文章信息
- 黄利斌, 陈庆生, 张敏, 钱猛, 窦全琴
- Huang Libin, Chen Qingsheng, Zhang Min, Qian Meng, Dou Quanqin
- 乌桕秋叶显色的生理生化与数字图像分析
- Analysis of Autumnal Leaf Colorization of Sapium sebiferum by Using Physiological and Digital Imaging Methods
- 林业科学, 2013, 49(11): 32-41
- Scientia Silvae Sinicae, 2013, 49(11): 32-41.
- DOI: 10.11707/j.1001-7488.20131105
-
文章历史
- 收稿日期:2013-04-15
- 修回日期:2013-08-15
-
作者相关文章
2. 南京农业大学生命科学学院生命科学实验中心 南京 210095
2. Life Science Laboratory Center School of Life Sciences, Nanjing Agricultural University Nanjing 210095
The appearance of leaf color was linked to differential expression of genes(Nothnagel et al.,2003),changes in tissue and cell structures(Matile,2000 ; Schaberg et al.,2008)and physiological conditions(Vitrac et al.,2000)in plant cells,as well as the effect of external environment(Chalker-Scott,1999). The prevailing view among plant physiologists is that foliage changes yellow and orange due to the breakdown of green chlorophyll molecules,which resulting in unmasking of other pigments,like carotenoids(Lee et al.,2002),and most red leaves result from de novo synthesis of anthocyanins(Field et al.,2001). Recent investigations have revealed that many factors,including UV-B radiation(Ranjbarfordoei et al.,2009),light stress(Merzlyak et al.,2000),abscisic acid(Hung et al.,2008),drought(Castellarin et al.,2007),senescence(Hoch et al.,2001)and ozone exposure(de Rezende et al.,2009)can influence biosynthesis of photosynthetic and non-photosynthetic pigments such as chlorophyll a,chlorophyll b,total chlorophyll,carotenoid and anthocyanins. The physiological significance of autumn leaf coloration through chlorophyll degradation and anthocyanins accumulation has been well documented(Field et al.,2001 ; Lee et al.,2002 ; Samuel et al.,2003). However,the linkage among foliar color,pigments contents and physiological conditions relating the biochemical and physiological basis of autumn coloration is not well understood(Schaberg et al.,2003). Furthermore,little is known concerning the differences in cellular ultrastructure and distribution of chloroplasts in leaves with different autumn hues.
Chinese tallow tree(Sapium sebiferum)belonging to Euphorbiaceae,is widely used for biomass-energy,timber and ornament. This tree species enriches in seed oil,grows rapid,and abounds in flowers. Chinese tallow trees show evidently autumnal color change,and the senescing leaves show various shades of yellow,orange,purple,or red. Sapium sebiferum cultivars with brightly colored leaves are valuable for ornament,which can enrich the landscape level as well as make up the urban color monotony.
In the present study,the differences in foliar pigments,nitrogen(N)and carbohydrate concentrations in various color leaves of Chinese tallow trees were investigated. Leaf color was quantified using digital imaging analysis. And the distribution and ultrastructure of chloroplasts were examined using transmission electron microscopy. Moreover,we took advantage of the unique autouorescence of chlorophyll,carotenoid and anthocyanins to study their distribution in leaf tissues by laser confocal microscopy. The purpose of this study was to find out the physiological and structural factors that influence autumn coloration of Sapium sebiferum. The results may provide basis for selective-breeding of Chinese tallow tree with better autumn colors.
1 Materials and methods 1.1 Plant materialsThe leaves were sampled from Sapium sebiferum with different color(green,yellow,orange,red and purple)autumn leaves growing in Yongxing nursery at Maoshan Town,Jurong County,Jiangsu,China. The leaves were harvested separately from 5 seedlings(3 -year old and 2 m high). All collections were made in the morning between 10:00-11:00 am on 13 November 2010.
1.2 Foliar color analysisLeaf color was measured by digital imaging analysis using Image J software,which was downloaded at http://imagej.nih.gov/ij/download.html. The leaves were scanned as a color photo at a resolution of 300 dpi with an Epson Perfection 1 260 color scanner and the images were saved in tag image file format(TIFF)(Fig. 1). Subsequently,the scanned color images were manipulated in Image J software. The full color images consist of red(R),green(G),and blue(B)channels. And then the mean values of the red,green and blue pixels for each leaf were calculated using the Color histogram tool.
