文章信息
- 孙瑾, 林锐航, 李晓增, 王晓波, 朱晓枫, 徐恩光
- Sun Jin, Lin Ruihang, Li Xiaozeng, Wang Xiaobo, Zhu Xiaofeng, Xu Enguang
- 杉木液化液交联苯酚-甲醛合成新型木材胶黏剂
- A Novel Plywood Adhesive Synthesized by Phenol-Formaldehyde Crosslinking Alkaline Chinese Fir Liquid
- 林业科学, 2014, 50(1): 140-148
- Scientia Silvae Sinicae, 2014, 50(1): 140-148.
- DOI: 10.11707/j.1001-7488.20140121
-
文章历史
- Received date: 2013-02-21
- Revised date: 2013-05-27
-
作者相关文章
2. 广州市质量监督检测研究院 广州 510110
2. Guangzhou Quality Supervision and Testing Institute Guangzhou 510110
The petrochemical synthetic phenol-formaldehyde resin adhesives, have commonly been used in the production of wood-based panels(He et al., 2012). However, the declining fossil fuel reserves combined with the increasing price of fossil fuel have become the obstacles of the development of wood composite boards(Schöene et al., 2009). Therefore, more and more exploitations aimed at adopting natural and economical products as substitutes for conventional wood resin adhesives have drawn many researchers’attention. In recent years, there have been many attempts to replace petrochemicals with renewable resources, such as lignin(Alonso et al., 2011), cornstarch(Moubarik et al., 2010), tannin(Jahanshaei et al., 2012), cellulose(Qing et al., 2012), cashew nut shell(Papadopoulou et al., 2011), wheat straw(Chen et al., 2012)for wood resin adhesives. However, few of them can be further applied for industrial process due to their inherent disadvantages in compromised adhesive strength, lowwater resistance or high formaldehyde emission.
Wood, the most abundant biomass in nature, has been regarded as the most promising renewable resource. It is a complex bio-composite mainly consisting of three structural components: cellulose, hemicellulose and lignin(Binder et al., 2009). Lignin is of particular interest because of its phenolic formation from which a wide variety of phenols and phenol derivatives and aromatic chemicals can be derived. Cellulose and hemicellulose can be converted into polyols, aldehydes and other small molecule compounds under certain conditions(Zhang et al., 2012). Hence, considerable attention has been given to the preparation of environmentally friendly polymeric products from liquefied woods and their derivatives.
Until now, there were two main methods of wood liquefaction. The first liquefaction method is called phenolysis, involving phenol with acids as catalysts, which resulted in liquefaction products rich in combined phenolic compounds. Further application in the preparation of phenolic adhesives is similar to the conventional phenol resins(Mohamad Ibrahim et al., 2011), mouldings(Lee et al., 2011) and others. The second method was achieved in existence of alcohols, especially polyhydric alcohols, and the gained products can be used as polyols for the preparation of polyurethane and epoxy products(Pan et al., 2012; Wu et al., 2010). But only little information can be found in the literature concerning controlled liquefaction of wood in alkaline medium. However, alkaline treatment at elevated temperature is often used to enhance the reactivity of the crystalline cellulose through decreasing DP(degrees of polymerization) and increasing accessibility of cellulose. Meanwhile, the lignin components depolymerize to form monomeric and oligomeric phenolic compounds. In view of the above, liquefaction of wood combining alkaline catalysts under high temperature appears to be an attractive way to obtain low molarcular weight compounds in the further use.
In this study, we evaluated the huge potential of waste Chinese fir as a biomass resource for the production of adhesives. In order to obtain a Chinese fir-based adhesive of desirable properties for bonding plywood in exterior use, the Chinese fir was liquefied in alkaline medium at 200 ℃ for pretreatment, then co-polymerized with phenol-formaldehyde to synthesize biomass adhesive. The Chinese fir-based adhesives were formulated with high percentages of Chinese fir liquefied resultant in the adhesive formulations to reduce the petrochemical dependency of conventional wood adhesives.
