3)。
|
|
与干旱阶段相比,在恢复正常供水之后,各个处理组的Pn,Sc,Tr,Ci都有一定程度的恢复,有的甚至显著高于对照水平。就Pn而言,CD,FSD 组显著高于C组,HS组与C,HSD组间均无显著差异。FSD组的Sc在所有处理组中最高,且显著高于其余各处理组; 与之相反,CD,HS,HSD组的Sc则与 C组无显著差异。各个处理组的Tr均显著高于C组。HSD组的Ci显著高于C组,这与CD组显著低于C组形成对照(图 4)。
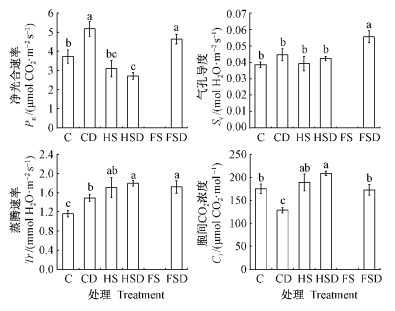 |
图 4 第3阶段末各处理组光合指标比较
Fig. 4 Comparison of photosynthetic indexes among
treatment groups at the end of phase Ⅲ
FS组因落叶,未能获得光合数据,但经检测,苗木仍然处于存活状态。 Photosynthetic parameter of the seedlings in the FS group was unavailable due to leaf abscission. However, the seedlings were alive upon examination. |
同样,各个处理组的叶、茎、根生物量及总生物量也有一定程度的恢复,FSD组与CD组之间分别均无显著差异,而HSD则分别高于CD组。HS组的根冠比在所有处理组中居于最低,CD,HSD组与C组的根冠比相互之间均无显著差异,FSD组与FS组间的根冠比 也无显著差异(表 4)。
|
|
三峡工程建成蓄水后,水库消落区某些区域淹没时间长达6个月,水淹深度达30 m。由于水库实行以年度为周期的水位调节方式,库岸两边部分区域会处于被淹没与出水暴露的周期性更替之中(类淑桐等,2009)。三峡库区消落带的水位变化使得原有植被大量退化,景观质量和环境健康受到威胁(New et al.,2008)。在消落带内构建人工植被是保护三峡库区消落带生态环境的重要措施之一。消落带水分条件的周期性变化,要求所栽种的植物不仅具有适应水分饱和与过剩的能力,还要具有适应水分亏缺与干旱的能力。
3.1 水杉对水淹胁迫的响应罗芳丽等(2006)认为水淹导致植株部分叶组织无法进行光合作用而使植株的整体光合受到影响,植株未被水淹的叶组织光合可能会增强。在水淹60天后,水杉HS组Pn显著高于C组(图 2),其原因可能是植物光合能力受到对光合产物需求的负反馈调节(Jeschke et al.,1997; Bragina et al.,2004),且与水杉叶片在水淹期间具有较高羧化能力有关(Gimeno et al.,2012)。短期水淹下植物保持较高的光合速率可能是植物能耐受水淹的重要特征之一(Chen et al.,2005)。
半淹60天后,与对照组C相比,水杉Pn增加33%,高于相同处理后耐淹物种水翁(Cleistocalyx operculatus)(Jing et al.,1999)和落羽杉(Taxodium distichum)(Li et al.,2006),这说明短期水淹对水杉光合能力影响较小; 但半淹120天后,水杉Pn显著降低(图 3),这说明长期水淹显著影响了水杉树苗的光合作用,水杉树苗Pn对长期水淹具有很强的负向响应能力(李昌晓等,2005)。
植物叶片气孔变小甚至关闭是植物对水淹胁迫的通常响应方式之一(Syvertsen et al.,1983),气孔导度是控制蒸腾速率的一个有效方式(Hessini et al.,2008)。Mielke等(2003)对美洲格尼帕树(Genipa americana)的研究发现,在水淹胁迫下,Tr随着气孔导度的降低而降低,本研究中水淹下水杉树苗的Tr与Sc也表现出相同的变化趋势。本研究还发现,水淹下HS组水杉的Ci在第1阶段末显著低于C组,而在第2,3阶段末却与C组并未出现显著差异,这与Pn在第1阶段末显著高于C组,在第2阶段末显著降低,第3阶段末基本恢复到C组的水平变化趋势存在一致性,这也许是水杉对水淹胁迫的一种光合生理适应机制。第1阶段水杉树苗Pn升高可能是在短期水淹胁迫下水杉树苗的光合酶活性和利用CO2能力增强所致(Gimeno et al.,2012);第2阶段水杉树苗Pn降低是由Sc降低所引起,也可能与长期水淹产生的超氧根离子影响到正常的光合功能有关(Yordanova et al.,2007);第3阶段Pn,Sc,Tr,Ci的恢复说明水杉树苗可以适应较长时间(120天)的水淹胁迫而存活。
水淹根部产生不定根和肥大的皮孔也是耐水淹植物的适应特性(Gibberd et al.,2001; Simova-Stoilova et al.,2012),本研究也发现类似的现象,这是许多树种应对水淹胁迫的有效方式之一(Pimenta et al.,2010; Calvo-Polanco et al.,2012)。
植物生物量的积累与其生长发育、营养物质的形成密切相关,对其所处的生长环境的综合表征作用明显(靖元孝等,2001; 杨静等,2008; 张晔等,2011; Tang et al.,1983)。在第1阶段末,HS组水杉树苗Pn显著升高,根生物量升高,根冠比显著增加,说明水杉在水淹下根部分配生物量较多,以有效应对水淹胁迫逆境条件(Ye et al.,2003; Larre et al.,2013)。在第2阶段末,即水淹120天后,HS组水杉树苗Pn显著降低,根生物量显著降低,根冠比降低,说明水杉树苗地上部分分配能量较多,以适应逆境胁迫,也有可能是水淹胁迫下产生的自由基对根造成了损害,导致根对水淹胁迫更为敏感(Peng et al.,2013)。在第3阶段末,即恢复阶段,水杉树苗Pn基本恢复到C组的水平,但生物量的响应有一定的后滞效应,生物量有一定程度的恢复但未达对照水平。经过3个阶段的锻炼,水杉树苗形成了一套应对水分胁迫的生理机制,即从忍耐到逃避,最终Pn基本恢复到C组的水平,存活率达100%。
