文章信息
- 郭剑芬, 杨玉盛, 钟羡芳, 贺旭东
- Guo Jianfen, Yang Yusheng, Zhong Xianfang, He Xudong
- 森林粗木质残体的贮量和碳库及其影响因素
- Storage, Carbon Pool of Coarse Woody Debris in Forest Ecosystems and the Influence Factors
- 林业科学, 2011, 47(2): 125-133.
- Scientia Silvae Sinicae, 2011, 47(2): 125-133.
-
文章历史
- 收稿日期:2009-07-08
- 修回日期:2010-11-24
-
作者相关文章
Coarse woody debris (CWD) includes material on the forest floor such as dead branches and whole fallen trees, standing dead trees (stags) and stumps. CWD is an important structural component of many forest ecosystems, and has a role in a number of aspects of ecosystem functioning (Harmon et al., 1986). It provides food and habitat for wildlife, reduces runoff, and is important in nutrient cycling (Grove et al., 2003). CWD also contributes substantially to the long-lived forest carbon pool that is often overlooked (Clark et al., 2002). As interest developed in forest carbon cycle and global warming, scientists in the late 1970s began to study CWD in forested ecosystems. From studies that have quantified CWD, the minimum size has varied considerably (Sturtevant et al., 1997; Fridman et al., 2000). Harmon et al., (1996) recommended the size breakpoint between fine and coarse woody debris be made at 10 cm at the large end of a piece for most forests. So the definition for CWD by Harmon et al.(1996) is used in this paper to enable comparisons of CWD amounts between different studies and ecosystems.
Up to now, most studies on CWD have been conducted in temperate forests in North America and North Europe, and a few of them have been conducted in tropical forests (Harmon et al., 1995; Santiago, 2000). However, much less information is available in subtropical zones. CWD was first studied in China in the early 1990s; except for some concerned reviews about CWD (Chen et al., 1991; Hou et al., 2001; Deng et al., 2002), so far, there has been a very limited amount of studies and most of them concern on coniferous forests (Yang et al., 2002), broad-leaved and Korean pine mixed forests (Dai et al., 2000), and Abies fargesii forests in temperate forests (Li et al., 1998). Studies on tropical and subtropical forests were only limited to humid evergreen broad-leaved forests in southwest and eastern China (Liu et al., 1995; Yan et al., 2007), monsoon evergreen broad-leaved forests (Tang et al., 2003), and the Castanopsis eyrie forest of Wuyi mountain (Li et al., 1996). Further, CWD-related researches have not been conducted systematically. Most of the studies have focused on its biomass, distribution and dynamics (Carmona et al., 2002; Tang et al., 2005), while few researches were done in understanding the role of CWD in forest biochemistry cycle. In spite of the increasing recognition of the importance of CWD for carbon sequestration, the studies dealing with carbon stock in CWD among forest types and regions are still scarce. The uncertainty in existing estimates of carbon stores in CWD has been identified as one of the major sources of discrepancies in the assessments of carbon pools and fluxes in forest ecosystems. In order to improve the accuracy of global forest carbon budgets, there is a need to evaluate global CWD storage and carbon stock.
The main objectives of this review are to summarize the recent literature regarding the storage and carbon pool of CWD in forests, and to identify the issues that should be addressed in future research.
1 CWD storageCWD stores in forest regions are difficult to assess because they vary significantly over succession and do not necessarily parallel the dynamics of live biomass. In unmanaged forests CWD generally comprises 5%-15% of total plant biomass (Krankina et al., 2002). Global studies on CWD storage in various forest ecosystems found the highest CWD storage in temperate coniferous forests ranging 30-200 t·hm-2, and the lowest in broad-leaved forests ranging 8-50 t·hm-2 (Harmon et al., 1986). Harmon et al. (1986) reported that coniferous forests generally contain a higher volume of downed CWD than deciduous forests. This difference in volume can be attributed to the slower decomposition rate of coniferous material, and the fact that coniferous forests produce about one-third more live volume than non-coniferous forests.
