文章信息
- 卜书海, 陈辉
- Bu Shuhai, Chen Hui
- 华山松16种小蠹(鞘翅目: 象虫科: 小蠹亚科) 前胃结构的形态学
- A Morphological Study on the Proventriculi of Sixteen Species of Bark Beetles (Coleoptera: Curculionidae: Scolytinae) Bred in Pinus armandi
- 林业科学, 2013, 49(5): 128-134
- Scientia Silvae Sinicae, 2013, 49(5): 128-134.
- DOI: 10.11707/j.1001-7488.20130517
-
文章历史
- 收稿日期:2012-02-21
- 修回日期:2012-10-10
-
作者相关文章
2. 西北农林科技大学林学院 杨凌 712100
2. College of Forestry, Northwest A&F University Yangling 712100
Scolytinae is a large and highly adapted group ofweevils of considerable economic significance becauseof their impact on trees and forestry industry(Rudinsky, 1966; Wood, 1982; Miller et al., 1989; Klepzig et al., 1991; Wallin et al., 2002). In Pinusarmandii(Chinese white pine)stands in Qinling, Shaanxi, China, there are about 22 species of bark andambrosia beetles forming a stable ecosystem by complexinteractions among spatial and trophic competition andcooperation(Chen et al., 2007).
The proventriculus is ectodermic in origin; it islined by a sclerotized cuticular intima. The structure of the proventriculus in bark beetles was briefly studied by several authors such as Balogun(1969), Nobuchi(1969), Yin et al.(1984), Lopez-Buenfil et al.(2001)and Díaz et al.(2000; 2003). Their studiescon centrated on the anatomy and histology of theproventriculus and formed preliminary work on thephysiology of its nutrition. In all species studied, display similar general pattern with each other, as well as between male and female. Taking the proventriculus of Dendroctonus armandi as an example, it looks like asmall lantern or bulb from an outer view(Fig. 1)(Bu et al., 2009). It is formed by eight thick folds of theproventricular wall, which is composed of circular and /or longitudinal muscles, an epithelial cell layer andchitinous plates projecting to the proventriculus lumen(Fig. 1). Each chitinous plate can be divided in tworegions: an anterior plate covered by cuticle with denticles, spines and a masticatory plate with the movable brushes carrying bristles.
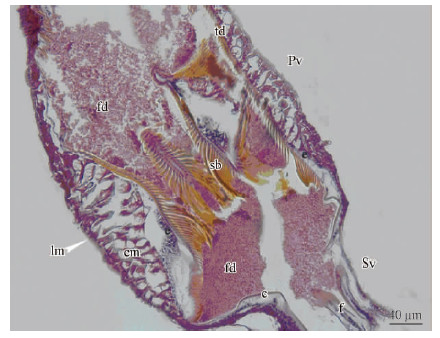 |
Fig. 1 Longitudinal section of the proventriculus of Dendroctonus armandi c:Cuticles; cm:Circular muscle; e: Epithelium; f: Fold; fd: Food; lm: Longitudinal muscle; Pv:Proventriculus; sb: Stopping bristles; Sv: Stomodeal valve; td: Transverse denticle. Light microscope.
|
In scolytid insects, the structures of masticatory plates are similar. Their cuticles are highlys clerotizated and in general have many bristles or hairsto form the comb-like movable brushes. These brushesand long bristles can stir and filter solid particles fromthe anterior plate contents down to the midgut as resultsof muscular action, while stopping bristles functioncontrolling the descending rate and quantity of food. Atthe anterior plates, their cuticles are sclerotized at thedifferent level, and form grindstone with many spinesor denticles, which can crush and triturate foods fromthe crop together with circle muscular action.
In our research, different types of theproventriculi are focused on, with comparision of thespatial distribution of bark beetles in the Chinese whitepine. Basically, as an inlet of midgut, provent riculusplays a protective function and primary digestion onsolid food with potential poisonous material.Meanwhile, microhabitat variations could stronglyindicate the microdifference of proventriculi. Thus weexamined the ultrastructures of the proventriculi from16 species of bark beetles in the Chinese white pine bylight microscopy and scanning electron microscopy(SEM)in this study. We attempted to finddifferences, by comparative morphology ofproventriculi, to reveal the morpho-functionaladaptations to the subcortical environment in the hosttree P. armandii.
