文章信息
- Yuan Fei, Luo Youqing, Shi Juan, Kari Heliövaara
- 袁菲, 骆有庆, 石娟, KariHeliövaara
- Different Attractive Effects and EAG Responses of Ips subelongatus(Coleoptera, Scolytidae) and Its Predator Thanasimus substriatus (Coleoptera: Cleridae) to Ipslures
- 不同含量引诱剂对落叶松八齿小蠹及其天敌红胸郭公虫的引诱
- Scientia Silvae Sinicae, 2012, 48(6): 89-94.
- 林业科学, 2012, 48(6): 89-94.
- DOI:
-
文章历史
- Received date: 2010-10-29
- Revised date: 2011-05-06
-
作者相关文章
2. Key Laboratory of Forest Protection of State Forestry Administration Research Institute of Forest Ecology, Environment and Protection, CAF Beijing 100091;
3. Department of Applied Biology, University of Helsinki Helsinki FIN-00014
2. 中国林业科学研究院森林生态环境与保护研究所 森林保护学国家林业局重点实验室 北京 100091;
3. 赫尔辛基大学应用生物学学院 芬兰 FIN-00014
The larch bark beetle Ips subelongatus (Coleoptera, Scolytidae) is a serious wood insect borer in northern China which attacks Larix sibirica, Larix gmelinii, Larix principis-rupprechtii, Larix olgensis and other larch species in its natural range (Zhang et al., 1992). This bark beetle prefers weakened, windthrown and burned trees, and also attacks healthy larch trees at high population densities (Zhang et al., 1989: 1992). Zhang et al. (2000) have reported several potential pheromone components from the hindgut of I. subelongatus males consisting of the major ipsenol and ipsdienol as well as minor 3-methyl-3-buten-1-ol. Furthermore, the racemic ipsenol can attract both sexes of I. subelongatus significantly (Zhang et al., 2007).
Predators of bark beetles, especially clerid beetles (Coleoptera, Cleridae) can also be attracted by the aggregation pheromone of bark beetle (Selander et al., 1980; Zumr, 1983; Schroeder, 1988; Avtzis, 1991; Zhao et al., 1993). For example, Norway spruce (Picea abies) logs baited with synthetic pheromone of the bark beetle Ips typographus, attracted about 17 times more of the clerids Thanasimus formicarius Linnaeus 1758. (Coleoptera: Cleridae) and T. rufipes Brahm 1797 than did logs without pheromone (Zumr, 1983). Both sexes of T. formicarius responded to the pheromone (Bakke et al., 1978). The predator T. formicarius also has olfactory receptor cells for many bark beetle pheromones, which indicated that T. formicarius is able to detect and discriminate between many bark beetle species (Tommeras, 1985). Unlike T. formicarius, little is known about the attractive effect of T. substriatus to aggregation pheromones of I. subelongatus.
1 Methods and materials 1.1 Field trapping experimentField trapping experiment was carried out in Aershan forest area (119°51′—120°57′E; 47°07′—47°55′N; 820-1 745 m a.s.l.), Inner Mongolia, China, from 20 June till 29 August 2008. Three compartments of forest growing Larix gmelinii were selected. The average age of the trees was 25 years, the height of the trees was 12-14 m and their diameter at breast height 11-15 cm. Each forest compartment was divided into two subcompartments so that the average age, tree height, diameter at breast height and crown density were similar. Because the attracting effect of ipslure is about 200 m, the distance between the two subcompartments was more than 200 m. Six traps were set at the height of 1.5 m on the tree trunk along a line in each subcompartment. The distance between the traps was 50 m. All the six traps in a subcompartment were baited either with Ipslure 1 (3 replicates) or with Ipslure 2 (3 replicates) using 36 traps in total. The ipslures were renewed in 1st August to ensure their attractiveness. The catch of the attracted I. subelongatus and T. substriatus adults was counted at the intervals of 7 days.