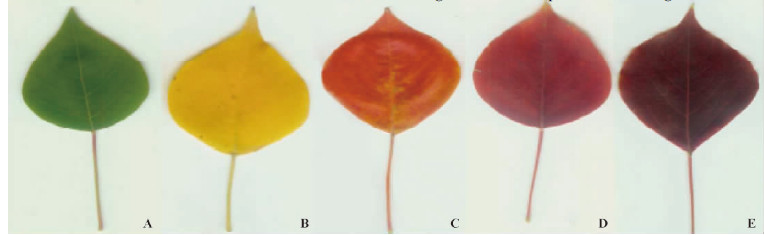 |
Fig. 1The scanogram of different color leaf of Sapium sebiferum
A. Green; B. Yellow; C. Orange; D. Red; E. Purple. |
Fresh leaf tissues were pulverized with liquid nitrogen and photosynthetic pigments were extracted with 80 % acetone for 24 h at-5 ℃. Pigments were determined spectrophotometrically with a Beckman DU 800 UV/VIS spectrophotometer. Contents of chlorophyll a and b and total carotenoid were determined according to Lichtenthaler(1987)formulae.
For the determination of anthocyanins,themethod of Pirie et al.(1976)was followed with minor modification. Briefly,the leaf samples were macerated initially in 10 mL of an aqueous methanol-HCl solvent(methanol:water:HCl,79:20:1,V/V,pH 3.5). Total anthocyanins were assayed by measuring absorbance at 530 nm using a Beckman DU 800 UV/VIS spectrophotometer and 10 -mm quartz cuvettes,and one absorbance unit was defined as the amount of anthocyanins giving an absorbance of 0.1 at 530 nm.
1.4 Nitrogen analysisThe fresh leaves were dried at 105 ℃ for 30 min to deactivate enzymes,and then the leaves were dried at 75 ℃ until attaining a constant weight. Total nitrogen(N)content in leaves was determined in accordance with the Kjeldahl method with a 25 mL aliquot of pure extract,followed by distillation and titration as described by Bremmer et al.(1982).
1.5 Carbohydrate determinationThe fresh leaves were oven dried at 70 ℃ until attaining a constant weight,and then the oven-dried leaves were powdered. Subsequently,0.15 g of the powder was mixed with 30 mL distilled water and stirred for 1 h. After then,50 mL distilled water was added to the mixture,mixed thoroughly and filtered. 20 mL filtrate was transferred into a 50 mL flask and 2.5 mL each of Carrez Ⅰ and Carrez Ⅱ was added and mixed,and 5 mL 0.1 mol·L-1 NaOH was added. After filtration,the filtrate was used for the measurement of glucose,fructose and sucrose with the Sucrose/D-Glucose/D-Fructose assay kit(Roche,Darmstadt,Germany).
For the measurement of starch,the Roche starch assay kit(Roche,Darmstadt,Germany)was used following the manufacturer’s instruction. In brief,0.15 g of the oven-dried leaf powder was added into 5 mL 40 % ethanol and centrifuged at 3 000 rpm for 10 min. After centrifugation,the supernatant was discarded,and the pellet was mixed with 5 mL of 40 % ethanol and centrifuged again under the same condition. The pellet was then washed with 8mL DMSO for 4 times(2 mL each time),and 2 mL 8 mol·L-1 HCl was added into the eluent and bathed at 60 ℃ for 30 min. After being cooled at room temperature for 30 min,10 mL distilled water was added and then the pH value of mixture was adjusted to 4-5 with 5 mol·L-1 NaOH. The final volume of the mixture was made up to 50 mL with distilled water. After filtration,the filtrate was used for the assessment of starch in the leaves.
1.6 Transmission electron microscopyFor transmission electron microscopy the leaves were cut into slices(2 mm × 3 mm)and fixed with 2.5 % glutaraldehyde,postfixed with 1 % OsO4,and then embedded in EP 812 resin. Ultrathin sections were cut on an ultramicrotome(LKB-V,Sweden)and stained with 2 % uranyl acetate followed by 6 % lead citrate. Chloroplast ultrastructure was observed with an H-600 transmission electron microscope(JEOL,Japan).
1.7 Laser confocal microscopyThe distribution of chlorophyll,anthocyanins and carotenoid in the leaf mesophyll cells were visualized in situ by their autofluorescence. Briefly,the fresh leaves were embedded in OCT medium(Sakura Finetek USA,Inc,Torrance,CA,USA)and 7 μm continuoussections were obtained with a Leica CM 1950 cryostat. And then the sections were mounted onto the poly-lysine coated slides. The 633 nm excitation ray line was used to image chlorophyll and the emitted uorescence was collected between 650 and 700 nm. For the detection of carotenoid,the 488 nm excitation ray line was used and the emitted uorescence was collected between 500 and 600 nm(Egea et al.,2011). The autouorescence of anthocyanins was excited using a helium-neon 543 nm laser(long pass 585 nm)(Gomez et al.,2011). And the sections were counterstained with DAPI(4’,6’-diamidino-2 -phenylindole). The samples were then examined by a Leica TCS SP5 confocal scanning microscope(Leica Microsystems,Heidelberg GmbH,Mannheim,Germany).