3.2 水杉对干旱胁迫的响应干旱是另一个限制植物生长和光合的重要非生物因子(Parida et al.,2007; Hamayun et al.,2010; Riaz et al.,2010)。一般而言,干旱胁迫会导致树木的气孔关闭(Eclan et al.,2002),净光合速率降低(De Simone et al.,2003; Nemani et al.,2003; Jackson et al.,2005; Haldimann et al.,2008),进而导致光合产物积累减少,使树木的生长发育受到影响(Johari-Pireivatlou et al.,2010)。在干旱处理阶段,2年生水杉树苗光合过程受到显著的影响(CD,HSD组的Pn,Sc显著下降)(图 3),这与Li等(2010)对落羽杉(Taxodium distichum)与池杉(Taxodium ascendens)、Doupis等(2013)对橄榄(Olea europaea)的研究结果相似。
在干旱胁迫条件下,净光合速率的降低与气体交换受到限制有关。气体交换限制包括气孔限制和非气孔限制2种(Lawlor,2002)。植物在胁迫环境条件下,气孔限制是一种很好的适应(Duan et al.,2005; Musila et al.,2009)。Farquhar等(1982)认为,气孔限制引起的净光合速率降低是因为气孔导度下降,阻止了CO2的供应; 而非气孔限制则是因为叶肉细胞光合能力下降,导致叶肉细胞利用CO2 的能力降低,进而使胞间CO2浓度升高所致。在干旱胁迫下,水杉CD与HSD组的Pn,Sc均显著下降,此为许多树种对干旱胁迫的典型响应(Ngugi et al.,2004; Fan et al.,2008; Liu et al.,2011)。然而,Sc的下降并未引起Ci的显著变化(图 3)。由此表明本研究中2年生水杉Pn降低可能是由非气孔限制引起,即干旱胁迫影响了卡尔文循环中的电子传递和生物化学反应(Yordanova et al.,2003; Cechin et al.,2007; Wolkerstorfer et al.,2011)。
蒸腾速率在估测植物的耐旱性方面是一个很重要的指标,通过气孔散失较少水分的物种应该是较为耐旱的物种(Riaz et al.,2013)。植物处于逆境胁迫下,气孔调节对植物的适应性是至关重要的(Dos Santos et al.,2013)。本研究中,在干旱阶段水杉树苗Ci下降,并与Sc表现出相同的变化趋势,这表明在干旱胁迫下,Tr下降的部分原因是气孔关闭(Baquedano et al.,2006; Silva et al.,2010)。
植物生物量的积累,对于其维持正常的生理活动、完成全部生活史至关重要。Xiao等(2005)对中间锦鸡儿(Caragana intermedia)的研究表明:中度干旱降低中间锦鸡儿根、茎、叶的生物量,这与本研究结果相似,但CD与HSD组之间无显著性差异,说明前期的水淹并未增加水杉树苗对后期干旱胁迫的敏感性。根冠比可以帮助评价植物是否处于健康生长状态,以及估测植物忍耐胁迫的潜力(Riaz et al.,2013)。干旱胁迫下,盆栽千层(Callistemon citrinus)的根冠比升高(Alvarez et al.,2013),以维持较高的根表面积,吸收更多的水分; 然而,在本研究中,干旱胁迫下,与C组相比,水杉根冠比并未出现显著变化(表 3),这表明2年生水杉具有一定的耐受干旱胁迫的能力。
本研究发现,在干旱胁迫下,CD与HSD组水杉Pn以及生物量均没有显著差异,由此说明前期的水淹并未影响水杉树苗对后期干旱胁迫的光合生理响应。本研究还发现,FSD组水杉树苗的Pn,Sc,Ci与C组间无显著差异,这可能是水杉树苗从水淹胁迫进入干旱胁迫,充足的气体供应以及光照加快了水杉树苗的光合同化,从而进一步证实水杉树苗前期的水淹并未影响其在后期干旱胁迫条件下的光合生理响应。这些均反映出水杉树苗具有应对水淹-干旱胁迫的有效策略及较强的适应能力(Striker,2012)。
3.3 移除干旱胁迫后的响应在自然条件下,一个物种对胁迫的忍耐性取决于其在移除胁迫后短期内恢复生长的表现(Fini et al.,2013),这个过程对于物种缓解前期胁迫的影响至关重要。本试验第3阶段,移除水分胁迫后,CD,HSD,FSD组水杉树苗的Pn显著上升,与毛竹栎(Quercus pubescens)(Galle et al.,2007)和麻疯树(Jatropha curcas)(Fini et al.,2013)等表现相似,这表明在干旱阶段水杉能够维持光合机构的功能完整性(Arend et al.,2013),表现出对干旱胁迫的良好适应能力; CD,HSD组水杉的Sc显著上升,表明干旱胁迫对水杉光合结构的生化限制是不显著的(Mottonen et al.,2005; Avila et al.,2012)。
干旱胁迫去除后,恢复的程度及幅度取决于干旱的强度、持续时间以及受胁迫的物种种类(Xu et al.,2010)。在干旱胁迫下,CD,HSD组的Pn显著下降,但其存活率却是100%。在移除胁迫后,不同水分处理下的水杉树苗生物量虽然得到一定程度的恢复,但未达对照水平,其原因可能是前期的干旱时间过长或是恢复时间不够(Galle et al.,2007),这是否是由于干旱胁迫导致水杉的生理过程出现滞后效应,以及该效应影响程度如何,还有待于进一步研究。
4 结论本研究发现前期的水淹并未影响水杉树苗对后期干旱胁迫的光合生理响应。水杉不仅表现出耐水湿的特点,还表征出一定程度的耐旱性,可以将其适当置于轻度干旱环境条件下,但应注意浇水抗旱,使水杉保持正常的净光合速率。从试验研究结果来看,水杉可以作为三峡库区消落带植被建设的候选树种之一。
| [1] |
白祯,黄建国.2011.三峡库区护岸林主要树种的耐湿性和营养特性.贵州农业科学,39(6): 166-169.( 1) 1)
|
| [2] |
郭相平,甄博,王振昌.2013.旱涝交替胁迫增强水稻抗倒伏性能.农业工程学报,29(12): 130-135.( 1) 1)