1.2 Insect collectionNewly emerged I. subelongatus and active T. substriatus adults were collected for antennal recordings from naturally attacked Larix gmelinii trees in mid July 2008. The sexes of I. subelongatus were separated on the basis of the second and third elytral spine differences (Yin, 1984; Zhang et al., 1992). The sexes of T. substriatus were separated on the basis of mating couples. Thousands of living bark beetles and hundreds of T. substriatus were maintained on fresh L. gmelinii bark in wooden collection boxes.
1.3 Electroantennogram responsesThe two different ipslures were tested on the antennae of I. subelongatus and T. substriatus by using electroantennogram (EAG) (Syntech Company). The tested ipslure samples were dissolved in hexane at a concentration of 0.5%. Pure hexane was used as control. Antennae of I. subelongatus and T. substriatus were cut off at the basal part, and a small part of the antennal end were cut away. Then the two ends of the antennae were connected to electrodes with electrode gel. The outlet for the EAG was inserted into a humidified airstream (100 mL·s-1) over the antennal preparations. Stimuli were given by applying 20 μL of ipslures or pure hexane onto a piece of filter paper (0.5 cm × 3 cm) in a Pasteur pipette. Gas flow for pulse stimulation was 2 mL·s-1 and the stimulation time was 0.2 s. Each stimulation was followed by a minimum of 60 s purge period of filtered air to ensure the recovery of antennal receptors. Each test had eight replicates both for females and males, and each antenna was tested three times.
1.4 GC-MS analysesThe two different liquid ipslure samples were tested by using Gas Chromatography-Mass Spectrometry (GC-MS). GC analysis conditions were as follows: Column AB-5/MS (30 m×0.25 mm×0.25 μm film thickness), 0.4 μL as the injection volume, pressure of carrier gas 20 kPa, programmed from 50 ℃, rising to 220 ℃ at 20 ℃·min-1 and held for 1 min; then rising to 290 ℃ at 10 ℃·min-1 and held for 15 min. MS conditions: EI-source, electron energy 70 eV, mass range 29~540 u, scanning speed 0.6 s·scan-1. GC/MS interface temperature 250 ℃, ion source temperature 200 ℃ with filament current 350 μA.
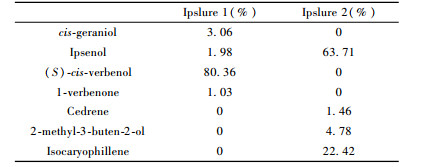
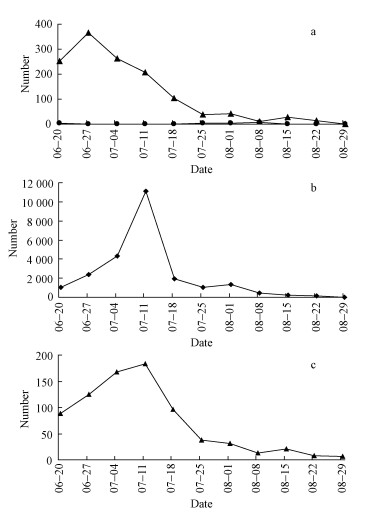
2 Results 2.1 Field trappingIn the field trapping experiment, 26 215 bark beetles and predators were caught with 36 traps. 16 I. subelongatus and 1 319 T. substriatus were attracted by Ipslure 1, while 24 104 I. subelongatus and 776 T. substriatus were attracted by Ipslure 2.
Ipslure 1 attracted much more T. substriatus than I. subelongatus. Only 16 I. subelongatus individuals were attracted by Ipslure 1 in 18 traps (Fig. 1a). On the contrary, the highest number of I. subelongatus attracted by Ipslure 2 exceeded 10 000 during the period of 7 days (Fig. 1b), but only 183 T. substriatus were caught (Fig. 1c). Not only I. subelongatus and its predators, but also representatives of other insect orders such as Hemiptera (10 individuals), Lepidoptera (5 individuals) and Hymenoptera (24 individuals) were attracted by Ipslure 1. Ipslure 2 attracted only 52 long horned beetles and 12 individuals of other insect orders showing high specificity to I. subelongatus. This phenomenon was surprising since both ipslures belong to aggregation pheromone components of I. subelongatus.
