文章信息
- 马金龙, 姜国斌, 姚善泾, 金华
- Ma Jinlong, Jiang Guobin, Yao Shanjing, Jin Hua
- 盐胁迫下杨树质外体离子对气体交换参数的影响
- Effects of Apoplastic Ion Contents in the Tender Stems of Two Populus Cultivars on Gas Exchange Parameters Under Salt Stress
- 林业科学, 2013, 49(7): 40-47
- Scientia Silvae Sinicae, 2013, 49(7): 40-47.
- DOI: 10.11707/j.1001-7488.20130706
-
文章历史
- Received date: 2012-05-16
- Revised date: 2013-02-19
-
作者相关文章
2. 大连民族学院环境与资源学院 大连 116600;
3. 大连民族学院生命科学学院 大连 116600
2. Environment and Resources College, Dalian Nationalities University Dalian 116600;
3. Life Science College, Dalian Nationalities University Dalian 116600
Approximately 1/3 of earth l and s are covered with saline soils, which seriously affect agricultural production and vegetation distribution(Munns et al., 2008). There are nearly 2.5×106 hm2 of various saline soils in China, and the situation still deteriorates every year(Zheng et al., 2008). Therefore, it is imperative to investigate the salt-tolerance mechanism of plants. Apoplast of higher plants is a dynamic space in which many important physiological and biochemical processes and reactions such as solute transportation, nutrient activation, adversity resistance and etc. occur(Bartels et al., 2005;Saglam et al., 2010). The dynamic changes of ions in apoplast not only directly affect the salt tolerance of plants, but also are closely associated with many physiological mechanisms such as plant transpiration, signal transduction, ion compartmentation in cells and etc.(Tuteia, 2007).
Oertli(1968)hypothesized that salt stress decelerated the ions in the apoplast of plant tissues before entering symplast, which resulted in the substantial accumulation of ions in apoplast that then dehydrated cells, eliminated turgor and damaged membrane structures, thereby inhibiting the growth and development of plants. However, this hypothesis is hardly proven. Sun(2000)reported that the apoplastis an essential part of plants in which different organs, tissues and cells interact and communicate that affect the physiological functions of cells as soon as they confront in vivo and in vitro biological or non-biological environmental changes. The reduced photosynthesis under salt stress commonly results from water potential and decreased stomatal conductance induced by osmotic stress, which thus prevents the movement of CO2(Zhu et al., 1999). Meanwhile, the increasedNa+content in leaves lowers stomatal conductance and prevents CO2 from being transported to the chloroplast, and CO2 assimilation was blocked as a result(Wei et al., 2004). The apoplast in tender stems, which aresimilar to those in leaves, are close to the growing point of plants. The roots, stems and leaves are closely related, and ions absorbed by roots will be transported to leaves through stems. The dynamic changes of apoplastic ions in tender stems will also indirectly affect photosynthesis. Therefore, clarifying the effects of apoplastic ion changes on gas exchange parameter sunder salt stress may reveal the relationship between apoplastic changes and photosynthetic physiology. On the other h and , photosynthesis provides fundmamental materials and energy for the growth and development ofplants(Rhors-Richey et al., 2011). However, the underlying reason accounting for the decrease of photosynthetic capacity(stomatal or non-stomatal limitation)is still controversial(Xia et al., 2005).
Thus far, crops including barley, rice, cotton and etc. have been mainly employed as the salt tolerancemodels(Chaves et al., 2009), whereas economic trees have seldom been studied. Meanwhile, the functions of symplast have been more frequently studied than those of apoplast. Moreover, apoplastic ions in the roots and leaves of plants have been most frequently accessed(Cuin et al., 2010;2011;Zeng et al., 2009).However, the previous studies on apoplast in plants suffer from several drawbacks(Narendra, 2007), which are in need of a rapid, convenient and accurate method. The micro-dialysis sampling technique that has been widely applied in different parts of animals and human is feasible to remedy the deficiency.Notably, micro-dialysis(MD)is a non-invasive, in situ sampling, real-time and online dialysis technology(Simon et al., 2009), which functions in plant detections in limited previous literatures(Eklund, 1991;1993;Eklund et al., 1991). In addition, our group has demonstrated that microdialysis could acquire pure apoplastic sap of plants rapidly and easily(Wang et al., 2009).
