文章信息
- 林树燕, 丁雨龙
- Lin Shuyan, Ding Yulong
- 异叶苦竹大小孢子及雌雄配子体的发育
- Observations on Megasporogenesis, Microsporogenesis and Development of the Male and Female Gametophytes of Arundinaria simonii f. heterophylla
- 林业科学, 2013, 49(8): 168-175
- Scientia Silvae Sinicae, 2013, 49(8): 168-175.
- DOI: 10.11707/j.1001-7488.20130824
-
文章历史
- 收稿日期: 2012-12-21
- 修回日期: 2013-04-25
-
作者相关文章
The life history of many bamboo species is characterized by an unusual flowering habit (Jiang, 2007; Yi, 1997; Wen, 1993). They often flower and die simultaneously over wide areas at long intervals (3-120 years), and then regenerate from seed or rhizome (Janzen, 1976; Liese, 1985; Li et al., 2006; Taylor et al., 1988; 1993; Abe et al., 2001). There were already quite lots of publications dealing with bamboo flowering cycles and some hypotheses were given (Lai, 2001; Xia et al., 1995; Du et al., 2000; McClure, 1993). However, there were fewer reports about embryonic development of bamboo (Qiao et al., 1984; Hu et al., 1994; Wang et al., 2006; Lin et al., 2009) due to the long flowering intervals.
Arundinaria simonii f. heterophylla was introduced from Japan in 1984 and grown in the bamboo garden of Nanjing Forestry University, China. It has been flowering sporadically since 2004, but few seeds can be captured. In order to determine whether the low seed-setting rate was caused by the abnormal development occurred in different embryonic development period, the floral morphology and the whole process of microsporogenesis, megasporogenesis and gametophyte development of A. simonii f. heterophylla were described and compared.
1 Materials and methodsField observation and collection of the material were carried out during four consecutive years (2009-2012) at the site of bamboo garden of Nanjing Forestry University, Jiangsu Province, China.
The materials of Arundinaria simonii f. heterophylla from the young inflorescences and flowers in various stages for embryological study were collected everyday between 8: 00-10: 00 am during the period March 2nd-April 23rd from 2009 to 2012. Flower buds and flowers were fixed in modified FAA (formalin : acetic acid: alcohol = 5: 6: 89). The morphology of the flowers was observed continuously during the flowering season from 2002 to 2004. The fixed material was treated with conventional methods of dehydration, transparency, and embedded in paraffin. Tissues were sectioned, both transversely and longitudinally, at 8-10 mm thickness with a Leica Rotary Sectioner using st and ard techniques. Sections were stained with a saffranin-Fast Green combination. The sections were observed with an Olympus BH-2 microscope and photomicrographs were taken with photographic facilities associated with the microscope.
For examining pollen fertility, two different methods were used. First, anthers were collected from spikelets at anthesis and stained with KI-I2 solution. The pollen fertility was estimated by counting the numbers of normal and abnormal pollen grains in the fields of microscopy per spikelet and the mean values from ten spikelets were calculated. Second, at 0.5 h after floret opening, pistils were collected and fixed in Carnoy’s solution for 24 h, treated with 6 mol·L-1 NaOH for 12 h, then stained with 0.1% aniline blue. The pistils were examined with a fluorescence microscope and the number of pollen grains attached to and germinated on the stigma was counted. The means from ten pistils were calculated. Additional details of the procedure are available in Hu (1994) .
2 Results and analysis 2.1 Inflorescence morphologyThe flower buds of A. simonii f. heterophylla often differentiate in March and bloom in April, which is semelauctant inflorescence. Spikelet is 5-7 cm long, which insert each node of flowering branches, linear, green, flat, glabrous and pedicel is 2-4 cm long, which often contain 6-10 florets, with 2 empty glumes at its base, rachilla disarticulated, 4-8 mm long, flat, puberulose. Lemma 14-20 mm long, purple and green. Palea 12-15 mm long, white lodicule 3, stamen 3, yellow, 7-10 mm long, filaments filiform, 10-16 mm long. Ovary long elliptic, 2-3 mm long, style single, 1-2 mm long, stigmas 3, white, plumose, 3-4 mm long. Caryopsis 12.9-20.3 mm (Fig. 1a-i).