Global patterns of CWD storage indicate large accumulations in cool coniferous forests of high latitudes, intermediate accumulations in temperate regions, and low accumulations in moist tropical regions (Muller, 2003). However, these patterns are complex and are regionally modified by temperature and moisture regime, with greater accumulations occurring in drier and/or cooler habitats (Harmon et al., 1995). Rice et al. (2004) estimated that CWD stocks in tropical forests vary widely from 0 to>60 t·hm-2, and can comprise up to 33% of the biomass of trees≥10 cm in diameter. The CWD amounts in worldwide subtropical and tropical evergreen forests were generally 9.1-173 t·hm-2 (Yan et al., 2007). For instance, the CWD storage of 70.3 t·hm-2 in the primary forest (natural montane evergreen broad-leaved forest) was reported in Ailao Mountains, which was much higher than that of forests in tropical area (1-30 t·hm-2) (Delaney et al., 1998) and other subtropical regions (7.3-25.3 t·hm-2) (Li et al., 1996; Tang et al., 2003), but it was somewhat lower than that of the temperate broad-leaved evergreen forest in Chiloe Island, Chile (58-381 t·hm-2) (Carmona et al., 2002). In temperate forests of the USA, estimates of CWD range from 1 to 490 t·hm-2 (mean of 89 t·hm-2) for logs and 0.25 to 157 t·hm-2 (mean of 42 t·hm-2) for stags in coniferous forests; and from 4.7 to 38.4 t·hm-2 (mean of 18 t·hm-2) for logs and 4 to 12.2 t·hm-2 (mean of 8.2 t·hm-2) for stags in deciduous forests (Harmon et al., 1986). While in Australian forests, mean of published values for estimated storage of down CWD ranged from 22 t·hm-2 in woodland to 109 t·hm-2 in wet sclerophyll forest. These values were generally within the range of those observed for similar ecosystems in other parts of the world. Overall, there were considerable differences in the CWD estimates from the literature for each forest type due to different climate regions.
The amount of CWD in a stand is determined by the balance between inputs through tree mortality and outputs through decomposition. Understanding the fluctuations in CWD abundance, and the rates of decomposition are important with regard to our understanding of forest and carbon dynamics (Aakala, 2010). In forest ecosystems, input rates of CWD range from 0.12 to 30 t·hm-2 a-1. Input rates vary considerably in time and space on a number of scales (e.g., stand, regional, seasonal, annual, and long-term successional) related to climate and disturbance regime (Harmon et al., 1986). Decomposition rates of CWD are influenced by temperature, moisture, substrate quality, decomposer organisms, and size and species of CWD (Harmon et al., 1986). Recently, CWD decay rates have been determined from both time series and chronosequence studies, the latter allowing for the rapid determination of decay rate (Garrett et al., 2010). Despite the accumulating number of studies, there is still a scarcity of information covering CWD pools and their rates of decomposition under natural conditions.
2 Carbon pool of CWDThere has been a growing appreciation of CWD as an essential component of ecosystems in recent decades; notably, this has led to assessments of the carbon budget in the light of global climate change (Krankina et al., 2002; Wang et al., 2003). Given its low surface area and high lignin content, CWD is also important to long-term C storage, particularly in ecosystems such as the boreal forest (Harmon et al., 1986). A recent review of available data on CWD stores and decomposition rates indicates that global stores of carbon in CWD may range from approximately 75 Pg (Matthews, 1997) to 114 or 157 Pg, depending on estimation procedures (Harmon et al., 2001). Regional-scale estimates of carbon stored in CWD showed that in boreal forests, it accounted for an average of about 5% of the total ecosystem C. The mean carbon pool sizes of CWD in temperate and tropical forests were roughly 18% and 10% of the total ecosystem C respectively (Pregizter et al., 2004). However, information on the quantity of carbon in the CWD within subtropical forests is relatively sparse.
Since larger pieces of CWD store more carbon over a longer period of time, old-growth forest generally has a greater storage capacity for carbon than younger forest. The stores of carbon in CWD can constitute as much as 20% of the total in old-growth forests (Weedon et al., 2009). Thus, old-growth forest and its associated large diameter CWD pieces play a particularly important role in the carbon cycle (Bradford et al., 2009). Harmon et al. (1990) determined that coarse woody debris in old-growth Douglas fir (Pseudotsuga menziesii) forests store 97 t·hm-2 of carbon. Carmona et al. (2002) estimated that carbon contents in snags and logs in old-growth and primary coastal rainforests in southern Chile can be more than 85 t·hm-2. Furthermore, over several forest rotations woody debris pool may gain more importance due to harvested residues left on the forest floor. Therefore, woody debris may considerably contribute to the long-term C pool. A greater understanding of the quantities and dynamics of these components of forest carbon stocks is required so that information can be used for carbon accounting and assessing the impacts of forest operations on greenhouse gas balances.
Estimates of CWD carbon stocks are currently based on measurements of the debris volume over bark, which are calculated from lengths and small-and large-end diameters, and an assumed density, with modifiers used to account for reductions in CWD density in subjectively assessed decay classes (Beets et al., 2008). However, a measurement based approach for estimating CWD carbon stock changes over time entails ongoing remeasurement costs and can be inaccurate. Furthermore, CWD decay classes are a subjective assessment of external features, and may not accurately depict CWD density (Beets et al., 2008). An alternative approach for estimating CWD carbon stocks and changes is through modeling processes. Although the process of decay is not constant over time, a constant decay rate was modeled. Four potential models for determining the decay rate constant of CWD were: single exponential model, multiple exponential model, lag-time model, and the linear model (Mackensen et al., 2003). The single exponential model is most often used (Olson, 1963). The multiple exponential decay model assumes that CWD components decompose at varying rates over time. The lag-time model takes into consideration the time required for microorganisms to colonize CWD. This assumption leads to slower initial rates of decomposition in CWD (Mackensen et al., 2003). If only a short time span is observed, a linear decomposition model can also be estimated. Further, it is possible to model the process based on sigmoidal and matrix functions (Harmon et al., 1986). Overall, understanding the dynamics of CWD carbon stocks in forests requires long-term and repeated measurements on the same sites. In addition, modeling method that estimates changes in carbon pool of CWD in both managed and unmanaged forest conditions is needed.