In this paper, Populus×wutunenses is a novel salt-resistant and drought-resistant tree speciesdistributed in Liaoning Province that was developed and cultivated by our group, and “P. simonii×P.euphratica”×P. sp. is a traditional salt-tolerant and drought-tolerant tree species widely planted, which were used to investigate the effects of apoplastic ions in the tender stems of Populus cultivars on gas exchange parameters under the salt stress of 100 mmol·L-1 NaCl and to explore the mechanisms of apoplast in photosynthetic physiology, which will be conductive toimproving the salt tolerance of plants. Micro-dialysis in the physiological and biochemical examinations of plant stress resistance to preliminarily study the changes of apoplastic ions under salt stress, to reveal the correlations between them by combining traditional instruments, and to interpret the experimental results by data processing and previous literatures, aiming to allow timely, online and non-invasive measurements practically.
1 Materials and methods 1.1 Treatment of plant materialsEighty cutting woods of P.×wutunenses and “P.simonii×P. euphratica”×P. sp. collected from YaopuTownship, Xinmin Country, Liaoning Province were put into pots(inner diameter: 20 cm;height: 16 cm;each pot: 1 strain)in April. Forest humus soils were used in the pots(Zeng et al., 2009).
After 40 days, three uniformly grown strains(height: 12~15 cm;leaves: 12~16 pieces)of each type were selected. The plants were irrigated every fourdays with a 100 mmol NaCl solution depending on the humid conditions of the soils. It is necessary to irrigate more water than that held in soil in order to ensure a constant NaCl concentration(Brinker et al., 2010).The plants were sampled by microdialysis and gas exchange parameters were determined after 1, 5, 9, 13 and 17 days of salt processing. Only the mature and recent fully-unfolded leaves were used for tests. Every test was repeated in triplicate.
1.2 Determination methodsThe color, salt spot and falling of leaves were observed on the 1st, 5th, 9th, 13th, 17th and 20th day of salt processing.
1.3 Microdialysis samplingA MD probe(340 μm OD;polyacrylonitrile with a molecular weight cutoff of 30 000 Da;BASi, Sweden)was connected to a pumping system and a fraction collector(Model FC203b, Gilson, Middleton, WI, USA)by an FEP tubing(CMA/MD, Stockholm, Sweden). The perfusate was delivered at a flow rate of1.0 μL·min-1 by a MD infusion pump(CMA 102;CMA/MD AB, Stockholm, Sweden)equipped with three 1 mL gastight syringes(Hamilton, Reno, NV, USA). Considering that poplar tender stems are harder than animal tissues, a guide wire forming an angle of 30° to 45° with the tender stem was first inserted intothe sampling part to prevent potential damages of the probe(Simon et al., 2009). To prevent the loss of perfusion fluid, complete dry mono-methylmethacrylate (MMA), which was prepared by mixing autocoagulating powders and a denatured base resin fluid, was used to seal the margin of the probe. Millipore-Qhyper-pure water was used as the perfusion fluid(flowrate: 1 μL·min-1). All the tests were performed at ambient temperature. The system was allowed to equilibrate for at least 20 min after eliminating the dead volume in the tubing. Then the dialysis fluid wascollected in 1.5 mL Eppendorf vials at appropriate time intervals(Fig. 1). Three different probes were used to ensure that the results were independent of the probe type. In order to perform microdialysis and to interpret the results correctly, it is also necessary to calibrate the microdialysis probes prior to the experiments.