|
Fig.1 Number of I. subelongatus and T. substriatus attracted by two ipslures from 20 June till 29 August, 2008 a:I. subelongatus (●) and T.substriatus (▲) attracted by Ipslure 1; b: I. subelongatus attracted by Ipslure 2; c:T. substriatus attracted by Ipslure 2. |
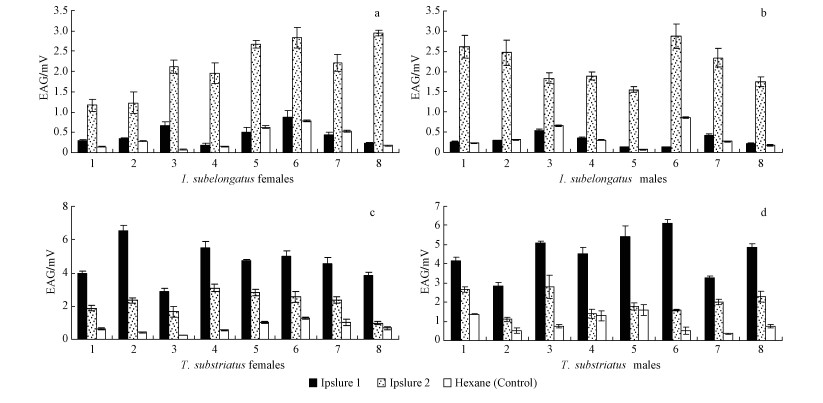
Ipslure 1, Ipslure 2 and pure hexane were tested on antennae of both sexes of I. subelongatus and its predator T. substriatus. The response of I. subelongatus (both sexes) was much stronger to Ipslure 2 than to Ipslure 1 (Fig. 2 a, b). In contrast, the response of T. substriatus (both sexes) to Ipslure 1 is much stronger than to Ipslure 2 (Fig. 2c, d). No significant differences were observed between the sexes of I. subelongatus or with the sexes of its predator (P>0.5 in each case, ANOVA). Both ipslures, but especially Ipslure 1, strongly attracted T. substriatus (Fig. 2c, d). These results are in accordance with the results from the field experiments: Ipslure 1 attracted much more T. substriatus than I. subelongatus, while reverse results were obtained for Ipslure 2.
|
Fig.2 EAG responses of both sexes of I. subelongatus (a, b) and T. substriatus (c, d) to ipslures Vertical bars show standard errors of the mean. Numbers refer to repetitions |
GC-MS determination showed that the current main effective components of Ipslure 1 were cis-geraniol, ipsenol, (S)-cis-verbenol and 1-verbenone, which account for 3.06%, 1.98%, 80.36% and 1.03%, respectively (Tab. 1). Obviously, (S)-cis-verbenol, which belongs to bark beetles components, was the major aggregation pheromone of Ipslure 1. Besides (S)-cis-verbenol, there were other bark beetles components such as ipsenol, 1-verbenone and plant volatile substances from the hosts such as cis-geraniol and a pinene. Results for Ipslure 2 were combined with the information from manufacturer and GC-MS analysis. The main effective components of Ipslure 2 were 2-methyl-3-buten-2-ol, ipsenol (purity 96.9%), cedrene and isocaryophillene, which account for 4.78%, 63.71%, 1.46% and 22.42%, respectively (Tab. 1). According to the present results, Ipslure 1 and Ipslure 2 could also be called (S)-cis-verbenol-dominant blend and ipsenol-dominant blend, respectively.
|
|
Both the field trappings and EAG experiments revealed that both sexes of T. substriatus showed strong responses to (S)-cis-verbenol-dominant blend. This indicated that a high concentration of (S)-cis-verbenol with minor surplus of ipsenol, 1-verbenone and volatile substances from the host plant could attract a high number of predators but nearly no I. subelongatus. However, both sexes of I. subelongatus showed strong responses to ipsenol-dominant blend. This, in turn, indicated that a high concentration of ipsenol with minor surplus of 2-methyl-3-buten-2-ol and plant volatile substances could efficiently attract I. subelongatus. Also the pedators T. substriatus could be attracted, although the effect was not as good as with the (S)-cis-verbenol-dominant blend.