|
| [3] |
靖元孝,程惠青,彭建宗,等.2001.水翁幼苗对淹水的反应初报.生态学报,21(5): 810-813.( 1) 1)
|
| [4] |
类淑桐,曾波,徐少君,等.2009.水淹对三峡库区秋华柳抗性生理的影响.重庆师范大学学报,26(3): 30-33.( 1) 1)
|
| [5] |
李昌晓,魏虹,吕茜,等.2010.不同水分处理对湿地松幼苗生长与根部次生代谢物含量的影响.生态学报,30(22): 6154-6162.( 1) 1)
|
| [6] |
李昌晓,钟章成.2005.三峡库区消落带土壤水分变化条件下池杉幼苗光合生理响应的模拟研究.水生生物学报,29(6): 712-716.( 1) 1)
|
| [7] |
罗芳丽,王玲,曾波,等.2006.三峡库区岸生植物野古草(Arundinella anomala Steud.)光合作用对水淹的响应.生态学报,26(11): 3602-3609.( 1) 1)
|
| [8] |
王朝英,李昌晓,张晔.2013.水淹对枫杨幼苗光合生理特征的影响.应用生态学报,24(3): 675-682.( 1) 1)
|
| [9] |
王振夏,魏虹,李昌晓,等.2012.土壤水分交替变化对湿地松幼苗光合特性的影响.西北植物学报,32(5): 980-987.( 1) 1)
|
| [10] |
王振夏,魏虹,吕茜,等.2013.枫杨幼苗对土壤水分"湿-干"交替变化光合及叶绿素荧光的响应.生态学报,33(3): 888-897.( 1) 1)
|
| [11] |
杨静,何开跃,李晓储,等.2008.淹水胁迫对两种栎树生长的影响.林业科技开发,22(4): 34-37.( 1) 1)
|
| [12] |
杨星宇,杨路路,余志伟,等.2011.水杉木材DNA提取及条形码分子鉴定.湖北大学学报:自然科学版,33(4): 397-403.( 1) 1)
|
| [13] |
张晔,李昌晓.2011.水淹与干旱交替胁迫对湿地松幼苗光合与生长的影响.林业科学,47(12): 158-164.( 2) 2)
|
| [14] |
Ahuja M R.2009.Genetic constitution and diversity in four narrow endemic redwoods from the family Cupressaceae.Euphytica,165(1): 5-19.( 1) 1)
|
| [15] |
Alvarez S,Sanchez-Blanco M J.2013.Changes in growth rate,root morphology and water use efficiency of potted Callistemon citrinus plants in response to different levels of water deficit.Scientia Horticulturae,156(8): 54-62.( 1) 1)
|
| [16] |
Anderson P H,Pezeshki S R.2000.The effects of intermittent flooding on seedlings of three forest species.Photosynthetica,37(4): 543-552.( 2) 2)
|
| [17] |
Anderson P H,Pezeshki S R.2001.Effects of flood pre-conditioning on responses of three bottomland tree species to soil waterlogging.Journal of Plant Physiology,158(2): 227-233.( 3) 3)
|
| [18] |
Arend M,Brem A,Kuster T M, et al.2013.Seasonal photosynthetic responses of European oaks to drought and elevated daytime temperature.Plant Biology,15(1): 169-176.( 1) 1)
|
| [19] |
Avila C,Guardiola J L,Nebauer S G.2012.Response of the photosynthetic apparatus to a flowering-inductive period by water stress in Citrus. Trees,26(3): 833-840.( 1) 1)
|
| [20] |
Bajpai V K,Na M,Kang S C.2010.The role of bioactive substances in controlling foodborne pathogens derived from Metasequoia glyptostroboides Miki ex Hu.Food and Chemical Toxicology,48(7): 1945-1949.( 1) 1)
|
| [21] |
Bajpai V K,Rahman A,Kang S C.2007.Chemical composition and anti-fungal properties of the essential oil and crude extracts of Metasequoia glyptostroboides Miki ex Hu.Industrial Crops and Products,26(1): 28-35.( 1) 1)
|
| [22] |