1 Materials and methodsThe adult specimens of bark beetles for this studywere collected directly from the bark of infested P.armandii over 30 years old on the south side of the Qinling Mountains(33° 36' N, 108° 28' E, 1 800-2 000 m a. s. l.)between Huoditang and Xunyangba of Ningshan County, Shaanxi Province, China, from Aprilto June 2009. In detail, twenty white pine trees underattack by bark beetles were random logged from Chinesewhite pine pure stand and mixed stands in three months. Two hundred forty square sample phloempieces, sampling unit the size of the diameter at thelocation on the tree trunk, were dissected from thelogged trees at 1 m intervals from the root-collar of thetrunk to upper trunk < 5 cm diameter upper of trunk.Each species of bark beetles was identified by maleseminal rods and external characters according to Yin etal.(1984). We examined 16 species of 10 generabelonging to six tribes: Hylastini, Hylurgini, Polygraphini, Dryocoetini, Ipini and Cryphalini(Wang et al., 2012; Bouchard et al., 2011; Yin et al., 1984)(Tab. 1).
Ten specimens of each species were dissected, and the chitinous plates of proventriculi were isolatedfrom the alimentary canal. Five proventriculi of eachspecies were transferred to Bouin's fixative solution for 24 h, dehydrated in ethanol series, and embedded inparaffin. Serial sections of 10 μm were cut on a rotarymicrotome, de-paraffinized and stained withhematoxylin-eosin. Five proventriculi of each speciesfor SEM(scanning electron microscopy)were digestedin 10% NaOH for 30 min before cleaning in Ultrasoniccleaners for 1h to washout food particles from the endosurfaceof chitinous plates. The chitinous plates weredried for 72 h at 4 ℃ and mounted on aluminium stubs, then sputter-coated with gold / palladium(40: 60)in a polaron E5400 high-resolution sputtercoater. Specimens were examinated in a JEOL T330 SEM operated at either 15 kV. Comparing proventriculiamong bark beetles, six characters were used fortaxonomic considerations(Tab. 1).
|
|
Within the basic morphological pattern describedin Dendroctonus armandi, some variation can beconsidered among the different bark beetle speciesanalyzed. Further more, the sclerotizated appendices ofeach chitinous plate, which are more or less differentin size, shape and number among these bark beetlesinclude transverse tooth lines, anterior margin, mediansuture, median denticles, basic denticles andsubstitute stopping bristles in anterior plate anddeclivity, masticatory brushes and stopping bristles inthe masticatory plate(Tab. 1).
Hylastes parallelus, Hylurgops longipillus, Tomicus piniperda and Dendroctonus armandi presentthe high density of tooth-like projections that formplates with 12-21 rows of transverse tooth lines(Fig. 2)in the proventriculi. Unlike 4 species above, Polygraphus polygraphus and Polygraphus sinensis havesharp median denticles and wide median suture withouttransverse tooth lines(Fig. 3A, 3B; Tab. 1). Dryocoetes hectographus and Dryocoetes autographu shave median denticles and wide median suture, andbasic denticles, which are arranged like a converse“V”shape, are located at the end of the column ofmedian suture(Fig. 3C, 3D; Tab. 1).