1.8 Statistical analysisStatistical analysis was carried out by one-way ANOVA using SPSS 13.0 software. And data presented were mean ± SEM of three experiments. When the ANOVA was significant at P<0.05,the Duncan multiple range test was used for mean comparison. Correlations between pigment contents and various functions using R,G and B values or foliar constituent concentrations were studied by multiple linear regression. In the analysis,the regression coefficients were showed in the equations.
2 Results 2.1 Foliar pigments contentsAll of the 5 individual trees of Sapium sebiferum changed color from green to yellow,orange,red or purple during senescence(Fig. 1). The contents of foliar pigments including chlorophyll,carotenoid and anthocyanins in different color leaves were shown in Tab. 1.
|
|
Chlorophyll a is the main green pigment,whichdirectly partakes in photosynthesis,while chlorophyll b and carotenoid are the accessory pigments. In the green leaves,chlorophyll a + b contents was 1.99 mg· g-1 FW,which was much higher than those in other colorized leaves. However,there was no significant difference in carotenoid contents among the five individual trees. Furthermore,the anthocyanins concentration was determined in our studies to gain better understand of the coloration mechanism of autumn leaves of Sapium sebiferum. It showed that the content of anthocyanins was significantly different in the five individual trees(P<0.05). The highest concentration of anthocyanins was 41.95 units·g-1 FW in the purple leaves,and the orange and red leaves were intermediate(18.05 and 20.95 units·g-1FW,respectively). The anthocyanins contents in the green and yellow leaves were significantly lower than those in purple,orange and red leaves,especially in yellow leaves,the concentration of anthocyanins was reduced by 91.23 % as compared with purple leaves.
2.2 Foliar nitrogen contentThe content of nitrogen in different colored leaves was presented in Tab. 1. It showed significant variations in leaf nitrogen content in the five individual trees. The total nitrogen content in green leaves was the highest,which was 2.88 -fold higher than that in the yellow leaves,and about twice higher than that in the orange leaves. In contrast,there were no significant differences in nitrogen concentrations between the red and purple leaves.
2.3 Foliar carbohydrates concentrationsTo find out whether the changes of carbohydrates influenced the development of autumn coloration in leaves of Sapium sebiferum during senescence,the contents of glucose,fructose,sucrose and starch were determined in different color leaves. It indicated that the concentrations of glucose were the highest in the green leaves and lowest in the purple leaves and there was significant difference between them(Tab. 1). Moreover,the fructose content in the yellow leaves was 1.72 mg·g-1 DW,which was significantly lower than those in other color leaves(P<0.05). Additionally,higher accumulation of sucrose in the purple leaves was observed as compared with other color leaves. Starch,another important carbohydrate,wasalso disparate in different color leaves. The concentration of starch was the highest in the green leaves,which was 15.45 -fold,7.73 -fold,12.14 -fold and 10.00 -fold higher than that in yellow,orange,red and purple leaves,respectively. However,there were no significant differences in starch contents among the yellow,orange,red and purple leaves. As for the total non-structural carbohydrate(TNC),the highest concentration was found in the green leaves,while the lowest content was present in the yellow leaves.
2.4 Foliar color analysis by R,G and B valuesLeaf color was quantified by digital imaging analysis and the R(red),G(green)and B(blue)values were presented in Tab. 2. The linear regression analysis was conducted to investigate the relationship between these data and various foliar pigments contents. Correlations between various functions derived from the R,G and B values and the parameters of foliar pigments including Chl a,Chl b,Chl a/b,Chl a + b,carotenoid and anthocyanins were examined(Tab. 3). It revealed that there was a significant negative relationship between R /(R + G + B)and chl b content with a correlation coefficient of-0.96. It suggests that R /(R + G + B),the red value normalized by the sum of the three channels,may be used as a good indicator to predict chlorophyll b content in various color leaves of Sapium sebiferum. In contrast,a significant positive relationship was found between R /(G-B)and anthocyanins concentration in the leaves(r= 0.92). Moreover,a strong positive relationship was observed between(G-B)/(R-B)and chl a content,G /(R-B)and chl b content,G /(R-B)and Chl a + b with the correlation coefficients of 0.90,0.95 and 0.90,respectively. However,a significant negative relationship was present in R /(G + B)and chl b,R /(R + G + B)and Chl a + b,and the correlation coefficients were-0.95 and-0.90,respectively.
|
|
|
|
To gain better understanding of the relationship between leaf constituents and foliar pigments,nitrogen and carbohydrates concentrations were analyzed against Chl a,Chl b,Chl a + b,carotenoid and anthocyanins. As shown in Tab. 4,the results of correlation analysis revealed that nitrogen and starch concentrations showed significant positive correlation with Chl a,Chl b,Chl a + b and carotenoid(r>0.8). There was a less strong correlation between fructose concentration and carotenoid,and sucrose and anthocyanins. The correlation coefficients were 0.747 and 0.77,respectively. We also observed a significant positive correlation between the TNC and carotenoid(r= 0.93). In contrast,weak negative correlation was found between glucose concentration and anthocyanins(r=-0.48),and starch concentration and anthocyanins(r=-0.42).