Baquedano F J,Castillo F J.2006.Comparative ecophysiological effects of drought on seedlings of the Mediterranean water-saver Pinus halepensis and water-spenders Quercus coccifera and Quercus ilex. Trees,20(6): 689-700.( 1) 1)
|
| [23] |
Bragina T V,Ponomareva Y V,Drozdova I S,et al. 2004.Photosynthesis and dark respiration in leaves of different ages of partly flooded maize seedlings.Russian Journal of Plant Physiology,51(3): 342-347.( 1) 1)
|
| [24] |
Calvo-Polanco M,Senorans J,Zwiazek J J.2012.Role of adventitious roots in water relations of tamarack(Larix laricina)seedlings exposed to flooding.BMC Plant Biology,12(1): 99.( 1) 1)
|
| [25] |
Carpenter L T,Pezeshki S R,Shields Jr F D.2008.Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra.Trees,22(5): 737-748.( 1) 1)
|
| [26] |
Cechin I,Fumis T F,Dokkedal A L.2007.Growth and physiological responses of sunflower plants exposed to ultraviolet-B radiation.Ciencia Rural,37(1): 85-90.( 1) 1)
|
| [27] |
Chen H J,Qualls R G,Blank R R.2005.Effect of soil flooding on photosynthesis,carbohydrate partitioning and nutrient uptake in the invasive exotic Lepidium latifolium. Aquatic Botany,82(4): 250-268.( 1) 1)
|
| [28] |
Chen X Y,Li Y Y,Wu T Y,et al. 2003.Size-class differences in genetic structure of Metasequoia glyptostroboides Hu et Cheng(Taxodiaceae)plantations in Shanghai.Silvae Genetica,52(3/4): 107-109.( 1) 1)
|
| [29] |
Cui M Y,Yu S,Liu M,et al. 2010.Isolation and characterization of polymorphic microsatellite markers in Metasequoia glyptostroboides(Taxodiaceae).Conservation Genetics Resources,2(1): 19-21.( 1) 1)
|
| [30] |
De Simone O,Junk W J,Schmidt W.2003.Central Amazon floodplain forests: root adaptations to prolonged flooding.Russian Journal of Plant Physiology,50(6): 848-855.( 1) 1)
|
| [31] |
Dong L B,He J,Wang Y Y,et al. 2011.Terpenoids and Norlignans from Metasequoia glyptostroboides.Journal of Natural Products,74(2): 234-239.( 1) 1)
|
| [32] |
dos Santos C M,Verissimo V,de Lins Wanderley Filho H C,et al. 2013.Seasonal variations of photosynthesis,gas exchange,quantum efficiency of photosystem Ⅱ and biochemical responses of Jatropha curcas L.grown in semi-humid and semi-arid areas subject to water stress.Industrial Crops and Products,41(1): 203-213.( 1) 1)
|
| [33] |
Doupis G,Bertaki M,Psarras G,et al. 2013.Water relations,physiological behavior and antioxidant defence mechanism of olive plants subjected to different irrigation regimes.Scientia Horticulturae,153(5): 150-156.( 1) 1)
|
| [34] |
Du K B,Xu L,Li M H,et al. 2013.Genetic variation in progenies of 23 relic trees of Metasequoia glyptostroboides in their original habitat.Forest Science and Practice,15(1): 1-12.( 1) 1)