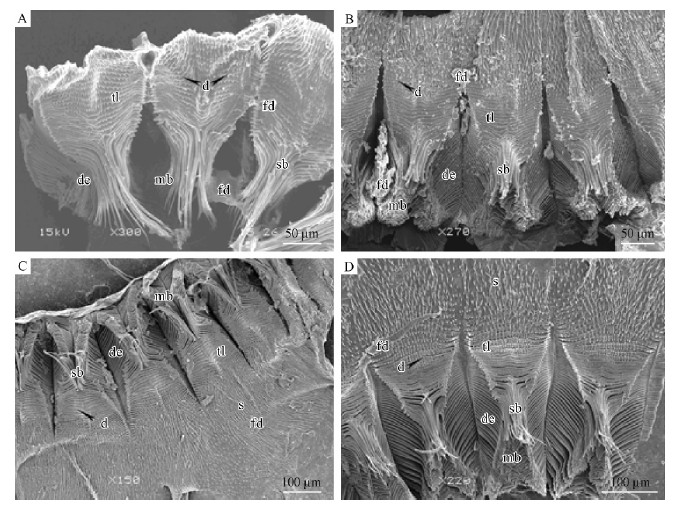 |
Fig. 2 SEM micrographs, chitinous plates of the proventriculi of stype A A. Dendroctonus armandi; B. Tomicus piniperda; C. Hylurgops longipilis; D. Hylastes parallelus. d: Denticles; de: Declivity; mb: Masticatory brushes; fd: Food; s: Spines; sb: Stopping bristles; tl: Transverse tooth lines. |
 |
Fig. 3 SEM micrographs, chitinous plates of the proventriculi of stype B A. Polygraphus sinensis; B. Polygraphus polygraphus; C. Dryocoetes hectographus; D. Dryocoetes autographus. am: Anterior margin; bd: Basic denticles; de: Declivity; mb: Masticatory brushes; md: Median denticles; ms: Median satures; fd: Food; s: Spines; sb: Stopping bristles. |
Among Pityogenes japonicus, Ips acuminatus, Ipssexdentatus, Ips mannsfeldi and Orthotomicus laricis, 2 columns of median denticles are linked with each otherby narrow median suture of the proventricular plate(Fig. 4A, 4B, 4C, 4D; Tab. 1), contrasting to widemedian suture and without median suture found in theother bark beetles analyzed. Cryphalus lipingensis, Cryphalus chinlingensis and Cryphalus pseudochinlingensis have 3 rows of basic denticles like3 rows of palisades, each row of which has long basicdenticles in the middle(Fig. 4F, 4G, 4H; Tab. 1).In Orthotomicus laricis, the appearance of mediansuture is sharper than those of Pityogenes japonicus, Ipsacuminatus, Ips sexdentatus and Ips mannsfeldi, andthe demarcation line between its basic denticles andmedian sutures is not distinguished because of theirsimilar appearance(Fig. 4E).
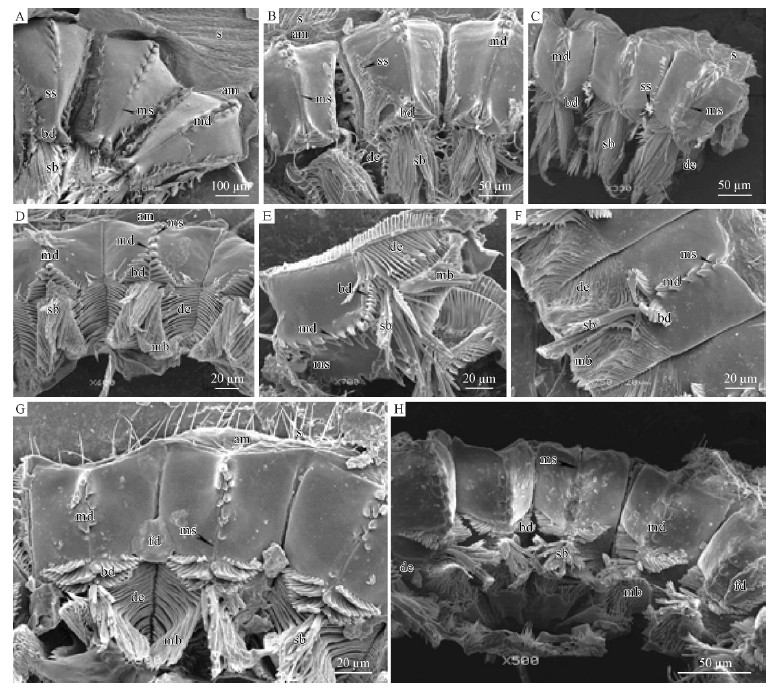 |
Fig. 4 SEM micrographs, chitinous plates of the proventriculi of stype C A. Ips sexdentatus; B. Ips acuminatus; C. Ips mannsfeldi; D. Pityogenes japonicus; E. Orthotomicus laricis; F. Cryphalus pseudochinlingensis; G. Cryphalus lipingensis; H. Cryphalus chinlingensis. am: Anterior margin; bd: Basic denticles; de: Declivity; mb: Masticatory brushes; md: Median denticles; ms: Median satures; fd: Food; s: Spines; sb: Stopping bristles; ss: Substitute stopping bristles. |
Similarly, three types of proventriculi areidentified according to the basic morphologicalcomparison of the chitinous plates.(1)Type A. Dendroctonus armandi as a representative share manycharacters with Tomicus piniperda, Hylastes parallelus, and Hylurgops longipilis. The obvious features in thistype are the developed transverse tooth lines withoutmedian denticles, basic denticles and substitutestopping bristles for weak degree of sclerotization(Fig 2).(2) Type B. This type appears as a medial degreeof sclerotization among Polygraphus polygraphus, P.sinensis, Dryocoetes hectographus, and D. autographus. Apparently, this type differs from Type Ain having the wide median sutures and developedmedian denticles, whereas the proventricular plates in Dryocoetes have developed basic denticles(Fig. 3).(3)Type C. The remaining 8 species belong to thistype in which the degree of sclerotization is high. Eightbeetles above all have the developed basic denticles, apart from developed median denticles. In Type C, themedian suture is very narrow, compared with Type B(Fig. 4).
