文章信息
- 王明霞, 袁玲, 周志峰, 杨红军, 黄建国
- Wang Mingxia, Yuan Ling, Zhou Zhifeng, Yang Hongjun, Huang Jianguo
- 铝对外生菌根真菌草酸分泌及氮磷钾吸收的影响
- Efflux of Oxalate and Uptake of Nitrogen, Phosphorus and Potassium by Ectomycorrhizal Fungal Isolates in Vitro in Response to Aluminum Stress
- 林业科学, 2012, 48(2): 82-88.
- Scientia Silvae Sinicae, 2012, 48(2): 82-88.
-
文章历史
- 收稿日期:2010-12-10
- 修回日期:2011-04-21
-
作者相关文章
It is universal in acidic soils that Aluminum(Al) resistant plants,especially for trees.There are many ways for trees to adapt to acidic soils; one of them is to develop ectomycorrhizal(ECM)symbioses with fungi to alleviate Al toxicity.Ectomycorrhizas can be found widely in subtropical and temperate forests.The beneficial effect of ECM fungi on the resistance of host trees to Al stress is widely reported in literature (Cumming et al., 1990; Ahonen-Jonnarth et al., 2003; Gu et al., 2005).
The survival rate of trees in acidic soils was enhanced greatly by ECM fungi roots colonization(Yu et al., 2002).Al3+ could replace the intracellular Ca2+ in plant roots,thereby to inhibit root elongation and subsequently the uptake of nutrients,for example,Mg2+,K+ and H2PO3-/HPO23-(Koyama et al., 2001; Barceló et al., 2002; Postma et al., 2005).However,ECM fungi could retain Al in the external fungal hyphae and mantle around the root tips and then reduced intracellular Al in mycorrhizal roots(AhonenJonnarth et al., 2003; Moyer-Henry et al., 2005).Gu et al.(2005) reported the growth promotion of Pinus massoniana seedlings following inoculation of Laccaria bicolor,the growth promotion was related to the increase of nutrient uptake such phosphorus,magnesium,calcium and potassium alleviating Al toxicity in acidic soils in southwest China.Phosphorus could precipitate extracellular Al3+ to reduce Al concentrations in plant cells.Bivalent cations,for example,Ca2+ and Mg2+,could repel Al3+ out of cells by charge balance(Smith et al., 1997).Cumming et al.(1990) noted that P.tinctorius inoculation protected pine seedlings(P.rigida)from the adverse effects of Al through the increment of phosphorus acquisition. The growth inhibition of P.sylvestris seedlings produced by Al was diminished with concomitant increment of nutrient uptake,particularly magnesium and calcium,following ectomycorrhizal fungus infection (Ahonen-Jonnarth et al., 2003).Moreover,low molecular weight organic acids(LMWOA)excreted by ECM fungi play a vital role in Al detoxification through Al chelation(Smith et al., 1997).Oxalate from ECM fungi is one of most important LMWOA,which has been found commonly in culture media of ECM fungi and in soils around the mycorrhizal roots(Lapeyrie et al.,1987;Lapeyrie,1988; Paris et al., 1996; Gharieb et al., 1999; Cumming et al., 2001; Arvieu2003; Casarin et al., 2003).This organic acid can form a stable Al chelate,[A1(C2O4)3]3- with 2.0× 1016 of stable coefficient,as a detoxification agent.Oxalate synthesis in EMC fungi could thus be one of most efficient ways to eliminate Al toxicity (Malajczuk et al., 1982).
Al toxicity occurs frequently in acid forest soils in southwest China.Though alleviation of Al toxicity to trees by ECM fungi is well known,the fungal species varied greatly in their abilities to improve the resistance of their hosts to Al toxicity.Southwest China is rich in ECM fungi but information on oxalate efflux and nutrient absorption by the local ECM fungi in response to Al stress is scarce.In the present study,we investigated the mycelial growth,nutrient uptake,and oxalate efflux by four ECM fungal isolates isolated from acidic and calcareous soils.The main objective is to prepare the Al resistance of EMC fungi from different ecosystems,to provide primary information on the fungal selection for nurse bed inoculation,and to give more insights into fungal Al resistance.