1 Experimental 1.1 MaterialsChinese fir(collected in Guangdong Province, China)was ground into flour in a rotating disintegrator. The wood flour with a dimension passing 100 meshes was dehydrated in an oven at 105 ℃ until oven-dried. Reagent grade phenol and formaldehyde(formalin, at concentration of 37%)solution were purchased from Guangdong Guanghua Chemical Factory Co. Ltd, China. Other analytical chemicals such as sodium hydroxide, hydrochloric acid, sodium thiosulfate pentahydrate, absolute ethyl alcohol, potassium iodide, iodine, hydrochloric acid, ammonium acetate, acetylacetone were obtained from Guangzhou chemical reagent factory, China.
1.2 Liquefaction of Chinese fir flourThe Chinese fir flour was liquefied in sealed reactor using the stainless steel instrument under the following conditions: column, 12 cm × 12 cm × 1. 5 cm(diameter × height × thickness); limit temperature, 500 ℃; limit pressure, 12 MPa. About 75 g Chinese fir flour was loaded into the reactor. A concentration of 25% sodium hydroxide solution(300 g in total)was gradually charged into the reactor with stirring. After all of the sodium hydroxide solution had been loaded, the reactor was sealed, and the mixture was kept in oil bath at(200 ± 2)℃ for 15 min. The liquefied resultant was then cooled and preserved for synthesis.
1.3 Preparation of Chinese fir-based adhesivesFor preparing Chinese fir-based adhesives, the Chinese fir liquid was used to prepare resins in the reaction flask. The three weight ratios of Chinese fir(CF)liquid to phenol-formaldehyde(PF)(60 /40, 55 /45 and 50 /50), three molar ratio of formaldehyde to phenol(the values are 2: 1, 2. 5: 1 and 3: 1)were chosen respectively as the analyzing variable factors. The typical synthesis procedure was described as below in which the PF /CF was 60 /40 with molar ratio of 2: 1. The preparation conditions of all adhesives are summarized in Tab. 1.
|
|
47 g of phenol and 60 g of formalin(the F /P molar ratio was 2)were charged into a three-necked, 500mL flask equipped with a condenser, a thermometer, a teflon stirrer and the reaction temperature was maintained at 45-50 ℃ for 30 min. Then the temperature was gradually raised to around 80 ℃, and 160. 5 g of Chinese fir liquid was then added during a period of 50 min. After all of the Chinese fir liquid had been infused, the reaction temperature was set at(92 ± 2)℃ immediately and maintained until the Gardner-Holdt viscosity reached(3 000 ± 200)mPa·s(20 ± 2)℃. The viscosity measurements were carried out at 10-min intervals. The finished adhesive was cooled to room temperature and kept in the refrigerator for use. About 250 g of each adhesive sample was prepared. The PF resin was prepared as control sample. The solid content, free formaldehyde content and free phenol content of prepared adhesives were determined according to the China Industry St and ard(GB /T 14074—2006).
1.4 Resin solid contentThe percent of resin solid content is calculated by the following equation.
| $ S\left( {\rm{\% }} \right){\rm{ = }}{S_{\rm{1}}}{\rm{ / }}{S_{\rm{0}}}{\rm{ \times 100}}{\rm{.}} $ |
where“S”was the percent of resin solid content and “S0” and “S1”were the weight of the resin before and after the test, respectively.
1.5 Free formaldehyde contentThe free formaldehyde content of the prepared resins was determined by the hydroxylamine hydrochloride method. Accurately weighed about 3 or 5 gm of resin sample was transferred into 250 mL beaker and dissolved in 50 mL methyl alcohol or 50 mL 75% isopropyl alcohol. Simultaneously, the pH value of the solution was adjusted to 3. 5 by 1 N hydrochloric acid solution. 25 mL of 10% hydroxylamine hydrochloride solution was added and stirred for 10 min. Finally, the mixture solution was titrated with 0. 1 N or 1 N sodium hydroxide solution. Free formaldehyde content was calculated by using following formula.