|
|
The ultrastructure of mesophyll cells of different color leaves,especially the differences in chloroplasts,was examined by transmission electron microscopy(Fig. 2). The architecture of green leaf mesophyll cells appeared to be intact with numerous chloroplasts and other organelles such as mitochondria and vacuoles(Fig. 2 A,B). Chloroplasts showed the typical architecture with thylakoid membranes arranged in granum and stroma lamellae(Fig. 2 C),and large starch grains and several osmiophilic granules were visible within chloroplasts(Fig. 2 B). In contrast,almost no organelles could be observed in mesophyll cells of yellow leaves,and some degrading chloroplasts were found occasionally(Fig. 2 D). The chloroplasts in orange and red leaves were found to be abnormal,and the microscopic data could not reveal obvious ultrastructural differences between them(Fig. 2 E,F). The irregular or degraded chloroplasts were distributed at the edge of the inside cell wall,and many of them contained several osmiophilic granules. In the palisade tissue cells of purple leaves,the number of chloroplasts were reduced,whose membranes disintegrated,and abundant fragments were found within the chloroplasts(Fig. 2 G). Concurrently,considerable larger osmiophilie globules,which might represent reserve materials,were found in chloroplast and cytoplasm(Fig. 2 G). Rare chloroplasts could be found in the spongy tissue cells,but enormous starch grains were present in the cytoplasm(Fig. 2 H).
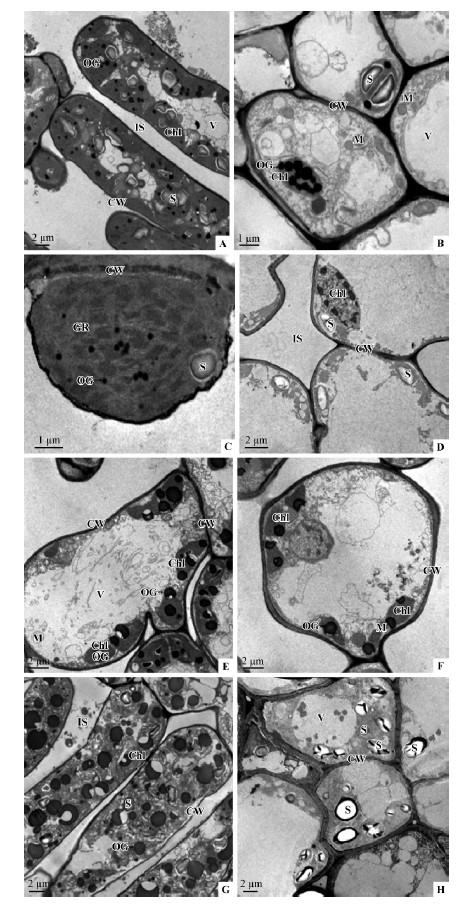 |
Fig. 2 Transmission electron micrographs of Sapium sebiferum leaf mesophyll cells showing chloroplast structure and arrangement
A.The cell of palisade tissue in green leaves; B.The spongy tissue cells of green leaves; C.Magnification of chloroplast; D.The ultrastructure of mesophyll cells of yellow leaves; E.The deformation or degradation of chloroplasts in the orange leaf cells; F.The cellular structures in red leaves; G.The cells of palisade tissue in purple leaves; H.The spongy tissue cells of the purple leaves. Chl. Chloroplasts; CW.Cell wall; GR.Thylakoid grana; M.Mitochondria; OG.Osmiophilic granules; S.Starch grain; V.Vacuole; IS. Intercellular space. |
In order to determine the subcellular localization of chlorophyll,carotenoid and anthocyanins,leaf tissues were examined using confocal microscopy. As seen in Plate Ⅱ,mesophyll chloroplasts showed redautofluorescence. Confocal microscopy observations revealed that in the green leaves both the palisade tissue and spongy parenchyma contained large numbers of chloroplasts exhibiting strong chlorophyll fluorescence(Plate Ⅰ A). In contrast,the intensity of the chlorophyll fluorescence was decreased in the yellow(Plate Ⅰ B),orange(Plate Ⅰ C),red(Plate Ⅰ D)and purple(Plate Ⅰ E)leaves. Especially in the yellow leaves,only very few chloroplasts were found in the spongy parenchyma. Moreover,in the orange leaves the palisade tissue contained more chloroplasts than that in the spongy parenchyma(Plate Ⅰ C). However,in the red and purpleleaves,the chloroplasts were mainly localized in the spongy parenchyma(Plate Ⅰ D). In contrast to chlorophyll,carotenoid was found to be distributed both in the palisade tissue and spongy parenchyma in the five different colored leaves(Plate Ⅱ). Furthermore,the fluorescence intensity in different colored leaves was similar.