|
| [35] |
Duan B,Lu Y,Yin C,et al. 2005.Physiological responses to drought and shade in two contrasting Picea asperata populations.Physiologia Plantarum,124(4): 476-484.( 1) 1)
|
| [36] |
Elcan J M,Pezeshki S R.2002.Effects of flooding on susceptibility of Taxodium distichum L.seedlings to drought.Photosynthetica,40(2): 177-182.( 1) 1)
|
| [37] |
Fan S,Grossnickle S C,Russell J H.2008.Morphological and physiological variation in western redcedar(Thuja plicata)populations under contrasting soil water conditions.Trees,22(5): 671-683.( 1) 1)
|
| [38] |
Farquhar G D,Sharkey T D.1982.Stomatal conductance and photosynthesis.Annual Review Plant Physiology,33(1): 317-345.( 1) 1)
|
| [39] |
Finin A,Bellasio C,Pollastri S,et al. 2013.Water relations,growth,and leaf gas exchange as affected by water stress in Jatropha curcas. Journal of Arid Environments,89(2): 21-29.( 2) 2)
|
| [40] |
Galle A,Haldimann P,Feller U.2007.Photosynthetic performance and water relations in young pubescent oak(Quercus pubescens)trees during drought stress and recovery.New Phytologist,174(4): 799-810.( 2) 2)
|
| [41] |
Gibberd M R,Gray J D,Cocks P S,et al. 2001.Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity,lateral root formation and aerotropic rooting.Annals of Botany,88(4): 579-589.( 1) 1)
|
| [42] |
Gimeno V,Syvertsen J P,Simon I,et al. 2012.Physiological and morphological responses to flooding with fresh or saline water in Jatropha curcas. Environmental and Experimental Botany,78(2): 47-55.( 2) 2)
|
| [43] |
Haldimann P,Galle A,Feller U.2008.Impact of exceptionally severe summer stress conditions on photosynthetic traits in oak(Quercus pubescens)leaves.Tree Physiology,28(5): 785-795.( 1) 1)
|
| [44] |
Hamayun M,Sohn E Y,Khan S A,et al. 2010.Silicon alleviates the adverse effects of salinity and drought stress on growth and endogenous plant growth hormones of soybean(Glycine max L.).Pakistan Journal of Botany,42(3): 1713-1722.( 1) 1)
|
| [45] |
Hessini K,Ghandour M,Albouchi A,et al. 2008.Biomass production,photosynthesis,and leaf water relations of Spartina alterniflora under moderate water stress.Journal of Plant Research,121(3): 311-318.( 1) 1)
|
| [46] |
Jackson M B,Colmer T D.2005.Response and adaptation by plants to flooding stress.Annals of Botany,96(4): 501-505.( 1) 1)
|
| [47] |
Jeschke W D,Hilpert A.1997.Sink-stimulated photosynthesis and sink-dependent increase in nitrate uptake: nitrogen and carbon relations of the parasitic association Cuscuta reflexa-Ricinus communis.Plant,Cell and Environment,20(1): 47-56.( 1) 1)