|
Fig.1 Flowering characters of Arundinaria simonii f. heterophylla a. The flowering clump of A. simonii f. heterophylla; b, c. The lateral inflorescence and the genuine spikelet with purple and green lemma and out-stretching stamens; d-h. The structure of one spikelet including three yellow-green anthers, one enlarged lodicule and one gynoecium with short style bearing two branches of stigma; i. Seeds of different size were typical caryopsis. |
The anthers of A. simonii f. heterophylla are tetrasporangiate structure and were the typical of monocotyledonous species. At the early developmental stage, a number rows of archesporial cells began to differentiate surrounding by the anther epidermis (Fig. 2a). It was noted that normal mitosis occurred in archesporial cells to form primary parietal cells and primary sporogenous cells (Fig. 2b).
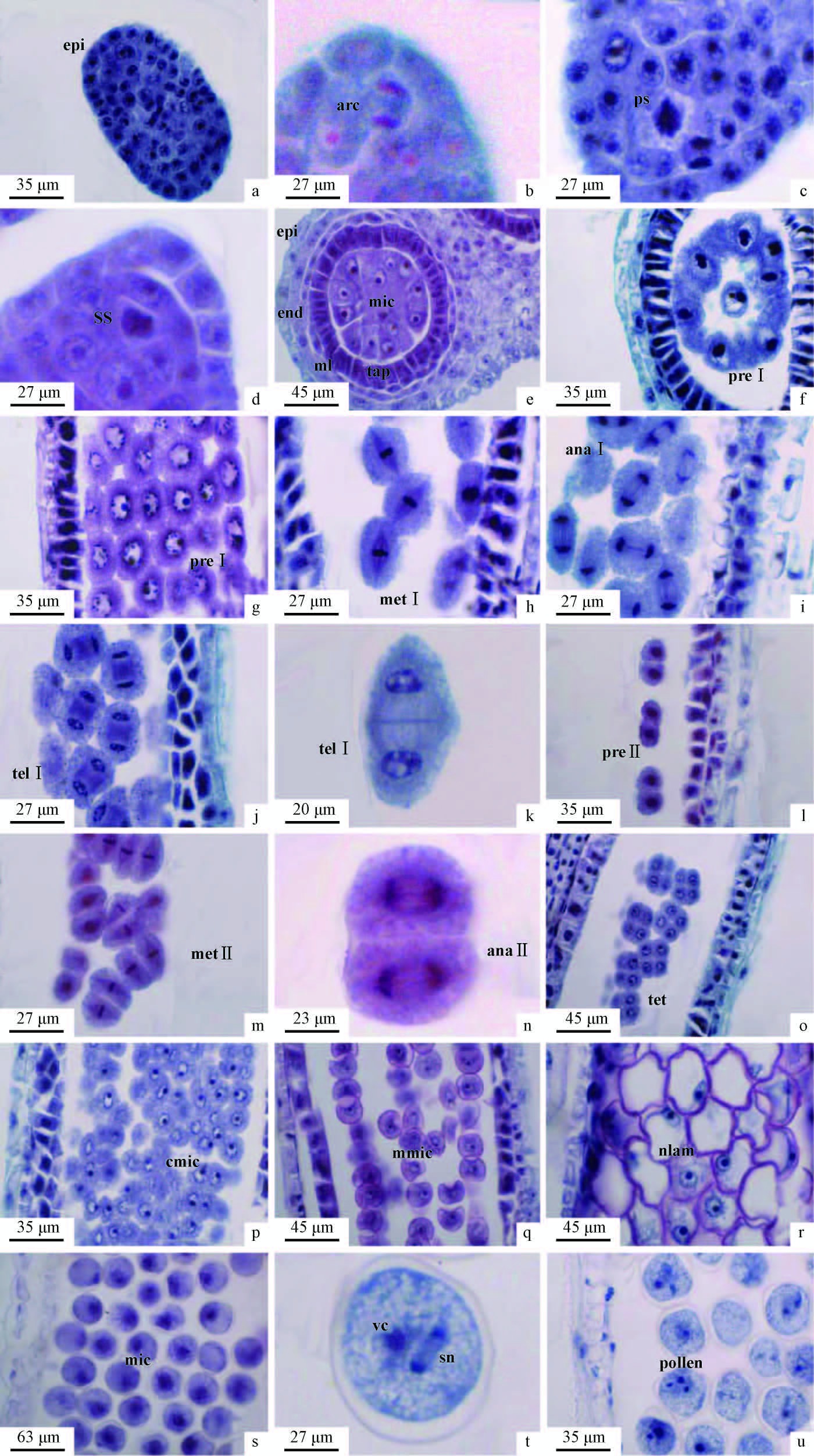
|
Fig.2 The microsporogenesis and development of male gametophyte of Arundinaria simonii f. heterophylla a. The transverse section of young anther in which archesporial cells were not easy to be found at this stage, the out layer is epidermis (epi); b. The mitosis of archesporial cell (arc); c. Primary sporogenous cell (ps); d. Secondary sporogenous cell (ss); e. Microspore mother cell (mic) and four layers of anther wall (epi, end, ml, tap). f, g. Premeitic phase Ⅰ (pre Ⅰ); h. Metaphase of meiosis Ⅰ (met Ⅰ); i. Anaphase of meiosis Ⅰ (ana Ⅰ); j, k. Telophase of meiosis Ⅰ (tel Ⅰ); l. Premeitic phase Ⅱ (pre Ⅱ); m. Metaphase of meiosis Ⅱ (met Ⅱ); n. Anapase of meiosis Ⅱ (ana Ⅱ); o. Tetrads (tet); p. The contraction microspore (cmic); q. The middle microspore (mmic); r. Microspore with the nucleus located aside (nlam); s. The mitosis of microspore (mic); t. Vegetative nucleus (vc) and two sperm nuclei (sn); u. Mature pollen. |