${\rm{Free formaldehybe}}\;{\rm{content(\% ) = }}\frac{{3c({V_1} - {V_0})}}{m}$
where “V1 ” and “V0 ” are volumes of sodium hydroxide solution required in the titration for sample and blank, respectively. “c”is the exact normality of sodium hydroxide solution. “m”is weight of sample in gm.
1.6 Free phenol contentThe percentage of free phenol was evaluated according to bromometry method. Accurately weighed about 2 gm of resin sample was taken in 1 000 mL round-bottomed flask. To that 100 mL distilled water was added and the pH value of the solution was adjusted to 4. 0 by 20% hydrochloric acid solution. Then steam distilled until the negative test for phenol with bromine water. The distillate was diluted to 1 000 mL with double distilled water. An aliquot of 50 mL was taken in Erlenmeyer flask and 25 mL of 0. 1 N bromate-bromide solution and 5 mL concentrated HCl was added. The flask was then closed and kept in dark for 15 min. After that, 1. 8 gm potassium iodide was added and kept in dark for another 10 min. Finally, the mixed solution was titrated with 0. 1 N sodium thiosulphate solution using starch as indicator. The free phenol was calculated by following formula
| $\begin{array}{l} {\rm{Pheppl}}\;{\rm{content}}\left( {{\rm{by}}\;{\rm{weight}}} \right) = \\ \frac{{\left( {{V_1} - {V_2}} \right){\rm{ \times }}c{\rm{ \times }}0.01568{\rm{ \times }}1000}}{{m{\rm{ \times }}50}}{\rm{ \times }}100\% \end{array}$ |
where “V1 ” and “V2 ” are the volumes of 0. 1 N sodium thiosulphate solution required for blank and sample, respectively. “m”is the weight of sample in gm and “c ” is the exact normality of sodium thiosulphate solution.
1.7 Evaluation of plywoodEucalyptus veneers with dimensions of 320 mm × 320 mm × 2. 1 mm were used to prepare 3-layer plywood panels. Chinese fir-based adhesive was applied to double sides of a veneer at a spread rate of 350 g·m-2(for double gluelines). The adhesivecoated veneer was then stacked between two uncoated veneers with the grain directions of two adjacent veneers perpendicular to each other. Thereafter, the assembled veneers were pre-pressed(pressure, 1 MPa)at room temperature for 1 h. After that, the prepressed veneers were hot-pressed at 160 ℃ for 15 min at the same pressure of 1 MPa. The specimens were maintained at room temperature, for 24 h, sawn to get samples for the test of bonding strength and formaldehyde emission. A total of 48 specimens of 100 mm × 25 mm(4 cycles’test, 12 specimens were prepared for each cycle)were cut from each panel for bond strength tests, according to JIS K6806—2004 st and ard. Ten specimens of 150 mm × 50 mm were cut from panel to determine the formaldehyde emission according to JIS A1460—2003 st and ard.
1.8 Fourier transform infrared(FT-IR)spectroscopy testThe adhesive sample was placed in an oven at(160 ± 2)℃ until a constant weight was obtained. FTIR spectra of both samples were performed in a Perkin Elmer model Spectrum V10 instrument. Each spectrum was recorded in a frequency range of 400-4 000 cm-1 using potassium bromide(KBr)disc. The KBr was previously oven-dried at 300 ℃ to reduce the interference of water.
1.9 Differential scanning calorimetry(DSC)testDSC measurements were conducted on a NETZSCH 204 F1 differential scanning calorimeter. Dynamic scans were conducted in a temperature range of 30-250 ℃, at constant heating rate of 10 ℃·min-1, under nitrogen atmosphere at a flux rate of 50 mL·min-1 . For sample preparation, the sample was placed in an oven at(40 ± 2)℃ until a constant weight was obtained. About 6 mg of the resin was used in an aluminum crucible of 40 μL with a perforated lid.