 |
Plate Ⅱ Red, carotenoid fluorescence signal; blue, DAPI. A. Green leaf; B. Yellow leaf; C. Orange leaf; D. Red leaf;E. Purple leaf. Bars = 50 μm. |
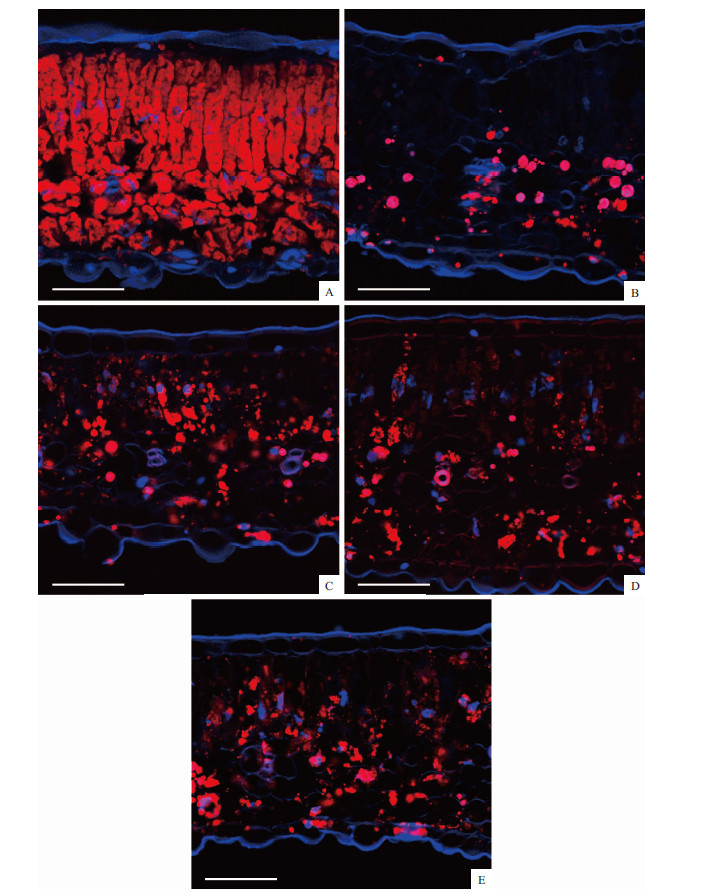 |
Plate Ⅰ Red, chlorophy Ⅱ fluorescence signal; blue, DAPI. A. Green leaf; B. Yellow leaf; C. Orange leaf; D. Red leaf;E. Purple leaf. Bars = 50 μm. |
Using the autofluorescence of anthocyanins,we observed numerous spherical vesicles in leaf mesophyll cells,especially in the red and purple leaves(Plate Ⅲ). In the green leaves,only faint redfluorescence was found in the palisade tissue,whereas,some intensely red colored structures filled with anthocyanins were present in the spongy parenchyma(Plate Ⅲ A).In the yellow and orange leaves,only a small number of vesicles were noticed in the spongy parenchyma(Plate Ⅲ B,C). These colored vesicles can be classified into two groups according to the size as the previous report,which are Group Ⅰ(G Ⅰ)corresponds to small particles,and Group Ⅱ(G Ⅱ)corresponds to larger spherical vesicles. Unlike the yellow and orange leaves,a great deal of vesicles was observed both in the palisade tissue and spongy parenchyma in red and purple leaves(Plate Ⅲ D-G). The GI structure was found in the palisade tissue,while the G Ⅱ structure was localized in the spongy parenchyma.