3 DiscussionFor bark beetles, carbohydrates, vitamins, fiberand minerals are the most important nutritionalconstituents from pine cambium. When they invadedthe host trees the bark beetles with the assisting of theirassociated fungus can break the physical structure ofphloem and xylem tissue, block resin ducts of xylemtissue, and cause resin and water metabolic confusion, which will cause the host trees weaken, then, secondary bark beetles were induced to attack thesuppressed and dying phase of the pine(Xie et al., 2008; Christiansen et al., 1990; Ren et al., 1959). Li et al.(2009)thought that the content of total protein, lipid, and glycogen of the barks were related linearly tophysical life(healthy, suppressed and dying phase)ofChinese white pine. The degree of sclerotization of theproventriculi, to some extent, could possibly becorrelated with major types(the hardness and sappydegree)and nutrition of food from different part ofhealthy, suppressed, diseased, dying and decayingtrees(Pu et al., 2007). Our studies showed that thetypes of the proventriculi were consistent with thespatial distributions among sixteen bark beetles for ashared host(Chinese white pine)resource, generally.In spatial distribution of bark beetles in trunks, thefirst group(Hylastes parallelus, Dendroctonusarmandi, I. sexdentatus and Hylurgops longipilis)werecommon species at the low trunk(1-6 m); Thesecond group consisted of Dryocoetes hectographus, D.autographus, Polygraphus sinensis, P. polygraphusand I. mannsfeldi, I. acuminatus, O. larici andPityogenes japonicus, were concentrated at the middletrunks(7-12 m); The third group(C. lipingensis, C. chinlingensis, C. pseudochinlingensis and T.piniperda)were predominant species at the upper trunkand branches of Chinese white pines(11-16 m).Similarly, the species of the first group have the weakdegree of sclerotization plates except for I. sexdentatus, and fed on the thicker texture of phloem with the highcontent of total protein and lipid from healthy orsuppressed trees. The species at the middle trunks is acomplex group with the middle and high degree ofsclerotization plates, who ate thick texture with lowcontent of total protein and lipid from suppressed trees.The third group which ate thin texture of phloem andxylem with lower content of total protein and lipid fromdying tree, have the high degree of sclerotization platesexcept T. piniperda. So the morphological variationand the degree of sclerotization of anterior platereflected the subtle feeding habits of these bark beetles.
Our results indicated various types of proventriculimight undertake different detoxification in digestiveprocess of bark beetles. The action and structure ofanterior plate can prolong retention time of food, whichimprove the effective rate of neutrolizing toxic materialsmixed with digestive enzyme(e.g. PAL, PPO, SOD)from saliva, midgut and symbiotic fungus. To type Asuch as D. armandi, the bark beetles had the complexstructures and showed the highest detoxifying degree, than weaker in type B such as Polygraphus species, and the weakest in Type C such as Ips species. Thedifferences of proventricular types reflected theirdefense resistance to special physical phase of Chinesewhite pine.