2 Results and discussion 2.1 Physical properties analysisAll of the Chinese fir-based adhesives had the dark color with the specific odor. The resin solid content, free formaldehyde content and free phenol content were measured and the results are presented in Tab. 2.
|
|
Resin solid content is an important property for phenolic resin. Low solid content adhesive will eject out more water during the hot pressing, which could reduce the bonding strength of the plywood. As expected, the control PF resin adhesives have higher solid content than the Chinese fir-based adhesives at the same F /P molar ratio. The solid content of Chinese fir-based adhesives ranges from 40. 78% to 48. 71%, which exceeded the minimum requirements of 35% in phenolic resin adhesive(Jin et al., 2010). With increasing the CF /PF mass ratio and F /P molar ratio, a decreasing solid content in Chinese fir-based adhesives was observed.
Free formaldehyde and free phenol are the archcriminal of toxicity in phenols or aldehydes synthetic resin. In general, low toxic PF resin follows with poor bond strength, while great bonding strength is often accompanied with high toxicity. Results in Tab. 2 showed that the free formaldehyde and free phenol of the synthetic Chinese fir-based adhesives would decrease along with the increasing addition of Chinese fir liquid. As the CF /PF rising from 50 /50 to 60 /40, the free formaldehyde content and free phenol content of the Chinese fir-based adhesives(F /P = 2: 1)decreased from 0. 276% to 0. 185% and 0. 081% to 0. 048%, respectively, which were far less than those of control PF resin. The free formaldehyde and free phenol were similar to those of the Chinese fir-based adhesives with higher F /P molar ratios(El Barbary Hassan et al.). As known, the alkaline liquefaction of Chinese fir breaks down lignin, cellulose and hemicellulose. The lignin was further hydrolyzed to three main structural units of benzyl propane: Guaiacyl(G), Hydroxyl Phenyl(H) and Syringyl(S)(Yang et al., 2012). G-type units have a free C5 position(ortho to the phenolic hydroxyl)in the ring, susceptible of reacting with formaldehyde, while in S-type units both C3 and C5 positions are linked to a methoxy group, resulting in low reactivity with formaldehyde. From this point of view, lignin with G groups as the principal structural units must be a priori more suitable for PF formulations, and the H groups take the second place. The units of H and G react with formaldehyde in alkaline medium producing H-hydroxymethyl and G-hydroxymethyl. Then the H-hydroxymethyl and Ghydroxymethyl happen dehydrolytic condensation and form the cross-linked structure. When exposed at high temperature(200-220 ℃), cellulose and hemicellulose were firstly degraded through hydrolysis path to oligosaccharide and then converted in alkaline pathway to glucose(Yin et al., 2011). Through diverse elimination reactions, glucose transformed to 5-hydroxymethyl furfural(5-HMF). Due to the similar chemical property to formaldehyde, the phenol was consumed by the condensation reaction of the 5-HMF, which occurred at three reactive sites. Hence, the use of an optimum amount of formaldehyde and phenol can form and improve the chance of Chinese fir liquid’s incorporation into the PF resin structures. As discussed above, the adhesive made from CF /PF = 60 /40 with F /P = 2 : 1 got the best physical properties.
2.2 Bonding properties analysisIn order to enhance the bonding strength and boiling water resistance, formaldehyde and phenol were used to co-polymerize with alkaline Chinese fir liquid. The boiling bonding strength and wood failure were evaluated for plywood panels bonded with various Chinese fir-based adhesives and control PF resin adhesives. The test results are presented in Tab. 3. To verify the boiling water resistance and durability, the test specimens were measured on more rigor conditions after the plywood specimens had been subjected to a 54-h, 76-h and 100-h cycle. The results showed that all the 28-h boiling-drying-boiling tests for panels prepared with Chinese fir-based adhesives with F /P = 2 exceeded 1. 1 MPa without evaluating the wood failure. This means that the Chinese fir-based adhesives formulation with F /P = 2 outdistanced the requirement set by JIS K6806—2004 for application in exterior use. The results in the 52-h boiling cycle test were also outst and ing because the values were greater than 1. 0MPa as before. Moreover, the 76-h boiling tests for panels prepared with the formulation of 50 /50-2 : 1 and the 55 /45-2 : 1 were(1. 23 ± 0. 05)MPa and (1. 02 ± 0. 03)MPa, which still met the JIS K6806— 2004 st and ard 0. 98MPa. In the 100-h boiling cycle procedure, nothing but the evaluated value of the Adhesive 50 /50-2 : 1 exceeded the minimum requirement of JIS K6806—2004 st and ard. All the results above indicated that the Chinese fir-based adhesive had wonderful water resistance and durability, especially the Adhesive 50 /50-2: 1.
