文章信息
- Li Changxiao, Geng Yanghui, Ye Bing, Schneider Rebecca
- 李昌晓, 耿养会, 叶兵, SchneiderRebcca
- Responses of Baldcypress and Pondcypress Seedlings to Multiple Stresses and Reforestation Implications for Riparian Zones of the Three Gorges Reservoir Region of China
- 落羽杉与池杉幼苗对多种胁迫环境的响应及其对三峡库区库岸防护林营建的启示
- Scientia Silvae Sinicae, 2010, 46(10): 144-152.
- 林业科学, 2010, 46(10): 144-152.
- DOI: 10.11707/j.1001-7488.20101024
-
文章历史
- Received date: 2009-10-26
- Revised date: 2010-07-21
-
作者相关文章
2. Chongqing Key Laboratory for the Protection and Restoration of Forest Ecology of the Three Gorges Reservoir Region Chongqing 400036;
3. Research Institute of Forestry Policy and Information, Chinese Academy of Forestry Beijing 100091;
4. Department of Natural Resources, College of Agriculture and Life Sciences, Cornell University Ithaca, NY 14853, USA
2. 三峡库区森林生态保护与恢复重庆市市级重点实验室 重庆 400036;
3. 中国林业科学研究院科技信息研究所 北京 100091;
4. 康乃尔大学农学与生命科学学院自然资源系 美国纽约州依萨卡市 14853
The newly-created Three Gorges Dam of the Yangtze River, for purposes of flood control, hydropower generation and navigation, has made a very large reservoir that began to hold water in 2003.To maximize the benefits of the Dam both for flood control and hydropower generation, an annual 30 m waterlevel fluctuation change was adopted, drawing down to 145 m ASL at the end of each May due to the impending rainy season and demand for holding flood waters, and rising to 175 m ASL at the end of each November for maximal hydropower generation.The Three Gorges Reservoir region encompasses about 400 km2 and 2 000 km of shoreline.
Because of high mountainous topography, rich karst geology and frequent acidic precipitation in the region, a series of eco-environment problems occur, such as soil erosion, vegetation fragmentation and biodiversity loss.Flow regulation imposed by the Three Gorges Dam further degrades the region's already fragile ecosystem.Although multifaceted socialeconomic factors might be attributed to concurrent ecosystem degradation, one of the root causes can be due to lower forest cover.Forests play a critical role in maintaining the ecological balance in a region.Thus, reforestation is of significance and necessary for increasing forest cover in order to facilitate restoration and rehabilitation of the ecosystems of the region. However, as far as reforestation in this region is concerned, suitable tree species selection has been an ongoing assignment in recent years.Adequate tree species selected for reforestation contributes to guaranteeing successful plantations and thus revegetation in the Three Gorges Reservoir region.
Tree species suitable for reforestation in the riparian zones of the Three Gorges Reservoir region are expected to tolerate and/or adapt to multiple stresses, such as higher soil moisture produced by rising waterlevels, and drought stress engendered by water-level drawdown, as well as other adverse environment conditions.Baldcypress (Taxodium distichum) and pondcypress (T.ascendens), native to southeastern North America, have become widespread throughout the Yangtze River valleys of China since their introduction 80 years ago.Previous studies showed that baldcypress and pondcypress perform well in wetlands (Wang et al., 2002), stream banks (Shu et al., 2003; Wang et al., 2002) and agroforestry (Huang et al., 1994).Both species are widely recognized tree species adaptable and/or tolerant to wet and drought soils, air pollution (Arnold, 2002; Cox et al., 1988; Wasowski et al., 1997), varying nutrient availability conditions, a wide range of soil aeration levels and somewhat extreme pH levels (Watson, 1983).So, baldcypress and pondcypress may be promising candidate tree species for reforestation in the Three Gorges Reservoir region.The purpose of this study was to review the research literatures related to baldcypress and pondcypress, so as to provide a reference to tree species selection for reforestation in the Three Gorges Reservoir region.