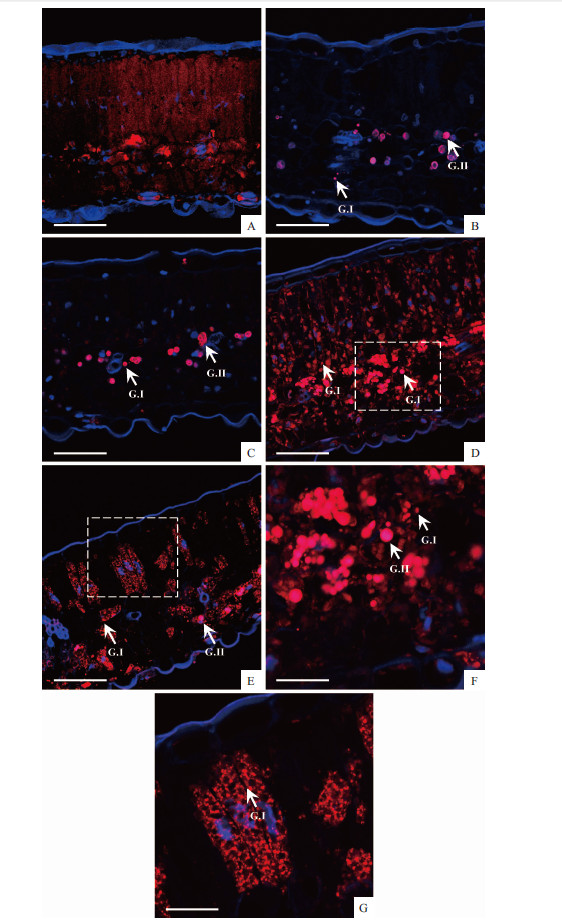 |
Plate Ⅲ Red autofluorescence was generated b anthoc anins and DNA is stained in blue with DAPI. A. Green leaf; B. Yellow leaf; c. Orange leaf; D. Red leaf; E. Purple leaf. F and G. Showed magnified images corresponding to the boxed regions in D and E, respectivel . G.Ⅰcorresponds to small particles and G.Ⅱ corresponds to larger spherical vesicles. Bars = 50 μm (A-E); 25 μm (F, G). |
Pigments are the foundation of the variation of leaf color. Autumn coloration was directly related to the categories and amounts of pigments. Chlorophylls are the most important pigments found in chloroplasts,and chlorophyll contents were dramatically higher in green leaves than other color leaves(Tab. 1). Carotenoid,as the “accessory”pigments of chlorophylls,was more stable during senescence,and its contents were similar in various leaves in our study. With declining autumn temperatures,the leaves of Sapium sebiferum stop producing chlorophyll,and in the yellow leaves the chlorophyll breakdown unmasked the yellow carotenoid pigments resulting in clear-yellow colored leaves(Field et al.,2001). Unlike the yellow leaves,most red leaves resulted from abundant synthesis of anthocyanins. Anthocyanins contents varied in different color leaves. There may be an admixture of decomposed chlorophyll,yellow carotenoid and red anthocyanins pigments to give the leaves various shades of orange,red or purple. In addition,correlation analyses showed a significant negative correlation between chlorophyll a + b contents and R /(R + G + B)(Tab. 3),and there was a markedly positive correlation between anthocyanins contents and R /(G-B). These algorithms may be used as an indicator to estimate the leaf color by digital imaging analysis,which could provide quantitative expression of color and be consistent with human color sensation(Laliberte et al.,2007).
In this study,N level was the highest in the green leaves,and there was a negative correlation between Chl a + b and N concentrations(r= 0.90). Much of the N in the leaves was translocated back to the spurs in the early autumn before leaf falling(Stephen et al.,2007),but after leaves turned color,the nitrogen concentration of the red and purple leaves was significantly higher than those of the yellow leaves,which indicated that red leaves may have a greater capacity for nutrient resorption than senescing yellow leaves during autumn(Schaberg et al.,2003).
Analyses of leaf carbohydrates concentrations in our study showed that: 1)the yellow leaves fixed a little amount of TNC than other color leaves; 2)a higher accumulation of sucrose was found in the purple leaves,and the correlation analysis indicated that sucrose contributed to the biosynthesis of anthocyanins; 3)there was a significant positive correlation between chl a and starch(r= 0.97). As is known,the anthocyanins pigments are related to the carbohydrates and carbohydrate accumulation favors their formation(Stephen et al.,2007). The contents of stored carbohydrates varied in different color leaves and changed seasonally as well(Hughes et al.,2005). It was suggested that trees of the same species growing together showed much difference in color might be due to variations in the amounts of soluble carbohydrates among individual trees(Stephen et al.,2007). Pigment contents,total N and TNC concentrations in yellow leaves were lower than other color leaves. The pigment contents were strongly correlated with photosynthetic capacity,thus the lower chlorophyll and anthocyanins contents in the yellow leaves may reflect its lower nitrogen and TNC concentrations(Kytridis et al.,2008). Among the soluble carbohydrates,sucrose is the primary transportable and storage carbohydrate. We assumed that the synthesis of carbohydrates or the conversion of insoluble to soluble carbohydrates,especially sucrose might favor anthocyanins formation and bright autumn colors in Sapium sebiferum. Starch is considered the most important reserve carbohydrate and has often been used as the sole indicator of the carbohydrate status of plants. Starch would convert to sugars when sugar contents were low or at low temperature(Kozlowski,1992),which could explain the correlativity of starch and chlorophyll.
Electron microscopic studies of different color autumn leaves of Chinese tallow tree revealed various pictures showing the differences in quantity,shape and structure of chloroplasts,as well as some contents such as starch grains and osmiophilic granules. Senescence of Chinese tallow tree in autumn progressed the chloroplast disaggregated seriously,and the drastic decrease in the number of chloroplasts led to reduction in chlorophyll concentration. Swelling of chloroplast,membrane reduction and accumulation of osmiophilic granules in orange,red and purple leaves were phenomena that were also observed in other plant leaves under many other stresses,such as ultraviolet-B(Peng et al.,2009),cadmium pollution(Baryla et al.,2001),freezing(Bourett et al.,1999)and low temperature(Holzingerl et al.,2007). Furthermore,we found starch grains were abundant in the green and purple leaves,but the cells in the yellow,orange or red leaves contained only negligible amounts of starch grains. The synthesis of assimilation starch ceased may in favor of the formation of various sugars(Sensor et al.,1975).