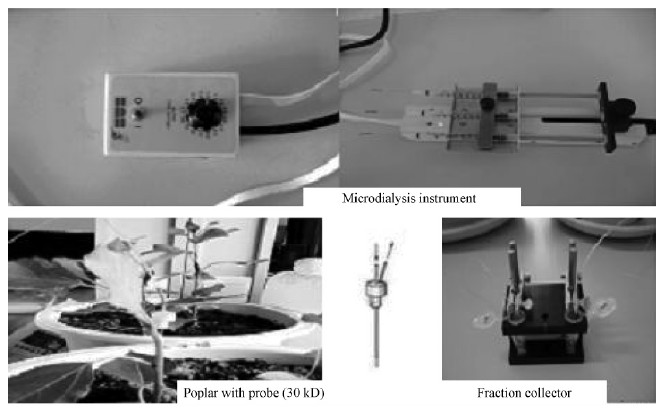 |
Fig. 1 Microdialysis system and tender stem of poplar with a probe
|
The dialysis fluids were diluted by 10 times with Millipore-Q ultrapure water to determine Na+ and K+concentrations by the atomic absorption spectrophotometer(AAS)(Jiang et al., 2010). The st and ard storage solution(1 000 mg·L-1 )was provided by theNational St and ard Material Research Center. AASanalysis was performed using a Hitachi Z2000(Japan)atomic absorption spectrophotometer system equipped with Potassium, Sodium hollow cathode lamp. The conditions of AAS are listed in Tab. 1.
|
|
In vitro relative recovery: The microdialysis probe was inserted into Na+st and ard solution with known concentration. 100 μL of dialysis fluid was collected 30 min after inserting the probe to determine Na+concentration. The in vitro relative recovery of Na+, which is the ratio of the measured concentration to thest and ard concentration, was determined as 8%.
C=C1×10/0.08
where C represents the concentration of a certain ion;C1 represents the concentration determined by AAS;10is the dilution factor;0.08 is the in vitro relative recovery. 1.5 Determination of gas exchange parametersThe gas exchange parameters of recent fullyunfolded leaves(4th-5th leaf from the top), includingnet photosynthetic rate(Pn), stomatal conductance(Gn) and intercellular CO2 concentration(Ci), were determined with a portable photosynthesis system(LICOR, 6400)during 10:00—11:00 AM. Stomatal limiting value(Ls)can be expressed as(1-Ci/Ca)(Ca: CO2 concentration in the sampling chamber).The conditions of 6400-02B LED source were set at:intensity: 800 μmol·m-2 s-1;temperature: 20 ℃;flow rate: 350 μmol·s-1;atmospheric CO2concentration: 370 μmol·mol-1.
1.6 Data analysisAll the data of each cultivar were measured in triplicate, and all the data were processed by correlation analyses(software: SPSS) and Duncan’s new multiple range test. The effects of apoptotic ions on Pn were analyzed by the multiple linear regression, and the variables were screened by the stepwise method(αinclude=0.05, αexclude=0.10).
2 Results 2.1 Growth status of the two populus cultivars under salt stressThe two populus cultivars grew healthily holding dark green leaves after being treated with salt stress for1d. Salt spots began to appear on the leaves of “P.simonii×P. euphratica”×P. sp. and P.×wutunenseson the 5th day and 13th day of salt processing, which were accompanied by falling leaves. Thereafter, the salt spots already existed on the leaves enlarged, and the leaves at higher positions were also subject to saltspots. Meanwhile, the leaves began to fall off from the bottom up. Accordingly, it has been initially verifiedthat“P. simonii×P. euphratica”×P. sp. suffered from more severe salt damage than P.×wutunenses did(Tab. 2).
|
|
With increasing salt processing time, Na+ concentrations in the apoplasts of the tender stems of the two populus cultivars gradually increased.Apoplastic Na+concentration in P.×wutunenses slightly decreased and differed apparently with that in“P. simonii×P. euphratica”×P. sp. after 17 days of salt processing(Fig. 2). Moreover, apoplastic Ca2+ content in P.×wutunenses continuously decreased until the 17th day, whereas that in “P. simonii×P.euphratica”×P. sp kept decreasing throughout the whole experiment. Furthermore, Na+/K+ratio of P.×wutunenses increased initially(5th day:≷1;17th day:~4) and slightly decreased on the 17th day of salt processing. In general, the contents of all the ions in the two populus cultivars differed significantly, and the ion contents in P.×wutunenses changed less than those in“P. simonii×P. euphratica”×P. sp..