|
|
The boiling bonding strength and wood failure of Adhesive 50 /50-2 : 1 for the 52-h cycle test, 76-h cycle test, and 100-h cycle test were(1. 35 ± 0. 03)MPa(70%), (1. 23 ± 0. 05)MPa(60%), and (1. 08 ± 0. 03)MPa(50%)apparently, while the results for the control PF were(1. 28 ± 0. 05)MPa(65%), (1. 12 ± 0. 05)MPa(50%), and (0. 91 ± 0. 05)MPa(40%)under the same test conditions. It indicated that the Adhesive 50 /50-2 : 1 had better boiling water resistance and durability than the control PF resin. And it is important that the Chinese fir liquid is not used as filler but raw material reacted with formaldehyde and phenol.
2.3 Formaldehyde emissionFormaldehyde emission of specimens was measured by 24-h desiccator method. Fig. 1 shows the formaldehyde emission results for the various specimens. Each specimen was tested twice and good repeatability of results was obtained with a maximum relative st and ard deviation of less than 2% . The formaldehyde emission of all the panels approached to the value of E0 specified in the JIS A1460—2003 st and ard(the value is less than 0. 5 mg·L-1)except the panel bonded with the control PF resin with the F / P ratio of 3 : 1. The quantity of formaldehyde emission of plywood bonded with Chinese fir-based adhesives decreased with the increasing CF /PF ratio as shown in Fig. 1. It can be seen that the sample in the group of F /P = 3 : 1, with a CF /PF ratio of 60 /40, achieving the lowest formaldehyde emission value of 0. 102 mg· L-1, which was only 1 /5 of the E0 specified value. Panels bonded with the Chinese fir-based adhesives emitted less formaldehyde than panels bonded with the control PF resin adhesive by 50%-80% . This may be due to the depolymerization products of lignin maintaining their aromatic character and high reactivity, which act as radical scavengers and can therefore readily react with free formaldehyde in the mixture, during crosslinking process(Kunaver et al., 2010). It is significant that formaldehyde emission reduction appears to a large extent, while sizable amounts of free formaldehyde present in the synthesized resins can be ignored before use in plywood manufacturing.
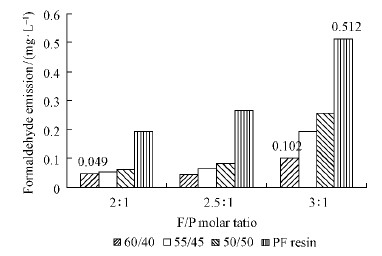 |
Fig. 1 Formaldehyde emission of plywood
|
As shown in Fig. 2, the Chinese fir-based adhesive showed a similar FT-IR absorbance to that of the control PF resin adhesive. The b and at 3 420 cm-1 in Chinese fir-based adhesive and the b and at 3 416 cm-1 in control PF resin are assigned to aromatic and aliphatic OH groups while the b and s at 2 948, 2 850 and 1 460 cm-1 in Chinese fir-based adhesive and the b and s at 2 946, 2 845 and 1 458 cm-1 in control PF resin are related to the C—H vibration of CH2 and CH3 groups. They are typical vibrations of methoxyl groups. However, with the incorporation of wood components, the FT-IR spectra of the Chinese fir-based adhesive contained some different b and s compared with that of the control PF resin. The major difference in the spectra between the Chinese fir-based adhesive and control resin adhesive is the absorbance in the carbonyl region. As shown in Fig. 3 the peaks at 1 733, 1 698, and 1 652 cm-1 in Chinese fir-based adhesive are ascribed to the ester carbonyl stretch, aryl ketone or aldehyde carbonyl stretch, and the di-substituted alkene C=CH2, respectively. However, the control PF resin showed no absorbance in this region as expected. Furthermore, the spectra of the Chinese firbased adhesive also showed two weak b and s at 1 473 and 879 cm-1 caused by tetra substituted(1, 2, 4, and 6)ring which did not occur in the spectra of control resin. It implies that the existence of some lignin fragments, most of which are tetra substituted aromatic rings in the Chinese fir-based adhesive. The other difference in the spectra between the Chinese firbased adhesive and control resin occurred at 1 077 cm-1(shown in Fig. 4), where the b and attributed to the ether linkage on the furan ring and the b and at 1 048 cm-1 attributed to the single bond C—O—C stretch with —CH2OH vibrations. Based on the above discussion, the b and s at 1 077, 1 048 cm-1 are associated with the 5-HMF that reacted with phenol during the polycondensation reaction.