Consistent with the TEM results,the amount of chloroplasts in yellow,orange,red and purple leaves detected by laser confocal scanning microscopy was apparently less than green leaves. Especially in the yellow and orange leaves it displayed very feeble fluorescence of chlorophyll,which may indicate decreased photosynthetic capacity in autumn. Moreover,the laser confocal microscopic observation revealed an asymmetric distribution of chlorophyll in the palisade tissue and spongy parenchyma in different color leaves. These results suggested chlorophylls have a specific cellular or subcellular location in different individual trees( ,2009). In contrast,the fluorescence intensity and subcellular location of carotenoid was similar in different color leaves. The confocal microscopy analysis of leaf tissues showed different patterns of anthocyanins compartmentation. In consistent with the observations of Gomez et al.(2011),we found that anthocyanins were present in two types of structures,differing mainly by the size. The authors suggested that GⅠ and GⅡ vesicles were membrane surrounded structures,and the larger G Ⅱ vesicles were formed by the fusion of the small size GI structures.
,2009). In contrast,the fluorescence intensity and subcellular location of carotenoid was similar in different color leaves. The confocal microscopy analysis of leaf tissues showed different patterns of anthocyanins compartmentation. In consistent with the observations of Gomez et al.(2011),we found that anthocyanins were present in two types of structures,differing mainly by the size. The authors suggested that GⅠ and GⅡ vesicles were membrane surrounded structures,and the larger G Ⅱ vesicles were formed by the fusion of the small size GI structures.
In summary,in the present study we utilized a quantitative method,digital color analysis,to explore autumnal leaf colorization of the woody plant S. sebiferum. Based on the RGB image analysis,foliar pigments of different color leaves can be quantitatively determined by measuring image(G-B)/(R-B),R /(R + G + B)or R /(G-B)value. Digital color analysis provides a simple and fast tool to estimate leaf colorization in comparison to traditional visual scale method,and the former is more accurate than the latter. Moreover,the physiological analysis also provides some clues for autumnal leaf colorization of S. sebiferum. However,autumn coloration may represent an integration of genetic potential,structural support and environmental stresses that influence leaf senescence and anthocyanins biosynthesis. Further study regarding the molecular mechanisms regulating the induction and progression of autumn leaf coloration as well as the biochemical and cell biological details involved in the pathways of pigments catabolism should be conducted. This could be useful to the selective-breeding of improved cultivars with better ornamental value.
| [1] |
Baryla A, Carrier P, Franck F, et al. 2001.Leaf chlorosis in oilseed rape plants (Brassica napus ) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth.Planta,212(5/6) : 696-709.( 1) 1)
|
| [2] |
Bourett T M, Czymmek K J, Howard R J. 1999. Ultrastructure of chloroplast protuberances in rice leaves preserved by high-pressure freezing. Planta, 208 (4): 472-479.( 1) 1)
|
| [3] |
Bremmer J M, Mulvaney C S. 1982. Nitrogen-total//Page A L, Miller R H, Keeney D R. Methods of Soil Analysis: Part 2. Madison, WI: American Society of Agronomy Soil Science Society of America, 595-624.( 1) 1)
|
| [4] |
Castellarin S D, Pfeiffer A, Sivilotti R, et al. 2007. Transcriptional regulation of anthocyanins biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant, Cell and Environment, 30 (11): 1381-1399.( 1) 1)
|
| [5] |
Chalker-Scott L. 1999. Environmental significance of anthocyanins in plant stress responses. Photochemistry and Photobiology, 70 (1): 1-9.( 1) 1)
|
| [6] |
de Rezende F M, Furlan C M. 2009. Anthocyanins and tannins in ozone-fumigated guava trees. Chemosphere, 76 (10): 1445-1450.( 1) 1)
|
| [7] |
Egea I, Bian W P, Barsan C, et al. 2011. Chloroplast to chromoplast transition in tomato fruit: spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Annals of Botany, 108 (2): 291-297.( 1) 1)
|
| [8] |
Field T S, Lee D W, Holbrook N M. 2001. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of redosier dogwood. Plant Physiology, 127 (2): 566-574.( 3) 3)
|
| [9] |