1 Materials and methods 1.1 Fungal strains and experimental treatmentsFour species of ectomycorrhizal fungi were used in the present study:Lactarius deliciosus,Suillus luteus,Suillus grevillei and Cenococcum geophilum were maintained in pure culture by successive transfer on Pachlewski agar medium.The strains of L.deliciosus and S.luteus were originally isolated basidiocarp growing in acidic soils(pH less than 4.5) in Chongqing(Jiang et al., 2002),Southwest China,and S.grevillei and C.geophilum from isolated basidiocarp growing in calcareous soils(pH 6.5~7.5) in Inner Mongolia,Northwest China(all from mycorrhizal Pinus tabulaeformis).Mycelia for inoculation were grown on Pachlewski agar medium for 3 to 4weeks prior to experiment.The medium contained tartrate 0.5g·L-1,KH2PO4 1.0g·L-1,MgSO4 0.5g·L-1,glucose 20.0g·L-1,maltose 5.0g·L-1,vitamin B1 0.1g·L-1,agar 20.0g·L-1 and 1 ml·L-1 microelement solution.[1L microelement solution contained H3BO3 8.45g,MnSO4 5.0g,FeSO4 6.0g,CuSO 40.625g,ZnCl2 2.27g,and(NH4)2MoO4 0.27].
Experimental treatments were established by adding Al2(SO4)3·18H2O into Pachlewski liquid medium at the following concentrations of Al3+ :0,0.20,0.40,and 1.00mmol·L-1,which were referred to as no Al(blank control),low Al,middle Al,and high Al,respectively,unless otherwise stated.Medium pH was adjusted to 4.50with HCl and NaOH,and 20 mL of the liquid medium was transferred to a 150mL Erlenmeyer flask and steamsterilized at 121℃ for 30min.Each flask was inoculated with a mycelial plug(6mm in diameter) and incubated without agitation for 3 weeks at(25± 1)℃in the dark.
1.2 Sampling and analysisThe fungal mycelium was harvested by filtration,washed with double distilled water and oven dried at (85± 2)℃ for 24h.The dry weights of fungal mycelia were recorded.The biomass was digested in H2SO4-H2O2 solution,then N concentration were obtained by Kjeldahl methods,P by the molybdenumblue method,and K by a flame photometry(Page,1982).The incubation solution together with washing filtrate was collected in a 25mL volumetric flask and analyzed for oxalate by high performance liquid chromatography(D-7000Hitachi,Japan)using detection at 210nm.Sample solutions(20μL)were injected into an Ion-300organic acid analysis column (Phenomenex,Torrance,CA,USA)with 2.5mmol·L-1 H2SO4 mobile phase at 0.5mL·min-1. Standards of oxalate was prepared and run before and after the Sample solutions.
1.3 Statistical analysisTreatment effects were evaluated by analysis of variance using the SAS(2004)statistical software package(SAS Institute, 2004, Cary,NC). Significant differences between means were tested by Fisher’s protected LSD.
2 Results and analysis 2.1 Fungal growthThe four fungal isolates varied significantly in growth(Fig. 1).S.luteus showed the highest mean biomass(64mg dw·flask-1),followed by L.deliciosus (43.05mg dw·flask-1),S.grevillei(26.25mg dw· flask-1),and C.geophilum(20mg dw·flask-1). Compared to the blank treatment,L.deliciosus and S. luteus showed less reduction in biomass than C. geophilum and S.grevillei in response to Al stress,particularly at higher Al concentrations.

|
图 1 Biomass of the four ectomycorrhizal fungal isolates in culture solution with different Al concentrations
 Blank control (without Al); Blank control (without Al);  Low Al; Low Al;  Middle Al; Middle Al;  High Al. By analysis of variance,the F ratios for fungal isolate,biomass and Al concentration were all highly significant (P<0. 05). The same below. High Al. By analysis of variance,the F ratios for fungal isolate,biomass and Al concentration were all highly significant (P<0. 05). The same below.
|
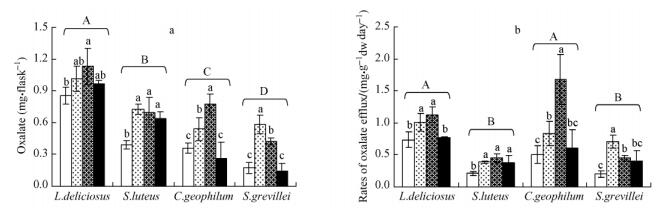
Accumulation of oxalate in culture solution varied significantly between the fungal isolates(Fig. 2a).L. deliciosus showed the highest mean of oxalate accumulation(0.99mg·flask-1),followed by Suillus luteus(0.61mg·flask-1),C.geophilum(0.48mg· flask-1),and S.grevillei(0.3mg·flask-1). Accumulation of oxalate first increased with increasing of Al concentrations from without Al to low Al or medium Al,then decreased at high Al.The highest accumulation of oxalate was 1.13mg·flask-1 for L. deliciosus,0.77mg·flask-1 for C.geophilum,0.73mg·flask-1 for S.luteus,and 0.58mg·flask-1 for S.grevillei under Al stress.