3 DiscussionThis is the first detailed study on the determination of which pheromone component or component blend plays an important role in attracting the bark beetle I. subelongatus and its predator T. substriatus. Volatile substances of host plants (mainly terpenic compounds) are the primary attraction cues to most bark beetles. After attacking the host plants many bark beetles release pheromones. These pheromones combined with the volatile substances of host plants then induce the secondary attraction of the bark beetles. Combination of the volatile substances of the host plants and sex or aggregation pheromones from the bark beetles form the attractive system of bark beetles (Borden et al., 1998; Erbilgin et al., 2000). According to the present analyses of Ipslure 2, ipsenol with minor surplus of 2-methyl-3-buten-2-ol and plant volatile substances cedrene and isocaryophillene (accounting together for 23.88%) could efficiently attract I. subelongatus. However, the volatile substances cis-geraniol and an unidentified pinene in Ipslure 1 only account for 6.27%. This concentration was so small (some compounds probably lost during the long-time preservation) that the combination compound attracted only a high number of predators T. substriatus but almost no I. subelongatus.
GC-MS determination of Ipslure 2 indicated that the high concentration of ipsenol had a crucial role in attracting high numbers of I. subelongatus. Previously, several species of bark beetles have been reported to have strong responses to ipsenol. For I. cembrae, ipsdienol and 3-methyl-3-buten-1-ol have been reported as parts of a synergistic mixture together with ipsenol, forming a three-component aggregation pheromone system (Stoakley et al., 1978; Renwick et al., 1979). When ipsdienol was left out of the three-component blend, the attraction of I. cembrae was totally eliminated (Stoakley et al., 1978). Field trapping experiments (Zhang et al., 2007) showed that the EAD-active, major male-hindgut component, racemic ipsenol, was the only individual compound that significantly attracted both sexes of I. subelongatus, whereas other compounds, ipsdienol and 3-methyl-3-buten-1-ol, were unattractive. However, different bark beetle species have shown varying responses to ipsenol and other aggregation pheromones. Studies of antennal olfactory receptors of T. formicarius and I. typographus showed that I. typographus is more sensitive to ipsdienol and (S)-cis-verbenol than ipsenol, but for both species (S)-(+)-ipsdienol is the most effective of the tested compounds (Hansen, 1983).
GC-MS determination of Ipslure 1 indicated that the high concentration of (S)-cis-verbenol has a crucial role in attracting high numbers of T. substriatus. However, previous research by Bakke et al. (1981) showed that a closely related clerid beetle T. formicarius responded mainly to racemic ipsdienol and ipsenol but less to (S)-cis-verbenol. Moreover, I. typographus was more sensitive to (S)-cis-verbenol than ipsenol (Hansen, 1983). Female and male antennae of I. paraconfusus were more sensitive to the pheromonal component, (S)-cis-verbenol, than to its antipode, (R)-cis-verbenol (Light, 1983). Our results are the first to show that T. substriatus responds strongly to (S)-cis-verbenol-dominant blend. This conclusion was further supported by EAG experiments showing that the response of T. substriatus (both sexes) to (S)-cis-verbenol-dominant blend is much stronger than to ipsenol-dominant blend.
The most striking result of the present investigation was the discovery that I. subelongtus and its predator T. substriatus respond in significantly different ways to (S)-cis-verbenol-dominant blend and ipsenol-dominant blend. Ipsenol-dominant blend attracted a high number of I. subelongtus and its predator T. substriatus. On the contrary, (S)-cis-verbenol-dominant blend attracted a high number of predators but almost no I. subelongtus. These results can be applied in population monitoring of the beetles and further in biological control of I. subelongatus.