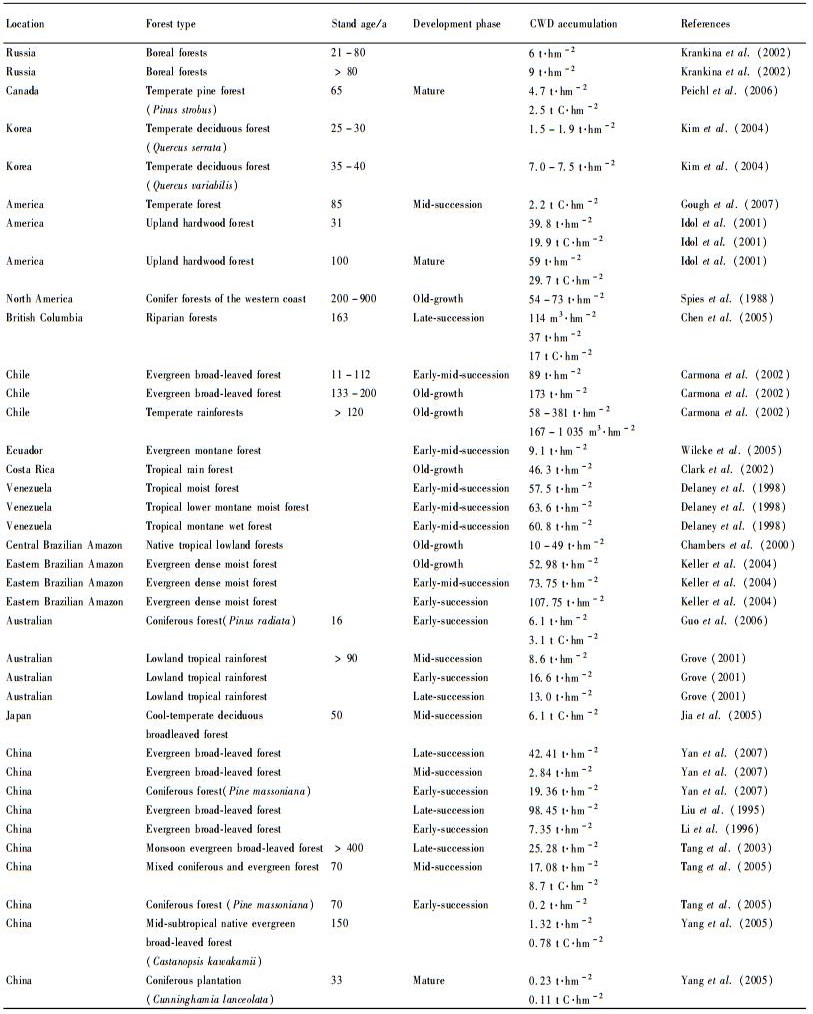
3 Influence factors 3.1 Stand age or successional stageStand age or successional stage has an impact on the storage and carbon pool of CWD in a forest ecosystem (Tab. 1). Most studies characterize CWD in stands in a single age class, or take a chronosequence approach by using stands in several age categories. The accumulation of CWD in the forest ecosystem approximately follows a U-shaped pattern. Generally, the amounts of CWD may be high in early successional stage, low in mature forests and considerably greater in old-growth forests (Spies et al., 1988). This is because as the stand ages, it undergoes a variety of disturbances causing a rise in tree mortality and therefore increased accumulation of CWD (Harmon et al., 1990; Lee et al., 1997; Krankina et al., 2002). Some international studies have demonstrated that old-growth forests can contain the largest quantities of CWD (Spies et al., 1988; Carmona et al., 2002). For example, CWD composed up to 56% of total aboveground detritus in the Changbai old-growth forest in China and between 71% and 81% of the aboveground detritus in Andrew’s old-growth forest in Oregon (Harmon et al., 2001). In mature 60-year old Douglas fir forests of the Pacific Northwest CWD accounted for 34% of the total aboveground detritus (Harmon et al., 1990). Brown (2002) reported that approximately 10%-20% of the aboveground biomass in mature forests is stored as coarse dead wood. However, the U-shaped trend might not apply to the uneven-aged stands, which have been influenced by small-scale natural disturbances and repeated silvicultural treatments (Fraver et al., 2002).