1 Relationship between baldcypress and pondcypressBased on allozyme analysis, Lickey et al. (2002a) found the possibility that these two taxa represent distinct species is unlikely due to the apparent existence of genetic flow and the possibility of clinal variation within populations.Thus, these two taxa are suggested to be best regarded as varieties of the same species.In addition, little genetic difference between baldcypress and pondcypress was found in DNA studies by Tsumura et al.(1995; 1999).In addition, Liu et al.(1990) reported that no genetic difference was detected in baldcypress between individuals remaining alive in the thermal effluent of the Savannah River Power Plant and the individuals not affected by the power plant's cooling waters.Some other authors also supported such a viewpoint by considering pondcypress as a botanical variety of T. distichum (Denny et al., 2007; Lickey et al., 2002b; Murphy et al., 1975; Schopmeyer, 1974; St Hilaire, 2001; Watson, 1986; 1993).However, some authors treat pondcypress as a distinct species (Arnold, 2002; Godfrey, 1988; Griffiths, 1994; Krüssmann, 1985; Turner et al., 1999) due to morphological and genetic variation compared with baldcypress (Allen et al., 1994; Faulkner, 1985; Neufeld, 1983, 1986; Sharma et al., 1978).Such an understanding of the intimate relationship between baldcypress and pondcypress might well explain why there are fewer specific studies on pondcypress than on baldcypress.
Besides the contrasting viewpoint on whether or not pondcypress should be considered a variety of T.distichum, such a debate concerning the existence and nature of intermediate phenotypes between baldcypress and pondcypress has remained unresolved for a long time.Hardin (1971) stated that these intermediates are in existence, while Godfrey (1988) insisted that trees and populations with intermediate morphologies are rarely seen in nature.
In addition, montezuma cypress under the same genus Taxodium as baldcypress and pondcypress, is treated as a distinct species in a name of T. mucronatum (Arnold, 2002; Chen et al., 1996; St. Hilaire, 2001; Turner et al., 1999).However, Watson (1983; 1993) and Denny (2007) treat montezuma cypress as a botanical variety, T.distichum var.mexicana.Furthermore, Denny (2007) insisted that the most appropriate treatment for baldcypress, pondcypress and montezuma cypress is three botanical varieties under one species.Thus, many more studies are needed to identify the true relationship among baldcypress, pondcypress and montezuma cypress.
2 Biological and ecological differencesAlthough leaves of both baldcypress and pondcypress exhibit the same kind of spiral arrangement around deciduous shoots, baldcypress is characterized by more linear leaves in contrast to awllike to scale-like leaves in pondcypress (Brown et al., 1986; Watson, 1986).Leaves of pondcypress are narrower and thicker than those of baldcypress, with an arrangement from radial in high light to distichous in low light (Neufeld, 1983).The foliage of pondcypress trees may confer greater tolerance to or avoidance of water stress effects than baldcypress trees (Brown, 1981).Furthermore, baldcypress typically has thinner bark than pondcypress (Brown et al., 1986).Neufeld (1986) documented that pondcypress have narrower crowns and are taller at a given diameter than baldcypress, and also have approximately twice as many branchlets as baldcypress in the distal 15 cm of branches with a higher leaf area ratio than baldcypress (2.60 versus 1.60 m2·kg-1 dw).In addition, pondcypress exhibit reduced internodal elongation and slower growth rates most likely due to the adaptation to the environment.
Baldcypress usually grows along nutrient-rich flood plains of large rivers, whereas pondcypress grows in nutrient-poor bays and creeks (Brown, 1981; Christensen, 1988).Watson (1986) speculated that pondcypress had evolved from those populations of Taxodium on the Florida peninsula where they had experienced a much drier climate during the recent glacial-interglacial cycle.Such drier climate selected drought tolerant, fire resistant individuals with characters typical of pondcypress, such as thicker bark, sclerophyllous, appressed leaves with sunken stomates and ascending branchlets (Lickey et al., 2002a).