|
| [48] |
Jiang H T,Xu F F,Cai Y,et al. 2006.Weathering characteristics of sloping fields in the Three Gorges Reservoir area,China.Pedosphere,16(1): 50-55.( 1) 1)
|
| [49] |
Jing Y X,Chen Z P,Cheng H Q, et al.1999.The relationship between photosynthetic character and adventitious roots in flooded Cleistocalyx operculatus seedlings.Journal of Tropical and Subtropical Botany,8(4): 361-364.( 1) 1)
|
| [50] |
Johari-Pireivatlou M,Qasimov N,Maralian H.2010.Effect of soil water stress on yield and proline content of four wheat lines.African Journal of Biotechnology,9(1): 36-40.( 1) 1)
|
| [51] |
Kolasinski M.2012.How can we propagate the Metasequoia glyptostroboides Hu et Cheng? Horticulture,15(2): 1-7.( 1) 1)
|
| [52] |
Larre C F,Fernando J A,Marini P,et al. 2013.Growth and chlorophyll a fluorescence in Erythrina crista-galli L.plants under flooding conditions.Acta Physiologiae Plantarum,35(5): 1463-1471.( 1) 1)
|
| [53] |
Lawlor D W.2002.Limitation to photosynthesis in water-stressed leaves: stomata vs.metabolism and the role of ATP.Annals of Botany,89(7): 871-885.( 1) 1)
|
| [54] |
Li C M,Zhang S,Liu J H,et al. 2006.Distribution of nutrients and chlorophyll a in the Three-Gorges Reservoir,China.Chinese Journal of Geochemistry,25(3): 295-300.( 2) 2)
|
| [55] |
Li C X,Zhong Z C,Geng Y,et al. 2010.Comparative studies on physiological and biochemical adaptation of Taxodium distichum and Taxodium ascendens seedlings to different soil water regimes.Plant and Soil,329(1/2): 481-494.( 2) 2)
|
| [56] |
Li C X,Zhong Z C,Liu Y.2006.Effect of soil water changes on photosynthetic characteristics of Taxodium distichum seedlings in the hydro-fluctuation belt of the Three Gorges Reservoir area.Frontiers of Forestry in China,1(2): 163-169.( 2) 2)
|
| [57] |
Li S,Martin L T,Pezeshki S R,et al. 2005.Responses of black willow Salix nigra cuttings to simulated herbivory and flooding.Acta Oecologica,28(2): 173-180.( 1) 1)
|
| [58] |
Li S,Pezeshki S R,Goodwin S.2004.Effects of soil moisture regimes on photosynthesis and growth in cattail(Typha latifolia).Acta Oecologica,25(1): 17-22.( 1) 1)
|
| [59] |
Liu C C,Liu Y G,Guo K,et al. 2011.Comparative ecophysiological responses to drought of two shrub and four tree species from karst habitats of southwestern China.Trees,25(3): 537-549.( 1) 1)
|
| [60] |
Mielke M S,De Almeida A A F,Gomes F P,et al. 2003.Leaf gas exchange,chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding.Environmental and Experimental Botany,50(3): 221-231.( 1) 1)