The structure of the proventriculus usually hasconsiderable variations among different insects that feedon solid food with chewing mouthparts such as in thecockroach(Blattodea), cricket(Orthoptera), Rhynchophorinae and Hydrophilidae beetles(Boudreaux, 1980; Chapman, 1998; Fontanetti et al., 2002). The diversity in the morphology of theproventriculus of insects has led several authors to useit as an auxiliary character in systematics andphylogeny, with special emphasis on theproventriculus, whose morphological variability isrelated to the diversity of insect feeding habits(Rentz et al., 1990; Bland et al., 1991; Fontanetti et al., 2002; Szinwelski et al., 2009; Serrão, 2001; 2007).In the present study, these results indicate that themorphological structure of the proventriculus exhibitedsome divergence, and may supply auxiliary taxonomycharacteristics for the bark beetles in Chinese whitepine, especially useful for groups, since for manyspecies the external morphology is insufficient forcharacterization of genera and species. We also believethat these differences may reflect an adaption tovariations in diet to which these populations attackedand brood developed in Chinese white pine.
| [1] |
Balogun R A. 1969. The anatomy and histology of the proventriculus of Ips cembrae Heer (Coleoptera: Scolytidae). Proc Royal Ent Soc Lond,44 (10-12):158-161.( 1) 1)
|
| [2] |
Bland T G,Rentz D. 1991. Studies in Australian Gryllacrididae: the proventriculus as a taxonomic character. Invertebr Taxon,5 (2): 443-455.( 1) 1)
|
| [3] |
Boudreaux H B. 1980. Proventricular acanthae and their phylogenetic implications. Ann Entomol Soc Am,73 (2):189-196.( 1) 1)
|
| [4] |
Bouchard P,Bousquet Y,Davies A E,et al. 2011. Family-group names in Coleoptera (Insecta). ZooKeys,88: 1-972.( 1) 1)
|
| [5] |
Bu S H,Chen H. 2009. The Alimentary Canal of Dendroctonus armandi Tsai and Li (Coleoptera: Curculionidae: Scolytinae). The Coleopterists Bulletin,63 (4):485-496.( 1) 1)
|
| [6] |
Chapman R F. 1998. The Insects: Structure and Function 4 th ed.Cambridge university Press,Cambridge,UK.( 1) 1)
|
| [7] |
Chen H,Tang M. 2007. Spatial and temporal dynamics of bark beetles in Chinese white pine in Qinling Mountains of Shaanxi Province,China. Environ Entomol,42 (5):1124-1130.( 1) 1)
|
| [8] |
Christiansen E,Solheim H. 1990. The bark beetle associated blue-stain fungus Ophiostoma polonicum can kill various spruces and Douglas fir. Eur J For Path,20 (6-7):436-446.( 1) 1)
|
| [9] |
Díaz E, Arciniega O, Sánchez L, et al. 2003. Anatomical and histological comparison of the alimentary canal of Dendroctonus micans, D. ponderosae, D. pseudotsugae pseudotsugae, D.rupifenis,and D. terebrans (Coleoptera: Scolytidae). Ann Entomol Soc Am,96 (2):144-152.( 1) 1)
|
| [10] |
Díaz E,Cisneros R,Zúñiga G. 2000. Comparative anatomical and histological study of the alimentary canal of Dendroctonus frontalis (Coleoptera: Scolytidae). Ann Entomol Soc Am,93 (2): 303 -311.( 1) 1)
|
| [11] |
Fontanetti C S, Zefa E, Passeti F, et al. 2002. Morphological characterization and comparative analysis of the proventriculus from three species of Endecous saussure,1878 (Orthoptera: Gryllidae: Phalangopsinae). Entomotropica,17 (1):15-23.( 2) 2)
|
| [12] |