 |
Fig. 2 Effects of salt stress on apoplastic ion contents in the tender stem of two Populus cultivars
|
The photosynthetic rates of plants will decreaseunder salt stress(Guo et al., 2001;Hui et al., 2004;Zhou et al., 2005), which is commonly attributed to stomatal and non-stomatal limitations(Zhu et al., 1999). Fig. 3 shows that Pn of the two Populus cultivars decreased with elapsed salt processing time.Pn of P.×wutunenses and “P.simonii ×P.euphratica”×P. sp. began to sharply decrease after 17 and 9 days of salt processing. Gn of P.×wutunenses increased and then conversely reduced, and that of “P. simonii×P. euphratica”×P. sp. continuously decreased. Ls of P.×wutunenses decreased initially and increased subsequently, and that of“P. simonii×P. euphratica”×P. sp. kept increasing. On the contrary, Ci of P.×wutunenses increased at first and then decreased, and that of “P. simonii×P.euphratica”×P. sp. gradually reduced. Generally speaking, the drastically changed gas exchange parameters of the two Populus cultivars all differed significantly.
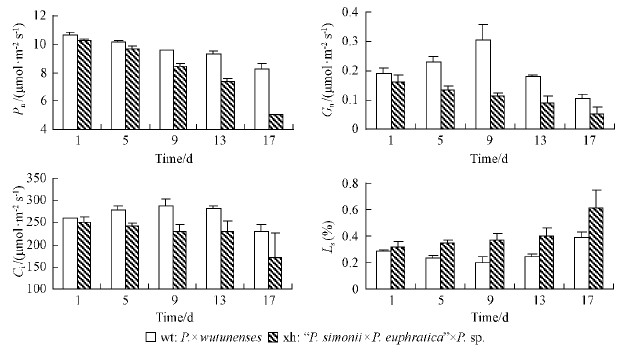 |
Fig. 3 Effects of salt stress on gas exchange parameters in the leaves of two Populus cultivars
|
The correlations between the concentrations of Na+, K+, Ca2+, Na+/K+ and Pn were analyzed by SPSS. Table 3 exhibits that Pn of P.×wutunenses is negatively correlated with Na+concentration and Na+/K+as well as is positively correlated with the concentrations of K+ and Ca2+, and Pn of “P. simonii×P. euphratica”×P. sp. is negatively correlated with Na+ concentration and Na+/K+extremely as well as is positively correlated with K+concentration extremely. Ls of P.×wutunenses is not significantly with any ion concentrations, whereas Ls of“P. simonii×P. euphratica”×P. sp. is positively correlated with Na+concentration and Na+/K+extremely as well as is negatively correlated with K+concentration extremely. Furthermore, a multiplelinear stepwise regression analysis was performed to obtain the corresponding equation utilizing Pn as the dependent variable and Na+, K+, Ca2+concentrations and Na+/K+as the independent variables, respectively.
|
|
The fitting equations can be expressed as follows:
P.×wutunenses: Y=5.306X+4.714(R2=0.580).
Y-Pn, X-K+, F=24.866, P=0.000<0.005, t=0.000<0.005(indicate K+ and Pn are correlated significantly).
“P. simonii×P. euphratica”×P. sp: Y=-1.819X+13.495(R2=0.952).
Y-Pn, X-Na+/K+, F=170.533, P=0.000<0.005, t=0.000<0.005(Na+/K+is correlated with Pn significantly).
Only Na+/K+ratio is included in the equation, and the concentrations of the three ions are excluded, which may be attributed to they are collinearly correlated.
3 DiscussionThe apoplast of higher plants consists of the outercell-membrane fibers and micro-crystal space that compose the cell wall, the intercellular space full of water and air, and the completely differentiated xylem(Cuin et al., 2011). Although apoplast only accounts for a small proportion of plant tissues(8%-15%), it plays essential roles in plant physiology. Manyreactions take place in the apoplastic space under saltstress(Mengle et al., 1988;Speer et al., 1991)responding to the changes of symplast(Ntsika et al., 1986). Oertli(1968)found that the accumulation of apoplastic Na+under salt stress destroys the balance between intracellular and extracellular water potentials, which leads to the dehydration of numerous protoplasts and thus results in osmotic stress of cells. In this study, apoplastic Na+concentrations in the tender stems of the two Populus cultivars accumulated in different ways. The apoplastic Na+concentration in P.×wutunenses decreased by self-regulation, but tha tin“P. simonii×P. euphratica”×P. sp. accumulated continuously, leading to the osmotic stress of cells and growth inhibition. Moreover, when the external salt concentrations increased, K+flowed from the roots into the ambient medium or got replaced by Na+, thereby decelerating photosynthesis. The results are inconsistent with those reported previously(Cramer et al., 1985;Sulian et al., 2012). In this study, K+concentration declined with increasing salt processing time, which may result from the increase of Na+concentration. As a result, the growth of plants was inhibited.