|
| [61] |
Mottonen M,Lehto T,Rita H,et al. 2005.Recovery of Norway spruce(Picea abies)seedlings from repeated drought as affected by boron nutrition.Trees,19(2): 213-223.( 1) 1)
|
| [62] |
Mou X L,Fu C,Wu H K,et al. 2007.Composition of essential oil from seeds of Metasequoia glyptostroboides growing in China.Chemistry of Natural Compounds,43(3): 334-335.( 1) 1)
|
| [63] |
Musila C F,Arnolds J L,Van Heerden P D R,et al. 2009.Mechanisms of photosynthetic and growth inhibition of a southern African geophyte Tritonia crocata(L.)Ker.Gawl.by an invasive European annual gras Lolium multiflorum Lam.Environmental and Experimental Botany,66(1): 38-45.( 1) 1)
|
| [64] |
Nakai A,Kisanuki H.2011.Stress responses of Salix gracilistyla and Salix subfragilis cuttings to repeated flooding and drought.Journal of Forest Research,16(6): 465-472.( 1) 1)
|
| [65] |
Nemani R R,Keeling C D,Hashimoto H,et al. 2003.Climate-driven increases in global terrestrial net primary production from 1982 to 1999.Science,300(5625): 1560-1563.( 1) 1)
|
| [66] |
New T,Xie Z.2008.Impacts of large dams on riparian vegetation: applying global experience to the case of China’s Three Gorges Dam.Biodiversity and Conservation,17(13): 3149-3163.( 1) 1)
|
| [67] |
Ngugi M R,Doley D,Hunt M A,et al. 2004.Physiological responses to water stress in Eucalyptus cloeziana and E.argophloia seedlings.Trees,18(4): 381-389.( 1) 1)
|
| [68] |
Parida A K,Dagaonkar V S,Phalak M S,et al. 2007.Alterations in photosynthetic pigments,protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery.Plant Biotechnology Reports,1(1): 37-48.( 1) 1)
|
| [69] |
Peng Y,Dong Y,Tu B,et al. 2013.Roots play a vital role in flood-tolerance of poplar demonstrated by reciprocal grafting.Flora-Morphology,Distribution,Functional Ecology of Plants,208(8): 479-487.( 1) 1)
|
| [70] |
Pimenta J A,Bianchini E,Medri M E.2010.Adaptations to flooding by tropical trees: morphological and anatomical modifications.Oecologia Australis,4(1): 157-176.( 1) 1)
|
| [71] |
Riaz A,Younis A,Hameed M,et al. 2010.Morphological and biochemical responses of turf grasses to water deficit conditions.Pakistan Journal of Botany,42(5): 3441-3448.( 1) 1)
|
| [72] |
Riaz A,Younis A,Taj A R,et al. 2013.Effect of drought stress on growth and flowering of marigold(Tagetes erecta L.).Pakistan Journal of Botany,45(1): 123-131.( 2) 2)
|
| [73] |
Silva E N,Ribeiro R V,Ferreira-Silva S L,et al. 2010.Comparative effects of salinity and water stress on photosynthesis,water relations and growth of Jatropha curcas plants.Journal of Arid Environments,74(10): 1130-1137.( 1) 1)