|
|
Compared to all other terrestrial biomes, forests have the greatest capacity for storing and cycling carbon (Pregizter et al., 2004). Overall, woody debris C pools show a “U” shape pattern across the same chronosequences. Mature forests store less carbon than old-growth forests. Carbon storage in mature forests of the Pacific Northwest ranges from 3.8 t C·hm-2 to 29.7 t C·hm-2 (Harmon et al., 1990; Idol et al., 2001). Old-growth forests normally have the greatest storage of CWD thus providing a significant carbon sink (Peichl et al., 2006). For example, in old-growth forests CWD carbon storage peaked and leveled off between 75.0 t C·hm-2 and 127 t C·hm-2 after 200 years of forest growth (Harmon et al., 1990). Coarse woody debris was the largest pool of stored carbon in the old-growth Douglas fir forest storing 95.5 t C·hm-2 (Harmon et al., 2004). However, the temporal dynamics of detritus C pools may be more complicated than those of biomass, because there are more influencing factors. For example, fire severity and history (frequency) interact with preburn stand characteristics, and confound ecosystem C dynamics, especially detritus C pools (Wang et al., 2003).
3.2 Decomposition stageLiterature on CWD amount with respect to decay class is limited. We follow the five decay-class system adapted from Sollins (1982) (Tab. 2). Stewart et al. (2003) reported that in old-growth stands in Nova Scotia, all stands contained abundant CWD in all decay classes, with the highest volumes occurring in decay classes Ⅲ and Ⅳ. The volume of snags was generally higher in decay classes Ⅰ and Ⅱ. McGee et al. (1999) also reported higher snag volumes in the less-decayed classes for mature and old-growth forests in the Adirondack Mountains. Several studies have shown an increase in the volume of snags and/or downed CWD in the early decay stages following harvest (Lee et al., 1997; Fraver et al., 2002).
|
|
Coarse woody debris decay stage also influences the amount of carbon stored. For example, Douglas fir logs in British Columbia had increased concentrations of carbon, with decay class I having 489 g C·kg-1 and decay class V logs having 532 g C·kg-1 (Preston et al., 1998). After 14 years of decomposition, pine CWD still contained 49.8% of its total carbon (Laiho et al., 1999). However, in Northern Patagonian forests of Chile carbon storage stayed relatively constant throughout decay classes I-V ranging from 49.5%-44.7% dry weight (Carmona et al., 2002).
3.3 Forest management practicesForest management practices such as thinning, clear-cutting and prescribed burning are considered anthropogenic disturbances that can affect the quantity, quality, and dynamics of CWD subsequently impacting its capability of storing carbon (Lee et al., 1997).
Thinning a forest ecosystem reduced the total volume and number of snags in a forest ecosystem (Lee et al., 1997). Duvall et al. (1999) found that after 90 years, mature forests managed for timber production had 85% less CWD than unmanaged forests. However, if the thinned trees were left on site, total volume of CWD on the forest floor increased. For example, from southern Tasmania an Eucalyptus regnans/Eucalyptus obliqua forest had approximately 616 t·hm-2 of forest floor CWD > 15 cm in diameter, mostly as logging residue (Woldendorp et al., 2002).
Clear-cutting increased input of CWD volume into a forest ecosystem and affected the decomposition class of the CWD (Tinker et al., 2001). Generally CWD in clear-cut stands will be disproportionately composed logs in the early stages of decay, and have fewer highly decayed logs (Idol et al., 2001). McCarthy et al.(1994) measured differences in volume of CWD inputs between clear-cuts, pole stage stands, mature stands, and old-growth forests. Coarse woody debris volume was significantly higher in the clear-cut stands compared with the other stands. Clear-cuts had 90.0 m3·hm-2 of CWD, old-growth stands had 65.0 m3·hm-2, mature stands had 45.0 m3·hm-2, and pole-aged stands had 40.0 m3·hm-2 . However, if much of the bole wood is harvested during the clear-cut these larger pieces of CWD are disproportionately lost from the forest ecosystem (Tinker et al., 2001). After one rotation managed forests in British Columbia decreased to 30% of the original amount of CWD in natural stands (Densmore et al., 2004). Similarly, significant dead wood losses caused by clear-cutting have been reported from Finnish Lapland, where the amount of all CWD in clear-cuttings was only 40% of the amount measured in old-growth forests (Sippola et al., 1998), and from Sweden, where logging operations decreased the amount of all CWD by almost 50% (Fridman et al., 2000). On the other hand, the production of CWD following clear-cutting will strongly influence the carbon balance of clear-cut sites. Keller et al. (2004) found that decay of CWD in the logged sites would emit about 1.5-4.5 t C·hm-2 a-1 about one year following logging. This represented a substantial portion of the gross primary production (26 t C·hm-2 a-1) for undisturbed forest. To maintain higher volumes of CWD over the regeneration phase, harvesting methods should be less intense than clear-fellings. Also, the damaging and removal of the logs in the felling area should be avoided during felling operations.