|
图 2 Effects of Al concentrations in culture solution on efflux of oxalate by ectomycorrhizal fungal isolates (a: Accumulation of oxalate in culture solution; b: Exudation rate for oxalate.) |
The mean exudation rate for oxalate calculated by dividing accumulation of oxalate by fungal growth rate. The exudation rates of oxalate by C.geophilum and L. deliciosus were0.91mg·g-1 dw day-1,higher than S. grevillei(0.44mg·g-1 dw day-1) and S.luteus (0.36mg·g-1 dw day-1)(Fig. 2b).The changes in exudation rate for oxalate in response to Al stress show a pattern similar to the accumulation.The exudation rate increased as Al concentrations increased and reached to the maximum at low or middle Al,and then decreased at high Al.C.geophilum showed the highest exudation rate about 1.7mg·g-1 dw day-1 and Suillus luteus the lowest about 0.5mg·g-1 dw day-1 in solution with middle Al.
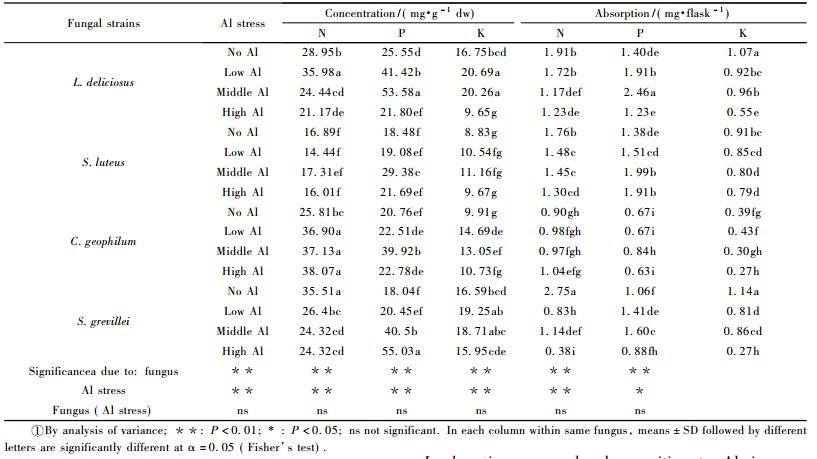
2.3 Concentration and accumulation of nitrogen,phosphorus and potassium by fungal isolatesThe concentrations of N,P and K fluctuated significantly between the fungal species(Tab. 1).The mean concentration of N ranged from 16.16mg·g-1 dw (S.luteus)to 32.93mg·g-1 dw(C.geophilum),P from 22.16mg·g-1 dw(S.luteus)to 35.59mg·g-1 dw(L. deliciosus),and K from 10.05mg·g-1 dw(S.luteus)to 17.54mg·g-1 dw(S.grevillei).The concentrations of N and K in response to Al stress vary from species to species.However,the concentration of P increased significantly in S.luteus and C.geophilum treated at middle Al,L.deliciosus at both low and middle Al,and S.grevillei at both middle and high Al as compared to the blank and other Al treatments.
|
|
Larger amount of N,P,and K were absorbed by L.deliciosus and S.luteus than by C.geophilum and S.grevillei in most cases of Al stress.N and K absorbed by fungal isolates decreased in response to Al stress except N in S.luteus which almost remained constant in the treatments with and without Al.However,P absorption increased significantly in C.geophilum at middle Al,L.deliciosus and S.grevillei at both low and middle Al,and in S.luteus at both middle and high Al as compared to the blank and other Al treatments.
3 Discussion and ConclusionsECM fungal species varaibly resist to Al stress. Jongbloed et al.(1992)found that Lactarius rufus and L.hepaticus were already sensitive to Al in pure culture at the concentration of 0.03mmol·L-1 Al3+ in contrast to Laccaria bicolor which was Al tolerant in vitro.In Vitro fungal growth could be an indicator of their sensitivity of Al resistance(Schier et al., 1996). ECM fungi with high abilities to adapt Al stress,for example Pisolithus tinctorius isolate,changed little even increased in the growth rate in liquid culture medium with Al less than 0.5mmol·L-1 (Cumming et al., 2001).