The anther wall was mainly derived from parietal cells consisting of (from the outer to the inner) one row of epidermis, one row of endothecium, one row of middle layer, and one row of tapetum that was in the form of secretory tapetum (Fig. 2e). The epidermis can be retained throughout the gametogenesis process. However, the middle layer cells degenerated gradually during the course of the formation of microspore mother cells, with only relics remaining by the time of the microspore tetrads developed. The tapetum cells were characterized by the presence of one or two nuclei and the dense cytoplasm. The tapetum reached its maximal size during meiosis division and then degenerated and even completely disintegrated until the formation of trinucleate pollen grains. When mature pollen grains developed, the anther wall only consisted of epidermis and endothecium and the endothecium cell wall thickened radially and became fibrous.
2.3 Microsporogenesis and male gametophyteIt was observed that during further development, the primary sporogenous cells were going through the mitosis division to form secondary sporogenous cells from which the microsporocytes were derived (Fig. 2c, d, e). The microsporocyte passed through each stage of meiosis division to form mostly bilaterally symmetrical tetrads (Fig. 2f-o) and the cytokinensis was successive. It was identified that the microspores either in neighbouring sporangia or within the same sporangium were not synchronized in their development. After the separation of tetrads, the mature microspores were formed with dense cytoplasm and one prominent and centrally placed nucleus (Fig. 2p, q). As the microspore developed further, a large vacuole pulled the cytoplasm and the nucleus to poles (Fig. 2r, s). During the subsequent microspore division, two unequally sized nuclei were produced. The larger one would be the vegetative nucleus and the smaller would be the reproductive one. The immature pollen grain was uninucleate and in the mature pollen grain two sperm nuclei were formed from the reproductive nucleus by going through a mitotic division (Fig. 2t). As a result that the mature pollen developed into a trinucleate grain with only one germinal aperture (Fig. 2u).