Prescribed burning has the capability of increasing the volume and biomass of CWD compared to non-disturbed early successional stands. For example, in the Northern Patagonian tropical forests of Chile there was 1 111 m3·hm-2 of CWD in a controlled burned forested ecosystem compared to 65.0 m3·hm-2 of CWD in an unburned tropical forest ecosystem (Carmona et al., 2002). Grier (1978) estimated that 36% of fallen logs in a hemlock-spruce forest in the USA were a remnant of the previous stand destroyed by fire. Further, a high severity, post harvest, slash burn can be detrimental to subsequent forest health because large amounts of CWD are burned, which releases carbon and other nutrients (Harmon et al., 2002). With growing awareness of climate change and the ratification of international agreements such as the Kyoto Protocol, forest managers will need to evaluate the effects of their actions on forest carbon stocks.
4 Conclusions and prospectIn conclusion, CWD storage must be estimated by forest type, age, and management practice to evaluate CWD functions in C pool and flux in the region. A great degree of consideration should be given specifically to belowground CWD, including the coarse root component. More research into CWD dynamics across the region is required. In particular, studies of CWD stocks and inputs are needed at more long-term sites to obtain a better understanding of the impacts of global climate change or disturbances (natural and/or anthropogenic) on the carbon balance of forests. Also, there should be a detailed examination of the worldwide CWD resources to address greenhouse gas offset accounting and biodiversity concerns. In addition, to gain estimates of C pool sizes in woody detritus at larger scales, two different approaches could be used. Landscape-scale field studies of pool sizes could be conducted, using an experimental design developed to be robust at the larger scale either through random sampling (Delaney et al., 1998) or sampling of areas stratified by forest types, ages, or management histories (Duvall et al., 1999). The second approach would use process models parameterized at intensive-study sites to extrapolate across landscapes. In this approach, it is critical that the controlling factors in producing landscape heterogeneity be captured in the models used to affect the scaling (Currie et al., 1997). The information obtained from such studies would be useful for determining proper management practices and for evaluating the effects of CWD management on the diverse ecological functions of CWD in forested ecosystems.
Considering the diversity of China’s forests and woodlands and the wide range of climatic conditions, it is no surprise that there is variation in the amount of CWD, and even the role of CWD in ecosystem functioning. However, CWD has not been as widely studied in China as in North America or Europe. There is growing interest in this component of biomass due to the increasing understanding of its importance in many processes within forests. We recommend that CWD levels in forests in China be inventoried and future CWD studies should address scale or temporal considerations. Additional studies that link feedbacks between climatic, physical, biotic, and anthropogenic factors to carbon dynamics of CWD in forest landscapes are also needed. We hope that further research into the amount, attributes and significance of CWD through a variety of field studies, data synthesis and modeling processes will assist our ability to manage forests in a way that promotes healthy ecosystems.
Aakala T. 2010. Coarse woody debris in late-successional Picea abies forests in northern Europe:Variability in quantities and models of decay class dynamics[J]. Forest Ecology and Management, 260(5): 770-779. DOI:10.1016/j.foreco.2010.05.035 |
Beets P N, Hood I A, Kimberley M O, et al. 2008. Coarse woody debris decay rates for seven indigenous tree species in the central north Island of New Zealand[J]. Forest Ecology and Management, 256(4): 548-557. DOI:10.1016/j.foreco.2008.05.036 |
Bradford J, Weishampel P, Smith M L, et al. 2009. Detrital carbon pools in temperate forests:magnitude and potential for landscape-scale assessment[J]. Canadian Journal of Forest Research, 39: 802-813. DOI:10.1139/X09-010 |
Brown S. 2002. Measuring carbon in forests:current status and future challenges[J]. Environmental Pollution, 116(3): 363-372. DOI:10.1016/S0269-7491(01)00212-3 |