Photosynthesis, which is an essential metabolic process in plants, remarkably affects plant growth and stress resistance(Hui et al., 2003). Photosynthetic inhibition may originate from stomatal or non-stomatal limitation. The relationship between Pn, Ci, Gs and Ls determines whether a decrease of the photosynthetic rate results from stomatal limitation. Only when Ci, Gs and Pn all decrease while Ls increases can it safely be concluded that photosynthetic inhibition is mainly induced by the decrease of stomatal conductance. Incontrast, if Pn, Gs and Ls all decrease while Ci increases, photosynthetic inhibition is primarily triggered by a reduced photosynthetic activity(Farquhar et al., 1989). Therefore, the results in our study show that the photosynthetic inhibition of P.×wutunenses is induced by stomatal limitation under along-term salt stress(100 mmol), which can be attributed to the stomatal contraction of leaves and the decrease of stomatal conductance that limited the transmission of CO2 into chloroplast and disrupted photosynthesis. Besides, the non-stomatal limitation of P.×wutunenses at the early stage probably results from the lowered activity of RuBP carboxylase under a shorttermsalt stress(Chen et al., 2006). However, thep hotosynthetic inhibition of “P. simonii×P.euphratica”×P. sp. primarily results from stomatal limitation. Moreover, Pn of the two populus cultivars decreased through apparently different ways. In summary, it has been demonstrated that the salt tolerance of P.×wutunenses is stronger than that of“P. simonii×P. euphratica”×P. sp..
It is well known that ion damage(damage induced by excessive or insufficient ions)is one of the reasons inhibiting photosynthesis. Ion redundancy and ion insufficiency refer to excessive Na+ and Cl- and insufficient K+ and Mg2+, respectively(Chen et al., 2010). The above experimental results and statistical analyses reveal that the variations of apoplastic ions in the tender stems of Populus under salt stress might influence the photosynthetic rate in leaves, which maybe ascribed to the accumulation of apoplastic Na+ratherthan K+ and Ca2+. As a result, the concentrations of K+ and Ca2+ lowered, and the photosynthesis of populus was inhibited. A multiple linear stepwise regression reveals that only K+ concentration is includedin the equation for P.×wutunenses and only Na+/K+ ratio is included in the equation for “P. simonii×P.euphratica”×P. sp. The results indicate that K+of P.×wutunenses is linearly correlated with Pn significantly, and Na+/K+of P. simonii×P. euphratica×P. sp. islinearly correlated with Pn significantly. The final fittingequation is expressed as:
Y=-1.819X+13.495(R2=0.952)
Y-Pn, X-Na+/K+, F=170.533, P=0.000<0.005, t=0.000<0.005.
Only Na+/K+ratio is included in the equation, and the concentrations of the three ions are excluded, indicating that they are collinearly correlated with Pn.
4 ConclusionsUnder salt stress, the apoplastic Na+concentration in P.×wutunenses decreased by self-regulation, but that in “P. simonii×P. euphratica ”×P. sp.accumulated continuously, leading to the osmotic stress of cells and growth inhibition. K+concentrations in the two Populus cultivars both decreases with increasing salt processing time. The changes of the four photosynthesis indexes of their leaves suggest that the photosynthesis of P.×wutunenses decreased owing tonon-stomatal limitation(initially) and stomatal limitation successively, whereas that of“P. simonii×P. euphratica”×P. sp. reduced due to stomatal limitation throughout the whole experiment.