Gomez C, Conejero G, Torregrosa L, et al. 2011. In vivo grapevine anthocyanin transport involves vesicle-mediated trafcking and the contribution of anthoMATE transporters and GST. Plant Journal, 67 (6): 960-970.( 2) 2)
|
| [10] |
Hoch W A, Zeldin E L, McCown B H. 2001. Physiological signification of anthocyanins during autumnal leaf senescence. Tree Physiology, 21 (1): 1-8.( 1) 1)
|
| [11] |
Holzingerl A, Buchner O, Lütz C, et al. 2007. Temperature-sensitive formation of chloroplast protrusions and stromules in mesophyll cells of Arabidopsis thaliana. Protoplasma, 230 (1/2): 23-30.( 1) 1)
|
| [12] |
Hughes N M, Neufeld H S, Burkey K O. 2005. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytologist, 168 (3): 575-587.( 1) 1)
|
| [13] |
Hung K T, Cheng D G, Hsu Y T, et al. 2008. Abscisic acid-induced hydrogen peroxide is required for anthocyanin accumulation in leaves of rice seedlings. Journal of Plant Physiology, 165 (12): 1280-1287.( 1) 1)
|
| [14] |
Kozlowski T T. 1992. Carbohydrate sources and sinks in woody plants. The Botanical Review, 58 (2): 107-222.( 1) 1)
|
| [15] |
Kytridis V P, Karageorgou P, Levizou E, et al. 2008. Intra-species variation in transient accumulation of leaf anthocyanins in Cistus creticus during winter: Evidence that anthocyanins may compensate for an inherent photosynthetic and photoprotective inferiority of the red-leaf phenotype. Journal of Plant Physiology, 165 (9): 952-959.( 1) 1)
|
| [16] |
Laliberte A S, Rango A, Herrick J E, et al. 2007. An object-based image analysis approach for determining fractional cover of senescent and green vegetation with digital plot photography. Journal of Arid Environments, 69 (1): 1-14.( 1) 1)
|
| [17] |
Lee D W, Gould K S. 2002. Why leaves turn red. Pigments called anthocyanins probably protect leaves from light damage by direct shielding and by scavenging free radicals. American Scientist, 90 (6): 524-531.( 2) 2)
|
| [18] |
Lichtenthaler H K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology, 148: 350-382.( 1) 1)
|
| [19] |
Matile P. 2000. Biochemistry of Indian summer: physiology of autumnal leaf coloration. Experimental Gerontology, 35 (2): 145-158.( 1) 1)
|
| [20] |
Merzlyak M N, Chivkunova O B. 2000. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. Journal of Photochemistry and Photobiology B: Biology, 55(2/3): 155-163.( 1) 1)
|
| [21] |
 E. 2009. Survey of plant pigments: Molecular and environmental determinants of plant colors. Acta Biologica Cracoviensia Series Botanica, 51(1): 7-16.( E. 2009. Survey of plant pigments: Molecular and environmental determinants of plant colors. Acta Biologica Cracoviensia Series Botanica, 51(1): 7-16.( 1) 1)
|
| [22] |
Nothnagel T, Straka P. 2003. Inheritance and mapping of a yellow leaf mutant of carrot (Daucus carota). Plant Breeding, 122 (4): 339-342.( 1) 1)
|
| [23] |
Peng Q, Zhou Q. 2009. Influence of lanthanum on chloroplast ultrastructure of soybean leaves under ultraviolet-b stress. Journal of Rare Earths, 27 (2): 304-307.( 1) 1)
|
| [24] |
Pirie A, Mullins M G. 1976. Changes in anthocyanin and phenolics content of grapevine leaf and fruit tissues treated with sucrose, nitrate abscisic acid. Plant Physiology, 58 (4): 468-472.( 1) 1)
|
| [25] |
Ranjbarfordoei A, Van Damme P, Samson R. 2009. Elevated ultraviolet-B radiation influences photosynthetic pigments and soluble carbohydrates of sweet almond [Prunus dulcis (Miller) D. Webb]. Electronic Journal of Environmental, Agricultural and Food Chemistry, 8 (11): 1077-1084.( 1) 1)
|
| [26] |
Samuel O, Gould S. 2003. Anthocyanins in leaves: light attenuators or antioxidants? Functional Plant Biology, 30 (8): 865-873.( 1) 1)
|
| [27] |
Schaberg P G, Murakami P F, Turner M R, et al. 2008. Associations between the red coloration and senescence of sugar maple leaves in autumn. Trees, 22 (4): 573-578.( 1) 1)
|
| [28] |
Schaberg P G, Van Den Berg A K, Murakami P F, et al. 2003. Factors influencing red expression in autumn foliage of sugar maple trees. Tree Physiology, 23 (5): 325-333.( 2) 2)
|
| [29] |
Senser M, Schötz F, Beck E. 1975. Seasonal changes in structure and function of spruce chloroplasts. Planta, 126 (1): 1-10.( 1) 1)
|
| [30] |
Stephen D R, Pallardy G. 2007. Physiology of woody plants. 3rd ed. Burlington, USA:Academic Press. ( 3) 3)
|
| [31] |
Vitrac X, Larronde F, Krisa S, et al. 2000. Sugar sensing and Ca2+ calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry, 53 (6): 659-665.( 1) 1)
|
 2013, Vol. 49
2013, Vol. 49