Carmona M R, Armesto J J, Aravena J C, et al. 2002. Coarse woody debris biomass in successional and primary temperate forests in Chiloé Island, Chile[J]. Forest Ecology and Management, 164(1/3): 265-275. |
Chambers J Q, Higuchi N, Schimel J P, et al. 2000. Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon[J]. Oecologia, 122: 380-388. DOI:10.1007/s004420050044 |
Chen Hua(陈华), Xu Zhenbang(徐振邦). 1991. History, current situation and tendency of CWD ecological research. Chinese Journal of Ecology(生态学杂志), 10(1), 45-50. |
Chen X Y, Wei X H, Scherer R. 2005. Influence of wildfire and harvest on biomass, carbon pool, and decomposition of large woody debris in forested streams of southern interior British Columbia[J]. Forest Ecology and Management, 208(1/3): 101-114. |
Clark D B, Clark D A, Brown S, et al. 2002. Stocks and flows of coarse woody debris across a tropical rain forest nutrient and topography gradient[J]. Forest Ecology and Management, 164(1/3): 237-248. |
Currie W S, Aber J D. 1997. Modeling leaching as a decomposition process in humid, montane forests[J]. Ecology, 78(6): 1844-1860. DOI:10.1890/0012-9658(1997)078[1844:MLAADP]2.0.CO;2 |
Dai Limin(代力民), Xu Zhenbang(徐振邦), Chen Hua(陈华). 2000. Storage dynamics of fallen trees in the broad-leaved and Korean pine mixed forest. Acta Ecologica Sinica(生态学报), 20(3), 412-416. |
Delaney M, Brown S, Lugo A E, et al. 1998. The quantity and turnover of dead wood in permanent forest plots in six life zones of Venezuela[J]. Biotropica, 30(1): 2-11. DOI:10.1111/btp.1998.30.issue-1 |
Deng Hongbing(邓红兵), Xiao Baoying(肖宝英), Dai Limin(代力民), et al. 2002. Advance in ecological studies on in-stream coarse woody debris. Acta Ecologica Sinica(生态学报), 22(1), 87-93. |
Densmore N, Parminter J, Stevens V. 2004. Coarse woody debris:inventory, decay modelling, and management implications in three biogeoclimatic zones[J]. BC Journal of Ecosystems and Management, 5(2): 14-29. |
Duvall M D, Grigal D F. 1999. Effects of timer harvesting on coarse woody debris in red pine forests across the Great Lakes States[J]. U.S.A. Canadian Journal of Forest Research, 29(12): 1926-1934. DOI:10.1139/x99-158 |
Fraver S, Wagner R G, Day M. 2002. Dynamics of coarse woody debris following gap harvesting in the Acadian forest of central Maine[J]. U.S.A. Canadian Journal of Forest Research, 32(12): 2094-2105. DOI:10.1139/x02-131 |
Fridman J, Walheim M. 2000. Amount, structure, and dynamics of dead wood on managed forestland in Sweden[J]. Forest Ecology and Management, 131(1/3): 23-36. |
Garrett L G, Kimberley M O, Oliver G R, et al. 2010. Decomposition of woody debris in managed Pinus radiate plantations in New Zealand[J]. Forest Ecology and Management, 260(8): 1389-1398. DOI:10.1016/j.foreco.2010.07.041 |
Gough C M, Vogel C S, Kazanski C, et al. 2007. Coarse woody debris and the carbon balance of a north temperate forest[J]. Forest Ecology and Management, 244(1/3): 60-67. |
Grier C C. 1978. A Tsuga heterophylla-Picea sitchensis ecosystem of coastal Oregon:decomposition and nutrient balances of fallen logs[J]. Canadian Journal of Forest Research, 8: 198-206. DOI:10.1139/x78-031 |
Grove S J. 2001. Extent and composition of dead wood in Australian lowland tropical rainforest with different management history[J]. Forest Ecology and Management, 154(1/2): 35-53. |
Grove S J, Meggs J. 2003. Coarse woody debris, biodiversity and management:a review with particular reference to Tasmanian wet eucalypt forests[J]. Australian Forestry, 66(4): 258-272. DOI:10.1080/00049158.2003.10674920 |
Guo L B, Bek E, Gifford R M. 2006. Woody debris in a 16-year old Pinus radiata plantation in Australia:mass, carbon and nitrogen stocks, and turnover[J]. Forest Ecology and Management, 228(1/3): 145-151. |
Harmon M E, Bible K, Ryan M G, et al. 2004. Production, respiration, and overall carbon balance in an old-growth Pseudotsuga-Tsuga forest ecosystem[J]. Ecosystems, 7: 498-512. |
Harmon M E, Ferrell W K, Franklin J F. 1990. Effects on carbon storage of conversion of old-growth forest to young forests[J]. Science, 247: 699-701. DOI:10.1126/science.247.4943.699 |
Harmon M E, Franklin J F, Swanson F J, et al. 1986. Ecology of coarse woody debris in temperate ecosystems[J]. Advances in Ecological Research, 15: 133-302. DOI:10.1016/S0065-2504(08)60121-X |
Harmon M E, Krankina O N, Yatskow M, et al.2001.Predicting broad-scale carbon stores of woody detritus from plot-level data// Lal R, Kimble J M, Follett R F.Assessment methods for soil carbon.New York:CRC Press, 533-552.