In the present experiment,the two isolates from acidic soils(pH<4.5)(L.deliciosus and S.luteus) showed less reduction in biomass,compared to those from the calcareous soils(S.grevillei and Cenococcum geophilum)in response to Al stress.Artificial reductions in pH and increases of active Al concentrations could affect the ectomycorrhizal species in soils,even those infecting the roots of Pinus sylvestris(Dighton et al., 1987).In Europe,acidification and Al toxicity in forest soils have been implicated in the decline of mycorrhizal fungi (Arnolds,1991).Such changes as a result of exposure of ECM fungi to Al may reflect the lost of Alsensitive species but survival of Al-resistant by evolution and natural selection in acidic soils. Therefore,it is reasonable to suggest that the fungal species,L.deliciosus and S.luteus originally isolated from acidic soils in southwest China could be more Al resistant than S.grevillei and C.geophilum.
While variations in Al tolerance among ectomycorrhizal species have been noted,the underlying mechanisms are still poorly elucidated.In the fungi,metal tolerance has been linked in some cases to extra-cellular chelation by organic compounds (Gadd,1993).Compared with other organic acids,oxalate has a high capacity to complex multivalent cations(Lapeyrie et al., 1987).Oxalate exudation is common among ECM fungi and its over excretion probably contributed to the metal tolerance of Beauveria caledoonica(Foramina,2005).In the present study,L.deliciosus and S.luteus showed higher oxalate accumulation in culture solution with and without Al added,than C.geophilum and S.grevillei.Due to the high stability coefficient of[A1(C2O4)3]3-(2.0×1016),oxalate ions can complex Al3+ ions and reduce the concentrations of active Al in soil solution near the hyphae and thus reduce the Al toxicity to the host trees by increasing oxalate efflux(Cromack et al., 1979). Similarly,the high accumulation of oxalate could also reduce Al3+ concentrations in liquid culture medium contained in Erlenmeyer flask and reduce the Al toxicity to the fungi.This result could explain the better growth of L.deliciosus and S.luteus under the exposure to Al.However,the exudation rate for oxalate by S.luteus was lowest in culture solutions with and without Al,indicating that the high accumulation of oxalate produced by S.luteus,the assumed Alresistant fungus,resulted from large fungal biomass. Therefore,the variable indices for scaling oxalate efflux could result in different explanations on the relationship between oxalate efflux and Al resistance of EMC fungi.The total amount of oxalate efflux could be more reasonable to comment on the role of oxalate effused from ECM fungi in Al stress.
ECM fungi play an important role in enhancing uptake of mineral nutrients for many tree species (Smith et al., 1997).The external hyphae from ectomycorrhizas have a suite of attributes to access N,P and K from recalcitrant sources(van Schöll et al., 2008; Yuan et al., 2004; 2005).Our data indicate generally higher accumulation of N,P,and K in Suillus luteus and L.deliciosus than S.grevillei and C. geophilum irrespective of the changes in Al3+ concentrations.The results demonstrate that Al stress had little influence on mineral nutrients uptake by EMC fungi resistant to Al.The enough nutrients absorbed by L.deliciosus and S.luteus could satisfy the growth of fungal hyphae and result in less biomass reduction under exposure to Al.The concentrations of N and K in fungal isolates changed in response to Al stress.There are still no unequivocal evidences for N and K to involve in Al resistance by both ECM fungal isolates and mycorrhizal trees(Meyer,1985). Analysis of N and K in fungal hyphae had also failed to demonstrate unequivocally the influence of Al on their concentrations in ECM fungal isolates.There was some evidence for increased concentrations of N and K by trees from soil following mycorrhizal inoculation in acidic soils,but in other cases N and K had been found at lower concentrations in mycorrhizal plant tissues compared to controls(Smith et al., 1997).The differences in concentrations of N and K among experimental fungal isolates in response to Al stress reflected their uncertain alterations and functions in ECM fungi for Al resistance.More investigations were necessary to carry out to understand the effects of Al on the concentration of N and K in ECM fungi.
Appropriate Al concentrations in culture solution increased significantly P concentrations in the four fungal isolates.Gerlitz(1996)noted that an Altolerant isolate of S.bovinus exhibited high P uptake capacity and formed large amount of intracellular polyphosphate(a strong Al chelator)under exposure to Al.Al concentrations in P.tinctorius isolate mycelia increased with Al concentrations increased in culture media(Cumming et al., 2001).The simultaneous increase of Al and P was associated with limited transfer of Al into the shoot in mycorrhizal plants (Egerton-Warburton et al., 1993; Gu et al., 2005). Therefore,it is reasonable to suggest that the increment of P concentration could benefit to reduce the concentrations of active Al in fungal cells and alleviate the Al toxicity.