2.4 Megasporangium and megasporogenesisThe archesporial cell below the nucellus epidermis was divided into a parietal cell and a sporogenous cell by the periclinal division and the sporogenous cell differentiated into a megasporocyte (Fig. 3a, b). The megasporocyte was surrounded by only one row of nucellus cells and therefore the ovule was tenuinucellatae. The megasporocyte was characterized by a large nucleus and dense cytoplasm. During the subsequent development the megasporocyte elongated lengthways and entered into meiosis division (Fig. 3c-g) to directly form the dyad (Fig. 3h) and the linear tetrad of megaspores (Fig. 3i). Only the megaspore at the chalazal end was the functional gamete, while others gradually degenerated (Fig. 3j).

|
Fig.3 The megasporogenesis and development of female gametophyte of Arundinaria simonii f. heterophylla a. Archesporium (ar); b, c. Megaspore mother cell (mmc); d - g. Meiosis of megaspore mother cell (mmc); h. Dyad; i, j. Tetrad of megaspore; k. Two-nucleus embryo sac (tnes); l. The mitosis of embryo nucleus (mit); m. Four-nucleus embryo sac (fnes); n. Eight-nucleus embryo sac (embs); o. Mature embryo sac (membs); p. Egg apparatus (ea); q. Two polar nucle (pn); r. Antipodal cells (ac). |
The functional megaspore with dense cytoplasm and a prominent nucleus formed a 2-nuclear embryo sac, which divided rapidly in further development and one 8-nucleate and 7-cellular embryo sac was formed after a 4-nuclear stage (Fig. 3k-n). Therefore, the embryo sac was of the Polygonum type, in which three nuclei lied at the micropylar end, two near the center, and three at the chalazal end. One micropylar nucleus became the egg and two became the synergids, which formed the egg apparatus (Fig. 3p). The two central nuclei became the polar nuclei, which later fused (Fig. 3q). The chalazal nuclei became three antipodal cells (Fig. 3o), which persisted up to the mature embryo sac stage. After the 8-nucleate stage, the antipodal cells divided rapidly to develop more antipodal cells (Fig. 3r).
2.6 The developmental correspondence relationship between stamens and pistilsThe flowering time of different florets in one spikelet was inconsistent and generally the florets close to the base bloomed earlier than others.
From Tab. 1, it can be seen that most of developmental stages between stamens and pistils were synchronized, except for the stage when the length of the spikelets were about 2-3 cm and the anthers were about 0-2 mm. When the length of anther is 4-5 mm, both the pistil and the stamen were in meiosis division. When anther maturing, the embryo sac also tended to mature, which indicated that the spikelets of A. simonii f. heterophylla belonged to adichogamy.
|
|
Over 70% of the pollen grains of A. simonii f. heterophylla were normal as indicated by staining with KI-I2 and aniline blue. About 30% sterile pollen grains were stained lightly and abnormally, whereas the typical normal pollen grains stained darkly with KI-I2 staining and were round in shape. Staining with aniline blue gave consistent results and fertile pollen grains fluoresced strongly. Thus most mature male gametophytes were indicated to be fertile.
3 DiscussionThe anther wall of A. simonii f. heterophylla consisted of four layers (epidermis, endothecium, middle layer and tapetum), which belonged to the monocotyledonous type. Meanwhile, the tapetum was of typical gl and ular type, the cytokinesis of microsporocyte in meiosis was of successive type. This finding is in agreement with that of Wang et al. (2006) . The microspore tetrads were bilaterally symmetrical type, which was significantly different from that of Wang et al. (2006) and Huang et al. (1999). They reported respectively that the microspore tetrads were tetrahedral type in both Dendrocalamus sinicus and Phyllostachys praecox. At the same time, Huang et al. (1999) pointed out that there were two rows of middle layer in the anthers of Ph. praecox and one of the middle layers degenerated and disappeared in subsequent development.
During pollen development tapetum layer serves mostly as a tissue for meiocyte /spore nutrition. In addition to this main function, the tapetum has other functions, namely the production of the locular fluid, the production and release of callase, the conveying of P. A. S. positive material towards the loculus, the formation of exine precursors, viscin threads and orbicules (= Ubisch bodies), the production of sporophytic proteins and enzymes, and of pollenkitt / tryphine (Pacini et al., 1985). In the present study, the tapetum cells began to degenerate at the premeiotic stage and were absorbed completely in the development of the microspores. Similar trends were reported by Huang et al. (1999) .