|
Harmon M E, Marks B. 2002. Effects of silvicultural practices on carbon stores in Douglas-fir-western hemlock forests in the Pacific Northwest.U.S.A.:results from a simulation model[J]. Canadian Journal of Forest Research, 32(5): 863-877. |
Harmon M E, Sexton J. 1996. Guidelines for measurements of woody detritus in forest ecosystems.Publication No.20[J]. Washington:US Long-Term Ecological Research Network Office, University of Washington. |
Harmon M E, Whigham D F, Sexton J, et al. 1995. Decomposition and stores of woody detritus in the dry tropical forests of the Northeastern Yucatan Peninsula, Mexico[J]. Biotropica, 27: 305-316. DOI:10.2307/2388916 |
Hou Ping(侯平), Pan Cunde(潘存德). 2001. Coarse woody debris and its function in forest ecosystem. Chinese Journal of Applied Ecology(应用生态学报), 12(2), 309-314. |
Idol W T, Figler R A, Pope P E, et al. 2001. Characterization of coarse woody debris across a 100 year chronoseqeuence of upland oak-hickory forests[J]. Forest Ecology and Management, 149(1/3): 153-161. |
Jia S G, Akiyama T. 2005. A precise, unified method for estimating carbon storage in cool-temperate deciduous forest ecosystems[J]. Agricultural and Forest Meteorology, 134(1/4): 70-80. |
Keller M, Palace M, Asner G P, et al. 2004. Coarse woody debris in undisturbed and logged forests in the eastern Brazilian Amazon[J]. Global Change Biology, 10: 784-795. DOI:10.1111/gcb.2004.10.issue-5 |
Kim R H, Son Y, Hwang J H. 2004. Comparison of mass and nutrient dynamics of coarse woody debris between Quercus serrata and Q.variabilis stands in Yangpyeong[J]. Korean Journal of Ecology, 27(2): 115-120. DOI:10.5141/JEFB.2004.27.2.115 |
Krankina O N, Harmon M E, Kukuev Y A, et al. 2002. Coarse woody debris in forest regions of Russia[J]. Canadian Journal of Forest Research, 32(5): 768-778. DOI:10.1139/x01-110 |
Laiho R, Prescott C E. 1999. The contribution of coarse woody debris to carbon, nitrogen, and phosphorus cycles in three Rocky Mountain coniferous forests[J]. Canadian Journal of Forest Research, 29: 1502-1603. DOI:10.1139/x99-090 |
Lee P C, Crites S, Nietfeld M, et al. 1997. Characteristics and origins of deadwood material in aspen-dominated boreal forests[J]. Ecological Applications, 7(2): 691-701. DOI:10.1890/1051-0761(1997)007[0691:CAOODM]2.0.CO;2 |
Li Linghao(李凌浩), Dang Gaodi(党高弟), Wang Tiejun(汪铁军), et al. 1998. Coarse woody debris in an Abies fargesii forest in the Qinling mountains. Acta Phytoecologica Sinica(植物生态学报), 22(5), 434-440. |
Li Linghao(李凌浩), Xing Xuerong(邢雪荣), Huang Daming(黄大明), et al. 1996. Storage and dynamics of coarse woody debris in Castanopsis eyrei forest of Wuyi mountain, with some considerations for its ecological effects. Acta Phytoecologica Sinica(植物生态学报), 20(2), 132-143. |
Liu Wenyao(刘文耀), Xie Shouchang(谢寿昌), Xie Kejin(谢克金), et al. 1995. Preliminary studies on the litterfall and coarse woody debris in mid-mountain humid evergreen broad-leaved forest in Ainao mountains. Acta Botanica Sinica(植物学报), 37(10), 807-814. |
Mackensen J, Bauhus J, Webber E. 2003. Decomposition rates of coarse woody debris:A review with particular emphasis on Australian tree species[J]. Australian Journal of Botany, 51(1): 27-37. DOI:10.1071/BT02014 |
Matthews E. 1997. Global litter production, pools, and turnover times:estimates from measurement data and regression models[J]. Journal of Geophysical Research, 102(D15): 1871-1880. |
McCarthy B C, Bailey R R. 1994. Distribution and abundance of coarse woody debris in a managed forest landscape of the central Appalachians[J]. Canadian Journal of Forest Research, 24: 1317-1329. DOI:10.1139/x94-172 |
McGee G G, Leopold D J, Nyland R D. 1999. Structural characteristics of old-growth, maturing, and partially cut northern hardwood forests[J]. Ecological Applications, 9(4): 1316-1329. DOI:10.1890/1051-0761(1999)009[1316:SCOOGM]2.0.CO;2 |
Muller R N. 2003. Landscape patterns of change in coarse woody debris accumulation in an old-growth deciduous forest on the Cumberland Plateau, southeastern Kentucky[J]. Canadian Journal of Forest Research, 33(5): 763-769. DOI:10.1139/x02-210 |