The concentrations of ions and the corresponding photosynthesis indexes of the two Populus cultivars all varied. The concentrations of almost all the ions(except for Ca2+ concentration in the tender stem of“P. simonii×P. euphratica ”×P. sp.)were correlated with Pn values significantly. A multiple linear stepwise regression reveals that K+of P.×wutunenses is linearly correlated with Pn significantly, and Na+/K+of“P. simonii×P. euphratica”×P. sp.is linearly correlated with Pn significantly. Only Na+/K+ratio is included in the equation, and the concentrations of the three ions are excluded, indicating that they are collinearly correlated with Pn .
| [1] |
Bartels D, Sunkar R. 2005. Drought and salt tolerance in plants. Critical Reviews in Plant Sciences, 24:23-58.( 1) 1)
|
| [2] |
Brinker M, Brosche M, Vinocur B. et al. 2010. Linking the salt transcriptome with physiological responses of a salt-resistant populus species as a strategy to identify genes important for stress acclimation. Plant Physiology, 154: 1697-1709.( 1) 1)
|
| [3] |
Chaves M M, Flexas J, Pinheiro C. 2009. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Annals of botany, 103: 551-560.( 1) 1)
|
| [4] |
Chen S, Polle A. 2010. Salinity tolerance of Populus. Plant biology, 12(2):317-333.( 1) 1)
|
| [5] |
Cramer G R, Lauchli A, Polito V S. 1985. Displacement of Ca2+ by Na+ from the plasmalemma of root cells. Plant Physiol, 79: 207-211.( 1) 1)
|
| [6] |
Cuin T A, Bose J, Stefano G, et al. 2011. Assessing the role of root plasma membrane and tonoplast Na+/H+ exchangers in salinity tolerance in wheat: in planta quantification methods. Plant, Cell and Environment, 34: 947-961.( 2) 2)
|
| [7] |
Cuin T A, Parsons D, Shabala S. 2010. Wheat cultivars can be screened for NaCl salinity tolerance by measuring leaf chlorophyll content and shoot sap potassium. Functional Plant Biology, 37(7): 656-664.( 1) 1)
|
| [8] |
Chen S F, Zhu Y L, Hu C M. 2006. Effects of NaCl stress on growth and photosynthetic characteristics in grafted tomato seedlings. Jiang su J of Agr Sci, 22(2): 145-149.( 1) 1)
|
| [9] |
Eklund L. 1991. Hormone levels in the cambial region of Picea abies during the onset of cambial activity. Physiol Plant, 82(3): 385-388.( 1) 1)
|
| [10] |
Eklund L. 1993. Movement and possible metabolism of ethylene in dormant Picea Abies. Plant Growth Regulation, 12: 37-41.( 1) 1)
|
| [11] |
Eklund L, Collin A K.1991. Microdialysis, a new tool for sampling and manipulating internal ethylene concentration in apples. Plant Physiol, 137: 155-157( 1) 1)
|
| [12] |
Farquhar G D, Ehleringer J R, Hubick K T. 1989. Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol, 40: 503-537.( 1) 1)
|
| [13] |
Guo S K, Zhao K F. 2001. The possible mechanisms of NaCl inhibit photosynthesis of Maize seedlings. Acta Phytophysiologica Sinica, 27(6):461-466.( 1) 1)
|
| [14] |
Hui H X, Xu X, Li Q R. 2003. Exogenous betaine improves the photosynthesis of Lycium barbarum under salt stress. Acta Bot Boreal-Occident.Sin, 23(12): 2137-2142.( 1) 1)
|
| [15] |
Hui H X, Xu X, Li S H. 2004. Possible mechanism of inhibition on photosynthesis of Lycium barbarum under salt stress. Chinese Journal of Ecology, 23(1): 5-9.( 1) 1)
|
| [16] |
Jiang B, Jiang G B, Liu C J. 2010. Determination of Metals in Ginkgo Biloba Leaves by Atomic Absorption Spectrometry with Microwave Digestion. Spectroscopy and spectral analysis, 30(3): 812-815.( 1) 1)
|
| [17] |