| [] | Avtzis N. 1991. Side captures in bark beetle pheromone traps in northern Greece. Anz. Schaedlingskd. Pflanzenschutz Umweltschutz, 64(1): 13–14. DOI:10.1007/BF01906191 |
| [] | Bakke A, Kvamme T. 1978. Kairomone response by the predators Thanasimus formicarius and Thanasimus rufipes to the synthetic pheromone of Ips typographus. Norw J Entomol, 25(1): 41–44. |
| [] | Bakke A, Kvamme T. 1981. Kairomone response in Thanasimus predators to pheromone components of Ips typographus. J Chem Ecol, 7(2): 305–312. DOI:10.1007/BF00995753 |
| [] | Borden J H, Wilson I M, Gries R, et al. 1998. Volatiles from the bark of trembling aspen, Populus tremuloides Michx. (Salicaceae) disrupt secondary attraction by the mountain pine beetle, Dendroctonus pseudotsugae Hopkins (Coleoptera: Scolytidae). Chemoecology, 8(2): 69–75. DOI:10.1007/PL00001806 |
| [] | Erbilgin N, Kenneth F, Raffa K F. 2000. Opposing effects of host monoterpenes on responses by two sympatric species of bark beetles to their aggregation pheromones. J Chem Ecol, 26(1): 2527–2548. |
| [] | Hansen K. 1983. Reception of bark beetle pheromone in the predaceous clerid beetle Thanasimus formicarius Coleoptera Cleridae. J Comp Physiol A Sens Neural Behav Physiol, 150(3): 371–378. DOI:10.1007/BF00605026 |
| [] | Light D M. 1983. Sensitivity of antennae of male and female Ips paraconfusus (Coleoptera: Scolytidae) to their natural aggregation pheromone and its enantiomeric components. J Chem Ecol, 9(5): 561–584. DOI:10.1007/BF00990410 |
| [] | Renwick J A A, Dickens J C. 1979. Control of pheromone production in the bark beetle, Ips cembrae. Physiol Entomol, 4(4): 377–381. DOI:10.1111/j.1365-3032.1979.tb00630.x |
| [] | Schroeder L M. 1988. Attraction of the bark beetle Tomicus piniperda and some other bark and wood-living beetles to the host volatiles alpha pinene and ethanol. Entomol Exp Appl, 46(3): 203–210. DOI:10.1111/eea.1988.46.issue-3 |
| [] | Selander J, Nuorteva M. 1980. Use of synthetic pheromones for the control of spruce bark beetles in a heavily infested spruce stand. Silva Fenn, 14(2): 113–121. |
| [] | Stoakley J T, Bakke A, Renwick J A A, et al. 1978. The aggregation pheromone system of the larch bark beetle Ips cembrae Heer. Z Angew Entomol, 86(1-4): 174–177. |
| [] | Tommeras B A. 1985. Specialization of the olfactory receptor cells in the bark beetle Ips typographus and its predator Thanasimus formicarius to bark beetle pheromones and host tree volatiles. J Comp Physiol A Sens Neural Behav Physiol, 157(3): 335–342. DOI:10.1007/BF00618123 |
| [] | 殷 惠芬, 黄 复生, 李 兆麟. 1984. Economic Insect Fauna of China. 北京, 科学出版社. |
| [] | Zhang Q H, Niemeyer H. 1992. Morphological characteristics for sexing living adults of Ips cembrae (Heer) (Col., Scolytidae). J Appl Entomol, 114: 403–409. DOI:10.1111/jen.1992.114.issue-1-5 |
| [] | 张 庆贺, 刘 篆芳, 孙 玉剑, et al. 1989. 东北林业大学学报, 18(6): 14–18. |
| [] | Zhang Q H, Schlyter F, Chen G F, et al. 2000. Pheromone components in the larch bark beetle, Ips cembrae, from China: quantitative variation among attack phases and individuals. J Chem Ecol, 26(4): 841–858. DOI:10.1023/A:1005447922939 |
| [] | Zhang Q H, Schlyter F, Chen G F, et al. 2007. Electrophysiological and behavioral responses of Ips subelongatus to semiochemicals from its hosts, non-hosts, and conspecifics in China. J Chem Ecol, 33(2): 391–404. DOI:10.1007/s10886-006-9231-8 |
| [] | 赵 博光, 张 松山. 1993. 南京林业大学学报, 17(1): 84–90. |
| [] | Zumr V. 1983. Effect of pheroprax synthetic pheromones on the coleopterous predators of the spruce bark beetle Ips typographus. Z Angew Entomol, 95(1): 47–50. |
 2012, Vol. 48
2012, Vol. 48