Olson J S. 1963. Energy storage and the balance of producers and decomposers in ecological systems[J]. Ecology, 44(2): 322-331. DOI:10.2307/1932179 |
Peichl M, Arain M A. 2006. Above-and belowground ecosystem biomass and carbon pools in an age-sequence of temperate pine plantation forests[J]. Agricultural and Forest Meteorology, 140(1/4): 51-63. |
Pregizter K S, Euskirchen E S. 2004. Carbon cycling and storage in world forests:biome patterns related to forest age[J]. Global Change Biology, 10(1/2): 2052-2077. |
Preston C M, Troymow J A, Niu J, et al. 1998. CPMAS-NMR spectroscopy and chemical analysis of coarse woody debris in coastal forests of Vancouver Island[J]. Forest Ecology and Management, 111(1): 51-68. DOI:10.1016/S0378-1127(98)00307-7 |
Rice A H, Pyle E H, Saleska S A, et al. 2004. Carbon balance and vegetation dynamics in an old-growth Amazonian forest[J]. Ecological Applications, 14(4). |
Santiago L S. 2000. Use of coarse woody debris by the plant community of a Hawaiian montane cloud Forest[J]. Biotropica, 32(4a): 633-641. |
Sippola A, Siitonen J, Kallio R. 1998. Amount and quality of coarse woody debris in natural and managed coniferous forests near the timberline in Finnish Lapland[J]. Scandinavian Journal of Forest Research, 13: 204-214. DOI:10.1080/02827589809382978 |
Sollins P. 1982. Input and decay of coarse woody debris in coniferous stands in western Oregon and Washington[J]. Canadian Journal of Forest Research, 12: 18-28. DOI:10.1139/x82-003 |
Spies T A, Franklin J F, Thomas T B. 1988. Coarse woody debris in Douglas-fir forests of western Oregon and Washington[J]. Ecology, 69(6): 1689-1702. DOI:10.2307/1941147 |
Stewart B J, Neily P D, Quigley E J, et al. 2003. Selected Nova Scotia old-growth forests:age, ecology, structure, scoring[J]. The Forestry Chronicle, 79(3): 632-644. DOI:10.5558/tfc79632-3 |
Sturtevant B R, Bissonette J A, Long J N, et al. 1997. Coarse woody debris as a function of age, stand structure, and disturbance in boreal Newfoundland[J]. Ecological Applications, 7(2): 702-712. DOI:10.1890/1051-0761(1997)007[0702:CWDAAF]2.0.CO;2 |
Tang Xuli(唐旭利), Zhou Guoyi(周国逸), Zhou Xia(周霞), et al. 2003. Coarse woody debris in monsoon evergreen broad-leaved forests of Dinghushan nature reserve. Acta Phytoecologica Sinica(植物生态学报), 27(4), 484-489. |
Tang Xuli, Zhou Guoyi. 2005. Coarse woody debris biomass and its potential contribution to the carbon cycle in successional subtropical forests of Southern China[J]. Acta Phytoecologica Sinica, 29(4): 559-568. |
唐旭利, 周国逸. 2005. Coarse woody debris biomass and its potential contribution to the carbon cycle in successional subtropical forests of Southern China[J]. 植物生态学报, 29(4): 559-568. |
Tinker D, Knight D. 2001. Temporal and spatial dynamics of coarse woody debris in harvested and unharvested lodge-pole pine forests[J]. Ecological Modelling, 141(1/3): 125-149. |
Wang C K, Bond-Lamberty B, Gower S T. 2003. Carbon distribution of a well-and poorly-drained black spruce fir chronosequence[J]. Global Change Biology, 9(7): 1066-1079. DOI:10.1046/j.1365-2486.2003.00645.x |
Weedon J T, Cornwell W K, Cornelissen J H C, et al. 2009. Global meta-analysis of wood decomposition rates:a role for trait variation among tree species?[J]. Ecology Letters, 12(1): 45-56. DOI:10.1111/ele.2008.12.issue-1 |
Wilcke W, Hess T, Bengel C, et al. 2005. Coarse woody debris in a montane forest in Ecuador:mass, C and nutrient stock, and turnover[J]. Forest Ecology and Management, 205(1/3): 139-147. |
Woldendorp G, Spencer R D, Keenan R J, et al. 2002. An analysis of sampling methods for coarse woody debris in australian forest ecosystems[J]. Canberra:Bureau of Rural Sciences. |
Yan E R, Wang X H, Huang J J, et al. 2007. Long-lasting legacy of forest succession and forest management:Characteristics of coarse woody debris in an evergreen broad-leaved forest of Eastern China[J]. Forest Ecology and Management, 252(1/3): 98-107. |
Yang Liyun(杨丽韫), Dai Limin(代力民), Zhang Yangjian(张扬建). 2002. Storage and decomposition of fallen wood in dark coniferous forest on the North slope of Changbai mountain. Chinese Journal of Applied Ecology(应用生态学报), 13(9), 1069-1071. |
 2011, Vol. 47
2011, Vol. 47