Neufeld (1983) found that baldcypress seedlings produced greater biomass, leaf area, height and diameter growth, while significant lower net photosynthetic rates, than pondcypress seedlings under light levels ranging from 5%-100% of full sunlight. The author illustrated that thicker cross-sections along with higher specific leaf weights in pondcypress leaves than in baldcypress leaves may have accounted for the differences in net photosynthetic rates due to a resultantly greater internal leaf surface area available for CO2 uptake.In contrast, Murphy (1974) reported that pondcypress seedlings grew 50% taller than baldcypress seedlings, and did not differ in diameter growth.Such conflictive results between Neufeld (1983) and Murphy (1974) reflected, at least partially, inherent differences in the maximum potential growth rates, as they both adopted almost the same kind of experiment conditions (Neufeld, 1983). Brown (1981) stated that lower growth rates in pondcypress compared to baldcypress may reflect an adaptation to anaerobic and/or nutrient deficient soils. Other reports documented that baldcypress had higher growth rate in the field (Harper, 1902; Mattoon, 1915; Langdon, 1965).
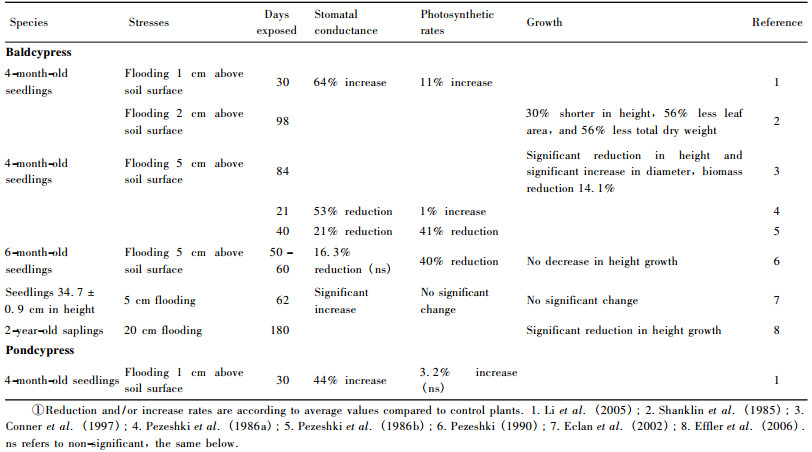
3 Tolerances to multiple stresses 3.1 Flooding toleranceBaldcypress and pondcypress survive and grow in flooded habitats partly because of root adaptation (Pezeshki et al., 1990), such as lenticel/aerenchyma formation, adventitious rooting and regeneration of new roots (Kozlowski, 1984).Hook et al.(1980) reported that Taxodium distichum (Taxodiaceae) produces water roots under flooding conditions.Grosse et al.(1992) found that pressurized gas transport improves oxygen supply to roots of T.distichum to help them survive the initial period of soil flooding. Intermittent flooding (flooded for 5 d and drained for 5 d) had no significant effect on stomatal conductance and net photosynthetic rate, growth and biomass in baldcypress seedlings, but root porosity increases 1.4 times as compared to the control (Anderson et al., 1999).Megonigal et al.(1992) also demonstrated that total biomass of periodically flooded baldcypress seedlings was comparable to continuously flooded seedlings due to the production of flood-adapted roots under the prolonged 3-year flood durations.Fisher et al.(1990) revealed that pondcypress is flood tolerant as well.In addition, baldcypress may rapidly resume gas exchange function after floodwater was drained (Anderson et al., 1999; Pezeshki, 1993).Yamamoto (1989) found that in baldcypress deep waterlogging inhibited growth of above-water-surface height and diameter, while facilitating growth of below-watersurface diameter.Furthermore, the tracheid of baldcypress under deep flooding became wider, along with thinner cell wall and bark as compared to the corresponding above-water portions.