Single ovule with parietal placenta was anatropous, double integument and tenuinucellatae. The megasporocyte underwent meiotic division to form four megaspores arranged in a line, and the micropylar megaspore was the functional one. The mature embryo sac possessed one central cell, one egg cell, and two synergids near the micropylar and many antipodal cells were observed near the chalazal end. Therefore, the development of embryo follows the Polygonum type, as described for Triticum aestivum (Hu, 1985; 2005) 。
According to the developmental correspondence relationship between stamens and pistils, it can be drawn that during the early development stage, the developmental stage of pistils was earlier than the anthers, which is similar with the report about Dendrocalamus sinicus by Wang et al. (2006) . However, the development of anthers and pistils began to be synchronized when the length of the spikelets was about 3-3.5 cm. It indicated that the development speed of the anthers was improved in this period. Wang et al. (2006) considered that it always was protogyny during the whole development in spikelets of D. sinicus unlike A. simonii f. heterophylla.
Usually bamboos have a long flowering period, but the number of fruits present at a given time point is usually very low and differs between species. Some research data is available, for example, seed set is 18.4% for Bashania fargesii, 5.8% for Phyllostachys violascens, and 6.1% for Phyllostachys iridescens (McClure, 1993). For some species, such as Bambusa vulgaris, which is widely cultivated around the world, and Bambusa balcoa in Bangladesh, seeds have never been seen so far. These results suggest that either fruit set, or its maturation, is relatively rare in both time and space. In most bamboos the florets producing mature fruits are more or less promptly released by abscission the rachilla and fall to the ground. Animals, particularly certain birds and rodents, harvest them from the plant or the ground. Any of these factors, either on their own or in combination, might be responsible for the low frequency of seed set by bamboo species. Generally, the major factors are likely to be climatic factors, abortion of a portion of the pollen grains, and the number of flowering plants growing at a site. Our previous study showed the self-fruitful rate of A. simonii f. heterophylla is low (unpublished data). In the present study, compared to other bamboo species, A. simonii f. heterophylla pollen appears to have a higher level of viability (above 70%), so it is impossible that the poor seed set of A. simonii f. heterophylla is associated with poor pollen viability. In the Nanjing Forestry University bamboo collection, only several plants flowered in the st and s of A. simonii f. heterophylla. We found that the number of florets opening in a single day is limited during the flowering period. Moreover, for a single floret, the period from the opening to the closure of the palea and lemma is short (only 1-2 h). Of course, the climate can also affect seed set; for example, constantly rainy weather during anthesis will reduce the frequency of seed set. At the same time the incidence of destruction of immature fruits by insects is very high in flowering natural st and s of A. simonii f. heterophylla.
In summary, we have described the basic reproductive developmental characteristics of Arundinaria simonii f. heterophylla and observed that the pollination effectiveness is poor because of the small number of flowering bamboo plants. We speculate that this might be a major cause for the species’low seed set.