 |
Fig. 2 FT-IR spectra of adhesive 50 /50-2: 1 and adhesive 2: 1
|
 |
Fig. 3 FT-IR spectra of adhesive 50 /50-2: 1 and adhesive 2: 1
|
 |
Fig. 4 FT-IR spectra of adhesive 50 /50-2: 1 and adhesive 2: 1
|
The isothermal DSC curves at a heating rate of 10 ℃·min-1 of Chinese fir-based adhesives and control PF resin adhesives are shown in Fig. 5 and Fig. 6. The results obtained are summarized in Tab. 4 containing onset temperature, peak temperature and ΔH.
|
|
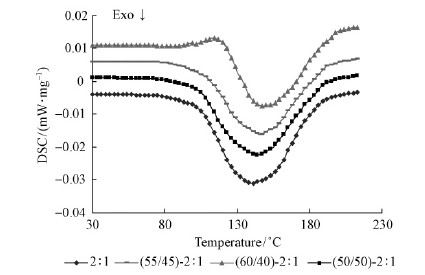 |
Fig. 5 DSC curves of adhesive at F /P = 2
|
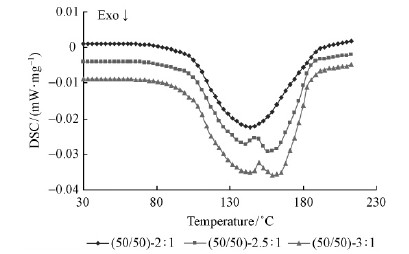 |
Fig. 6 DSC curves of Chinese fir-based adhesive with CF /PF = 50 /50
|
Fig. 5 and Fig. 6 show that the resin samples gave a single or two exothermic peaks in the range of 141. 4-157. 5 ℃. Both of the exotherms obtained in the resin systems were attributable to the curing reaction. According to the previous researchs(Perez et al., 2011)the lower exothermic peak in the range of 141. 4-144. 8 ℃ has been attributed to the addition reaction of free formaldehyde to phenolic ring, and the upper exothermic peak in the range of 143. 8-157. 5 ℃ was associated with the chain-building condensation reactions, involving hydroxymethyl groups attached to various phenolic species. The appearance of single exothermic peak is mainly due to the F /P molar ratio below 2. 3 that resulted in the overlapping of exothermic signals, two well-separated exothermic peaks are revealed when the F /P molar ratio was larger than 2. 3(Holopainen et al., 1997).