Klepzig K D,Smalley E B,Raffa K F. 1991. Combined chemical defenses against insects and fungi associated with a forest decline disease. J Chem Ecol,22: 1376-1388.( 1) 1)
|
| [13] |
Li Z (李臻),Chen H (陈辉),Wang S J (王胜军). 2009. Utilization of nutritients and mineral elements in Pinus armandii by Chinese white pine beetle (Dendroctonus armandi). Scientia Silvae Sinicae (林业科学),45 (11):98-103.( 1) 1)
|
| [14] |
Lopez-Buenfil J A,Valdez-Carrasco J,Equihua-Martinez A,et al.2001. El proventriculo como estructura para identificar generos Mexicanos de Scolytidae (Coleoptera). Folia Entomologica Mexicana,40:325-372.( 1) 1)
|
| [15] |
Miller D R,Borden J H,Slessor K N. 1989. Inter- and intrapopulation variation of the pheromone, ipsdienol produced by male pine engravers,Ips pini (Say)(Coleoptera: Scolytidae). J Chem Ecol,15: 233-247.( 1) 1)
|
| [16] |
Szinwelski N,Rodrigues M S,Pereira M R,et al. 2009. Proventriculus of Three Nemobiinae Crickets (Orthoptera: Grylloidea: Trigonidiidae). J Orthop Res,18 (1):59-63.( 1) 1)
|
| [17] |
Nobuchi A. 1969. A comparative morphological study of the proventriculus in the adult of the superfamily Scolytoidea (Coleoptera). Gov For Exp Sta Bull,224: 39-110.( 1) 1)
|
| [18] |
Pu X J (蒲晓娟),Chen H (陈辉). 2007. Relations between attacking of Dendroctonus armandi and nutrition and resistance materials of host trees (Pinus armandi). Journal of Northwest A&F University (西北农林科技大学学报),35 (3):106-110.( 1) 1)
|
| [19] |
Ren Z F (任作佛),Dang X D (党心德). 1959. Investigation and control of Dendroctonus armandi Tsai et Li in Qinling Mountain. J Northwest Agric Coll (西北农学院学报),(2):59-89.( 1) 1)
|
| [20] |
Rentz D C F,John B. 1990. Stuclies in Australian Gryllacrididae: Taxonomy,biology ecology and cytology. Invertebrate Taxonomy,3: 1053-1210.( 1) 1)
|
| [21] |
Rudinsky J A. 1966. Host selection and invasion by the Douglas-fir beetle,Dendroctonus pseudotsugae Hopkins,in coastal Douglas-fir forests. Can Entomol,98: 98-111.( 1) 1)
|
| [22] |
Serrão J E. 2001. A comparative study of the proventricular structure in corbiculate Apinae (Hymenoptera: Apidae). Micron,32 (4):379-385.( 1) 1)
|
| [23] |
Serrão J E. 2007. Proventricular structure in the bee tribe Augochlorini (Hymenoptera: Halictidae). Org Divers Evol,7 (3):175-180.( 1) 1)
|
| [24] |
Wallin K F,Raffa K F. 2002. Density-mediated responses of bark beetles to host allelochemicals: A link between individual behavior and population dynamics. Ecol Entomol,27 (4):484-492.( 1) 1)
|
| [25] |
Wang Z L (王志良),Zhang R Z(张润志). 2012. Taxonomic status of bark beetles (Coleoptera,Curculionidae). Acta Zootaxonomica Sinica(动物分类学报),37 (2):291-295.( 1) 1)
|
| [26] |
Wood S L. 1982. The bark and ambrosia beetles of north and central America (Coleoptera: Scolytidae),a taxonomic monograph. Great Basin Nat Mem,6: 1-1359.( 1) 1)
|
| [27] |
Xie S A (谢寿安),Lu S J(吕淑杰),Axel S,et al. 2008. Anatomical characteristics in xylem tissue of Pinus armandi infected by the bark beetle Dendroctonus armandi (Coleoptera: Scolytidae)and its associated blue-stain fungus Ceratocystis polonica. Acta Entomologica Sinica (昆虫学报),51 (12):1327-1333.( 1) 1)
|
| [28] |
Yin H F (殷慧芬),Huang F S (黄复生),Li Z L (李兆麟). 1984.Economic insect fauna of China. Fasc. 29. Coleoptera. Scolytidae.Science Press (科学出版社),Beijing,China.( 2) 2)
|
 2013, Vol. 49
2013, Vol. 49