Isolates of L.deliciosus and S.luteus collected from acidic soils in southwest China showed less reduction in biomass,higher absorption of N,P and K,and higher oxalate accumulation than S.grevillei and C.geophilum from calcareous soils in northwest china in response to Al stress.Increased P concentrations in the four fungal isolates with appropriate concentrations of Al added into culture solution could be one of patterns to reduce intracellular Al and alleviate the Al toxicity.
| [] | Ahonen-Jonnarth U, Göransson A, Finlay R D. 2003. Growth and nutrient uptake of ectomycorrhizal Pinus sylvestris seedlings in a natural substrate treated with elevated Al concentrations. Tree Physiology, 23(3): 157–167. DOI:10.1093/treephys/23.3.157 |
| [] | Arnolds E. 1991. Decline of ectomycorrhizal fungi in Europe. Agriculture Ecosystems Environment, 35(2/3): 209–244. |
| [] | Arvieu J C, Leprince F, Plassard C. 2003. Release of oxalate and protons by ectomycorrhizal fungi in response to P-deficiency and calcium carbonate in nutrient solution. Annals of Forest Science, 60(8): 815–821. DOI:10.1051/forest:2003076 |
| [] | BarcelóJ, Poscherrieder C. 2002. Fast rootgrowth responses, not exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance:a review. Environmental and Experimental Botany, 48(1): 75–92. DOI:10.1016/S0098-8472(02)00013-8 |
| [] | Casarin V, Plassard C, Souche G, et al. 2003. Quantification of oxalate ions and protons released by ectomycorrhizal fungi in rhizosphere soil. Agronomie, 23(5): 461–469. |
| [] | Cromack K J, Sollins P, Graustein W C, et al. 1979. Calcium oxalate accumulation and soil weathering in mats of the hypogeous fungus Hysterangium crassu. Soil Biology Biochemistry, 11(5): 463–468. DOI:10.1016/0038-0717(79)90003-8 |
| [] | Cumming J R, Swiger T D, Kurnik B S, et al. 2001. Organic acid exudation by Laccaria bicolor and Pisolithus tinctorius exposed to aluminum in vitro. Canadian Journal Forest Research, 31(4): 703–710. |
| [] | Cumming J R, Weinstein L H. 1990. Aluminum-mycorrhizal interactions in the physiology of pitch pine seedlings. Plant and Soil, 125: 7–18. DOI:10.1007/BF00010739 |
| [] | Dightor J, Skeffington R A. 1987. Effects of artificial acid precipitation on the mycorrhizas of scots pine seedings. New Phytologist, 107(1): 191–202. DOI:10.1111/nph.1987.107.issue-1 |
| [] | Egerton-Warburton L M, Kuo J, Griffin B J, et al. 1993. The effect of aluminum on the distribution of calcium, magnesium and phosphorus in mycorrhizal and non-mycorrhizal seedlings of Eucalyptus rudis:a cryo-microanalytical study. Plant and Soil, 155-156(1): 481–484. DOI:10.1007/BF00025088 |
| [] | Foramina. 2005. Role of oxalic acid oversectretion in transformations of toxic metal minerals by Beauveria caledonica. Applied and Environment Microbiology, 71(1): 371–381. DOI:10.1128/AEM.71.1.371-381.2005 |
| [] | Gadd G. 1993. Interactions of fungi with toxic metals. New Phytologist, 124(1): 25–60. DOI:10.1111/nph.1993.124.issue-1 |
| [] | Gerlitz T G M. 1996. Effects of aluminum on polyphosphate mobilization in the ectomycorrhizal fungus Suillus bovinus. Plant and Soil, 178(1): 133–140. DOI:10.1007/BF00011171 |
| [] | Gharieb M M, Gadd G M. 1999. Influence of nitrogen source on the solubilization of natural gypsum (CaSO4·2H2O) and the formation of calcium oxalate by different oxalic and citric acid-producing fungi. Microbiological Research, 103(3): 473–481. |
| [] | Gu X R, Liang G S, Yang S P, et al. 2005. Influences of Laccaria bicolor on the growth, nutrient uptake and aluminum resistance of Pinus massoniana seedlings. Scientia Silvae Sinicae, 141(14): 199–203. |