|
| [74] |
Simova-Stoilova L,Demirevska K,Kingston-Smith A,et al. 2012.Involvement of the leaf antioxidant system in the response to soil flooding in two Trifolium genotypes differing in their tolerance to waterlogging.Plant Science,183(2): 43-49.( 1) 1)
|
| [75] |
Striker G G.2012.Time is on our side: the importance of considering a recovery period when assessing flooding tolerance in plants.Ecological Research,27(5): 983-987.( 1) 1)
|
| [76] |
Syvertsen J P,Zablotowicz R M,Smith Jr M L.1983.Soil temperature and flooding effects on two species of citrus.Plant and Soil,72(1): 3-12.( 1) 1)
|
| [77] |
Tang C Q,Yang Y,Ohsawa M,et al. 2011.Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China.Biological Conservation,144(1): 279-289.( 1) 1)
|
| [78] |
Tang Z C,Kozlowski T T.1983.Responses of Pinus banksiana and Pinus resinosa seedlings to flooding.Canadian Journal of Forest Research,13(4): 633-639.( 1) 1)
|
| [79] |
Wolkerstorfer S V,Wonisch A,Stankova T,et al. 2011.Seasonal variations of gas exchange,photosynthetic pigments,and antioxidants in Turkey oak(Quercus cerris L.)and Hungarian oak(Quercus frainetto Ten.)of different age.Trees,25(6): 1043-1052.( 1) 1)
|
| [80] |
Xiao C W,Sun O J,Zhou G S,et al. 2005.Interactive effects of elevated CO2 and drought stress on leaf water potential and growth in Caragana intermedia. Trees,19(6): 712-721.( 1) 1)
|
| [81] |
Xu Z,Zhou G,Shimizu H.2010.Plant responses to drought and rewatering.Plant Signaling and Behavior,5(6): 649-654.( 1) 1)
|
| [82] |
Ye Y,Tam N F,Wong Y S,et al. 2003.Growth and physiological responses of two mangrove species Bruguiera gymnorrhiza and Kandelia candel to waterlogging.Environmental and Experimental Botany,49(3): 209-221.( 1) 1)
|
| [83] |
Yordanova R Y,Alexieva V S,Popova L P.2003.Influence of root oxygen deficiency on photosynthesis and antioxidant status in barley plants.Russian Journal of Plant Physiology,50(2): 163-167.( 1) 1)
|
| [84] |
Yordanova R Y,Popova L P.2007.Flooding-induced changes in photosynthesis and oxidative status in maize plants.Acta Physiologiae Plantarum,29(6): 535-541.( 1) 1)
|
| [85] |
Zeng Q,Cheng X R,Qin J J,et al. 2012.Norlignans and phenylpropanoids from Metasequoia glyptostroboides Hu et Cheng.Helvetica Chimica Acta,95(4): 606-612.( 1) 1)
|
| [86] |
Zhao Y,Thammannagowda S,Staton M,et al. 2013.An EST dataset for Metasequoia glyptostroboides buds: the first EST resource for molecular genomics studies in Metasequoia.Planta,237(3): 755-770.( 1) 1)
|
 2014, Vol. 50
2014, Vol. 50