Mengle K, Geurtzen G. 1988. Relationship between iron chlorosis and alkalinity in Zea mays. Physiologia plantarum, 72(3): 460-465.( 1) 1)
|
| [18] |
Munns R, Tester M. 2008. Mechanisms of salitnity tolerance. Annu Rev Plant Biol, 59:651-681.( 1) 1)
|
| [19] |
Narendra T. 2007. Mechanisms of High salinity tolerance in plants. Methods in enzymology, 428: 419-438.( 1) 1)
|
| [20] |
Ntsika G, Delrot S. 1986. Changes in apoplastic and intracellular leaf sugars induced by the blocking of export in Vicia faba. Physiologia plantarum, 68(1): 145-153.( 1) 1)
|
| [21] |
Oertli J J. 1968. Extracellular salt accumulation, a possible mechanism of salt injury in plants. Agrochernica, 12:461-469.( 2) 2)
|
| [22] |
Rhors-Richey J K, Mulder C P H, Winton L M, et al. 2011. Physiological performance of an Alaskan shrub (Alnus fruticosa) in response to disease (Valsa melanodiscus) and water stress. New Phytologist, 189: 295-307.( 1) 1)
|
| [23] |
Saglam A, Terzi R, Nar H, et al. 2010. Inorganic and organic solutes in apoplastic and symplastic spaces contribute to osmotic adjustment during leaf rolling in Ctenanthe setosa. Acta biologica cracoviensia seies botanica, 52(1):37-44.( 1) 1)
|
| [24] |
Simon N Z, Ken D O, Mark R S, et al. 2009. Use of simultaneous dual-probe microdialysis for the determination of pesticide residues in a jade plant (Crassula ovata). Analyst, 134: 748-754.( 2) 2)
|
| [25] |
Sulian L, Nie L L, Fan P X, et al. 2012. Sodium plays a more important role than potassium and chloride in growth of Salicornia europaea. Acta physiol plant, 34(2): 503-513.( 1) 1)
|
| [26] |
Sun D Y. 2000. Apoplanst-The important signal source for fate decision of cell development. Acta Botanica Sinica, 42(5): 441-445.( 1) 1)
|
| [27] |
Speer M, Kaiser W M. 1991. Ion relations of symplastic and apoplastic space in leaves from Spinacia oleracea L. and Pisum sativum L. under Salinity. Plant Physiol, 97: 990-997.( 1) 1)
|
| [28] |
Tuteja N. 2007. Mechanisms of High salinity tolerance in plants. Methods in enzymology, 428: 419-438.( 1) 1)
|
| [29] |
Wang W, Liu M G,Yin W L. 2009. Extracting apoplastic succus in a Schefflera octophylla tender stem by microdialysis. Journal of Beijing Forestry University, 31(4):41-44.( 1) 1)
|
| [30] |
Wei G Q, Zhu Z J, Fang X Z. 2004. The effects of NaCl stress on plant growth, chloropyll fluorescence characteristics and active oxygen metabolism in seedings of two cucumber cultivars. Scientia Agricultura Sinica, 37(11): 1754-1759.( 1) 1)
|
| [31] |
Xia Y, Sun M G, Li G L, et al. 2005. The effects of salt stress on the contents of chlorophyll in seedling leaves of four garden tree species. Journal of Shandong Agricultural University (Natural science), 36(1):30-34.( 1) 1)
|
| [32] |
Zheng Y H, Wang Z L, Sun X Z, et al. 2008. Higher salinity tolerance cultivars of winter wheat relieved senescence at reproductive stage. Environmental and Experimental Botany, 62: 129-138.( 1) 1)
|
| [33] |
Zhu X G, Zhang Q D. 1999. Advances in the Research on the Effects of NaCl on Photosynthesis. Chinese Bulletin of Botany, 16(4): 332-338.( 2) 2)
|
| [34] |
Zhou X Y, Cao F L. 2005. Effects of soil salt stress on the photosynthesis of Zoysiagrass and Centipedegras. Acta Agriculturae Universitatis Jiangxiensis, 27(3): 408-412.( 1) 1)
|
| [35] |
Zeng F J, Yan H, Stefan K A. 2009. Leaf and whole tree adaptations to mild salinity in field grown Populus euphratica. Tree Physiology, 29: 1237-1246.( 2) 2)
|
 2013, Vol. 49
2013, Vol. 49