| [1] |
Abe M, Miguchi H, Nakashizuka T. 2001. An interactive effect of simultaneous death of dwarf bamboo, canopy gap, and predatory rodents on beech regeneration. Ecology, 127(2): 281-286.( 1) 1)
|
| [2] |
Du Fan(杜凡), Xue Jiarong(薛嘉榕). 2000. Study on flowering phenomenon and its type of bamboo in Yunnan in past fifteen years. Sci Sil Sin(林业科学),36(6): 51-68.( 1) 1)
|
| [3] |
Hu Chenghua(胡成华),Yu Fugen(喻富根). 1994. Observation and study on embryology of Chimonobambusa marmorea. J Bamboo Research(竹子研究汇刊),13(4):6-13.( 1) 1)
|
| [4] |
Hu Shiyi(胡适宜). 1985. Embryology of the angiosperms(被子植物胚胎学). Beijing: Higher Education Press(高等教育出版社), 58-70.( 1) 1)
|
| [5] |
Hu Shiyi(胡适宜). 1994. Method of preparation of slides used to examine the pollen germination on the stigma and pollen tube growth in the style. Chinese Bulletin of Botany(植物学通报), 11(2):58-60.( 1) 1)
|
| [6] |
Hu Shiyi(胡适宜). 2005. Reproductive biology of angiosperms(被子植物生殖生物学). Beijing: Higher Education Press(高等教育出版社), 108.( 1) 1)
|
| [7] |
Huang Jianqin(黄坚钦), Huang Huahong(黄华宏), He Jifu(何福基), et al. 1999. The formation of microspore and the development of male gametophyte of Phyllostachys praecox. Journal of Bamboo Research(竹子研究汇刊), 18(3): 55-58.( 2) 2)
|
| [8] |
Janzen D H. 1976. Why bamboos wait so long to flower? Ann Rev Ecol Syst, 7: 347-391.( 1) 1)
|
| [9] |
Jiang Zehui(江泽慧). 2007. Bamboo and rattan in the world(世界竹藤). Beijing: China Forestry Publishing House(中国林业出版社), 15-17.( 1) 1)
|
| [10] |
Lai Guanghui(赖广辉). 2001.Supplementary description of floral morphology of ten species in the genus Phyllostachys (Bambusoideae). J Wuhan Bot Res(武汉植物学研究), 19(1): 7-13.( 1) 1)
|
| [11] |
Li Zhaohua, Denich M, Borsch T. 2006. Simultaneous flowering of umbrella bamboo (Fargesia murieliae) at its native home in Central China. Journal of Forestry Research, 17(4):293-297.( 1) 1)
|
| [12] |
Liese W. 1985. Bamboos—Biology, silvics, properties, utilization. GTZ, Eschborn,103-120.( 1) 1)
|
| [13] |
Lin Shuyan(林树燕), Hao Juanjuan(郝娟娟), Xin Hua(辛华). 2009.The megasporogenesis, microsporogenesis and the development of their female and male gametophyte in Menstruocalamus sichuanensis. J Nanjing Forestry Univ(南京林业大学学报), 33(3):9-12.( 1) 1)
|
| [14] |
McClure F A. 1993.The Bamboos.Washington and London: Smithsonian Institution Press, 95-97.( 2) 2)
|
| [15] |
Pacini E, Franchi G G, Hesse M. 1985. The tapetum: Its form, function, and possible phylogeny in Embryophyta. Plant Systematics and Evolution, 149(3/4):155-185( 1) 1)
|
| [16] |
Qiao Shiyi(乔士义), Liao Guanglu(廖光庐). 1984. Embryology observation of Phyllostachys pubescens. Bamboo Research(竹类研究), 3 (1):15-23.( 1) 1)
|
| [17] |
Taylor A H, Qin Z S. 1988. Regeneration from seed of Sinarundinaria fangiana, a bamboo, in the Wolong Giant Panda reserve, Sichuan, China. Am J Bot, 75: 1065-1073.( 1) 1)
|
| [18] |
Taylor A H, Qin Z S. 1993. Bamboo regeneration after flowering in the Wolong Giant Panda reserve. Biol Cons, 6(3): 231-234.( 1) 1)
|
| [19] |
Wang Shuguang(王曙光), Pu Xiaolan(普晓兰), Ding Yulong(丁雨龙). 2006.The structures of reproductive organs and development of the female and male gametophyte of Dendrocalamus sinicus. Bulletin of Bot Research(植物研究), 26 (3): 270-274.( 4) 4)
|
| [20] |
Wen Taihui(温太辉). 1993. Colored illustrations of Bambusoideae in China(中国竹类彩色图鉴). Taipei: Shuxin Press(淑馨出版社), 93-95.( 1) 1)
|
| [21] |
Xia Nianhe(夏念和), Jia Liangzhi(贾良智). 1995.Some new and imperfectly known species of bamboos from China. J Trop Subtrop Bot(热带亚热带植物学报), 4(1): 23-30.( 1) 1)
|
| [22] |
Yi Tongpei(易同培). 1997. Bamboos flora of Sichuan(四川竹类植物志). Beijing: China Forestry Publishing House(中国林业出版社), 284-286.( 1) 1)
|
 2013, Vol. 49
2013, Vol. 49