Fig. 5 hows the DSC curves of the Chinese firbased adhesives(F /P = 2)with a CF /PF ratio of 50 / 50, 55 /45 and 60 /40, respectively. As the data listed in Tab. 4, the extrapolated onset temperatures for the Chinese fir-based adhesives at F /P = 2 were found to be 114. 6, 115. 6 and 116. 4 ℃, respectively. It was close to the control PF resin adhesive of the same molar ratio, but slightly higher than the Chinese fir-based adhesive with larger F /P molar ratio. This indicated that with larger F /P molar ratio Chinese fir-based adhesives were more reactive at the low temperatures. As far as the effect of CF-to-PF ratio is concerned, it can be seen from Fig. 5 that the onset temperature and peak temperature of the Chinese fir-based adhesives shifted to higher temperatures with an increase of CF / PF mass ratio. However, the ΔH of cure were significantly reduced. Compared with the control PF resin at the same molar ratio, the Chinese fir-based adhesives had higher peak temperature and lower ΔH. This is probably because lignin has less free ring positions than phenol and more steric impediments what delays the hardening of Chinese fir-based adhesives. Furthermore, lignin introduces an extra amount of methylol groups similar to those produced by formaldehyde during curing, what will act in the opposite way.
Fig. 6 shows the DSC curves of the Chinese firbased adhesives(CF /PF = 50)with F /P molar ratios of 2: 1, 2. 5 : 1 and 3 : 1. Due to the F /P molar ratio lower than 2. 3, the DSC curve of the Chinese fir-based adhesive(F /P = 2: 1)reveals only a single exothermic at the peak temperature of 146. 1 ℃. The DSC curves of the Chinese fir-based adhesives(CF /PF = 50)with a F /P molar ratio of 2. 5: 1 and 3: 1 manifested that the curing behavior is completed in two steps which are signified by two exothermic curves. The peak temperature for the Chinese fir-based adhesive with a molar ratio of 2. 5 : 1 was 142. 1 ℃ and 155. 0 ℃, respectively. And they were 144. 8 ℃ and 157. 2 ℃ for the Chinese fir-based adhesive with a molar ratio of 3: 1. It implied that the Chinese fir-based resins with larger F /P molar ratio needed a higher temperature for completely curing. The ΔH in Tab. 4 suggested that the Chinese fir-based adhesive(CF /PF = 50 /50 and F /P = 3: 1)reacted more strongly than the other resins due to the larger ΔH released during curing.
3 ConclusionsThe alkaline Chinese fir liquid is proved a wonderful raw material for plywood adhesives. The process of liquefaction is easy to prepare which make it possible for industrial production. The synthetic Chinese fir-based adhesives exhibit excellent improvements for free formaldehyde content and free phenol content. More important, the properties of plywood glued with the the Chinese fir-based adhesives(CF /PF = 50 /50, F /P = 2 : 1)after 100-h boiling drying cycle still meet the requirement of the JIS K6806—2004 st and ard in exterior application and the formaldehyde emission is only 0. 074 mg·L-1 which is much less than that of the E0 specified in the JIS A1460—2003 st and ard(the value is less than 0. 5 mg·L-1). In addition, the cost of this adhesive appears much lower than that of the synthetic phenolic resins traditional used for wood adhesives. Hence, we can predicate that the Chinese fir-based adhesive will not only help reduce the polymer industry ’ s dependence on petro-chemical industry but also be applied for industrial production.