| [] | Jiang W H, Zhang S, Chen G C, et al. 2002. Effect of acid deposition on soil and vegetation of forest ecosystem in Nanshan of Chongqing. Research of Environmental Sciences, 15(6): 8–11. |
| [] | Jongbloed R H, Borst-Pauwels G W F H. 1992. Effects of aluminium and pH on growth and potassium uptake by three ectomycorrhizal fungi in liquid culture. Plant and Soil, 140(2): 157–165. DOI:10.1007/BF00010593 |
| [] | Koyama H, Toda T, Hara T. 2001. Brief exposure to low-pH stress causes irreversible damage to the growing root in Arabidopsis thaliana:Pectin-Ca interaction may play an important role in proton rhizotoxicity. Journal of Experimental Botany, 52(355): 361–368. |
| [] | Lapeyrie F. 1988. Oxalate synthesis from soil bicarbonate by the mycorrhizal fungus Paxillus involutus. Plant and Soil, 11(1): 3–8. |
| [] | Lapeyrie F, Chilvers G A, Bhem C A. 1987. Oxalic acid synthesis by the mycorrhizal fungus Paxillus involutus (Batsch.ex Fr.)Fr. New Phytologist, 106(1): 139–146. DOI:10.1111/nph.1987.106.issue-1 |
| [] | Malajczuk N, Cromack K J, Sollins P, et al. 1982. Accumulation of calcium oxalate in the mantle of ectomycorrhizal roots of Pinus radiata. New Phytologist, 92(4): 527–531. DOI:10.1111/nph.1982.92.issue-4 |
| [] | Meyer F H. 1985. Effect of the nitrogen factor on the mycorrhizal complement of Norway spruce seedlings in humus from a damaged site. Allgemeine Forst zeitschrift, 9(27): 208–219. |
| [] | Moyer-Henry K, Silva I, Macfall J, et al. 2005. Accumulation and localization of aluminium in root tips of loblolly pine seedlings and the associated ectomycorrhiza Pisolithus tinctorius. Plant Cell Environment, 28(2): 111–120. DOI:10.1111/pce.2005.28.issue-2 |
| [] | Page A L. 1982. Methods of soil analysis. Soil Sci Soc Am, Madison Wisconsin: 178–185. |
| [] | Paris F, Botton B, Lapeyrie F. 1996. In vitro weathering of phlogopite by ectomycorrhizal fungi.Ⅱ.Effect of K+ and Mg2+ deficiency and N sources on accumulation of oxalate and H +. Plant and Soil, 179(1): 141–150. DOI:10.1007/BF00011651 |
| [] | Postma J W M, Keltjens W G, van Riemsdijk W H. 2005. Calcium-(organo)aluminum-proton competition for adsorption to tomato root cell walls:experimental data and exchange model calculations. Environmental Science and Technology, 39(14): 5247–5254. DOI:10.1021/es048138v |
| [] | Schier G A, McQuattie C J. 1996. Response of ectomycorrhizal and nonmycorrhizal pitch pine (Pinus rigida)seedlings to nutrient supply and aluminum:growth and mineral nutrition. Canadian Journal Forest Research, 26(12): 2145–2152. DOI:10.1139/x26-243 |
| [] | Smith S E, Read D J. 1997. Mycorrhizal symbiosis. 2nd Edition. London, Academic Press. |
| [] | Yuan L, Huang J G, Li X L, et al. 2004. Biological mobilization of potassium from clay minerals by ectomycorrhizal fungi and eucalypt seedling roots. Plant and Soil, 262(1/2): 351–361. DOI:10.1023/B:PLSO.0000037055.67646.97 |
| [] | van Schöll L, Thomas W K, Mark M S, et al. 2008. Rock-eating mycorrhizas:their role in plant nutritio and biogeochemical cycles. Plant and Soil, 303(1): 35–47. |
| [] | Yuan L, Huang J G, Li X L, et al. 2005. Influence of potassium supply on growth and uptake of nitrogen, phosphorus, and potassium by three ectomycorrhizal fungal isolates in vitro. Journal of Plant Nutrition, 28(2): 271–284. DOI:10.1081/PLN-200047614 |
| [] | Yu F Q, Liu P G. 2002. Reviews and prospects of the ectomycorrhizal research and application. Acta Ecologica Sinica, 22(2): 2217–2226. |
 2012, Vol. 48
2012, Vol. 48