| [1] |
Alonso M V, Oliet M, Dominguez J C, et al. 2011. Thermal degradation of lignin-phenol-formaldehyde and phenol-formaldehyde resol resins. Journal of Thermal Analysis and calorimetry, 105(1): 349-356.( 1) 1)
|
| [2] |
Binder J B, Raines R T. 2009. Simple chemical transformation of lignocellulosic biomass into furans for fuels and chemicals. Journal of the American Chemical Society, 131(5): 1979-1985.( 1) 1)
|
| [3] |
Chen H,Zhang Y,Xie S. 2012. Selective liquefaction of wheat straw in phenol and its fractionation. Applied Biochemistry and Biotechnology, 167(2): 250-258.( 1) 1)
|
| [4] |
He Z K, Zhang Y P, Wei W J. 2012. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Building and Environment, 47:197-204.( 1) 1)
|
| [5] |
Holopainen T, Alvila L, Rainio J, et al. 1997. Phenol-formaldehyde resol resins studied by 13C NMR spectroscopy, gel permation chromatography and differential scanning calorimetry. Journal of Applied Polymer Science, 66(6): 1183-1193.( 1) 1)
|
| [6] |
Jahanshaei S, Tabarsa T, Asghari J. 2012. Eco-friendly tannin-phenol formaldehyde resin for producing wood composites. Pigment & Resin technology, 41(5): 296-301.( 1) 1)
|
| [7] |
Jin Y, Cheng X, Zheng Z. 2010. Preparation and characterization of phenol-formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresource Technology, 101(6): 2046-2048.( 1) 1)
|
| [8] |
Kunaver M, Medved S,Čuk N, et al. 2010. Application of liquefied wood as a new particle board adhesive system. Bioresource Technology, 101(4): 1361-1368.( 1) 1)
|
| [9] |
Lee W J, Yu C Y, Chang K C, et al. 2011. Spherical PF resin beads prepared from phenol-liquefied Bambusa dolichoclada with suspension polymerization. Holzforschung, 65(2): 163-169. ( 1) 1)
|
| [10] |
Mohamad Ibrahim M N, Zakaria N, Sipaut C S, et al. 2011. Chemical and thermal properties of lignins from oil palm biomass as a substitute for phenol in a phenolformaldehyde resin production. Carbohydrate polymers, 86(1): 112-119.( 1) 1)
|
| [11] |
Moubarik A, Charrier B, Allal A, et al.2010. Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive. European Journal of Wood and Wood Products, 68(2): 167-177. ( 1) 1)
|
| [12] |
Pan H, Zheng Z F, Hse C Y. 2012. Microwave-assisted liquefaction of wood with polyhydric alcohols and its application in preparation ofpolyurethane (PU) foams. European Journal of Wood and Wood Products, 70(4): 461-470.( 1) 1)
|
| [13] |
Papadopoulou E, Chrissafis K. 2011. Thermal study of phenol-formaldehyde resin modified with cashew nut shell liquid. Thermochimica Acta, 512(1/2): 105-109.( 1) 1)
|
| [14] |
Perez J M, Rodriguez F, Alonso M V, et al. 2011. Time-temperature-transformation cure diagrams of phenol-formaldehyde and lignin-phenol-formaldehyde novolac resins. Journal of Applied Polymer Science, 119(4): 2275-2282.( 1) 1)
|
| [15] |
Qing Y, Wu Y Q, Cai Z Y. 2012. Effect of freeze dry on the properties of cellulose nanofibrils/phenol formaldehyde nanocomposites. Biobase Material Science and Engineering, 217-221. ( 1) 1)
|
| [16] |
Schöene H. 2009. Direct, high-yield conversions of cellulose into biofuel and platform chemicals-on the way to a sustainable biobased economy. Chem Sus Chem, 2(2): 127-128.( 1) 1)
|
| [17] |
Wu C C, Lee W J. 2010. Synthesis and properties of copolymer epoxy resins prepared from copolymerization of bisphenol A, epichlorohydrin, and liquefied dendrocalamus latiflorus. Journal of Applied Polymer Science, 116(4): 2065-2073.( 1) 1)
|
| [18] |
Yang Q L, Shi J B, Lin L. 2012. Characterization of structural changes of lignin in the process of cooking of bagasse with solid alkali and active oxygen as a pretreatment for lignin conversion. Energy & Fuels, 26(11): 6999-7004.( 1) 1)
|
| [19] |
Yin S, Mehrotra A K, Tan Z. 2011. Alkaline hydrothermal conversion of cellulose to bio-oil: Influence of alkalinity on reaction pathway change. Bioresource Technology, 102(11): 6605-6610.( 1) 1)
|
| [20] |
Zhang H R, Pang H, Shi J Z, et al. 2012. Investigation of liquefied wood residues based on cellulose, hemicellulose, and lignin. Journal of Applied Polymer Ccience, 123(2): 850-856.( 1) 1)
|
 2014, Vol. 50
2014, Vol. 50