Under rhizosphere hypoxia imposed by flooding, baldcypress seedlings reduced ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBPCO) activity at 48 h, which is among the early signals of flood-stress contributing to the loss of leaf net photosynthetic rate (Pezeshki, 1994).The recovery of baldcypress seedlings in stomatal conductance and net carbon assimilation rate can reach 97% and 87% of control by day 28 (Pezeshki, 1994).Although baldcypress and pondcypress are commonly recognized as two water-tolerant tree species due to their adaptation capabilities, previous studies showed conflictive results in responses to flooding as indicated in table 1.Such conflictive results might be caused by disparity among individuals within the population of the seedlings used in different experiments.Some seedlings/saplings may develop adaptation systems antecedently, thus leading to an earlier production of water roots and development of intercellular air spaces. On the other hand, the effect of the flooding treatments on plant growth and biomass was different for 1-yearold seedlings and 3-year-old saplings (Megonigal et al., 1992).Improved growth in the continuously flooded environment began in the second growing season in coincidence with morphological adaptations to flooding (Megonigal et al., 1992).As all of those studies listed in the table 1 used 1-year-old seedlings or 2-year-old saplings, the results may not be in agreement with those obtained using 3-year-old or older saplings.Planting 3-year-old saplings may be better than 1-or 2-year-old saplings in terms of their adaptive performances in the Three Gorges Reservoir region.
|
|
The highest water level of 175 m ASL will be maintained for about 35-40 days annually in the riparian zones of the Three Gorges Reservoir region. Plants grown on the riparian zones will be subjected to full submergence during the time period of highest water-level maintenance.Souther et al.(2000) reported that newly-germinated baldcypress seedlings (10.9 ± 0.3 cm height, under two weeks of age) could be sustained for at least 45 days of full submergence, in contrast to 1-year-old seedlings (52.8 ± 1.9 cm height) subjected to complete submergence up to 150 days with 75% survival.Thus, older and bigger saplings such as 2-or 3-year-old baldcypress and pondcypress, with more established root systems, can be expected to better tolerate continuous flooding and waterlogging, but further verification studies are still needed.Nevertheless, both baldcypress and pondcypress are promising candidates for reforestation owing to their water tolerance.
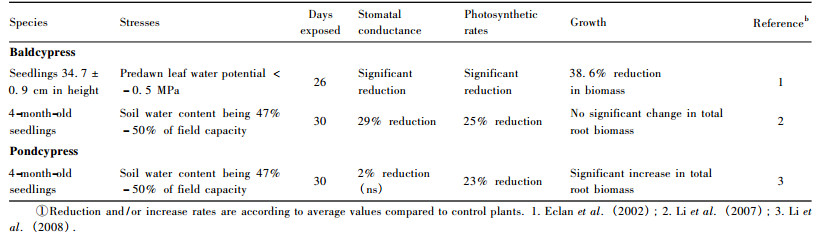
3.2 Drought toleranceAlthough baldcypress seedlings are droughtsensitive (Dickson et al., 1972), root biomass under mild drought stress can still be maintained at normal level (Li et al., 2007; see also table 2).However, root biomass of pondcypress showed significant increase under mild drought condition (Li et al., 2008).Eclan et al.(2002) found significant reductions in net photosynthetic rate and stomatal conductance in baldcypress under mild drought stress.In addition, Li et al.(2005) also found that net carbon assimilation rate was significantly reduced in baldcypress and pondcypress seedlings under mild drought conditions. However, under mild water deficit stress, stomatal conductance in pondcypress seedlings was not significantly affected, in contrast to a significant decline in baldcypress seedlings (Li et al., 2005).
|
|
Pondcypress seedlings appear more resilience than baldcypress in response to soil water deficit.Thus, pondcypress should be a high priority candidate tree species for reforestation under drought-prone conditions in the riparian zone of the Three Gorges Reservoir region.Unlike the seedlings that are to some extent drought-sensitive, adult trees of baldcypress and pondcypress may perform well when subjected to drought stresses (Wang et al., 1995).Likewise, saplings are also expected to be more drought-tolerant than seedlings partially due to more established root systems (Islam et al., 2003; 2004).When reforestation in drought areas is needed, saplings and/or adult trees of baldcypress and pondcypress might be a better choice for plantation than the seedlings.
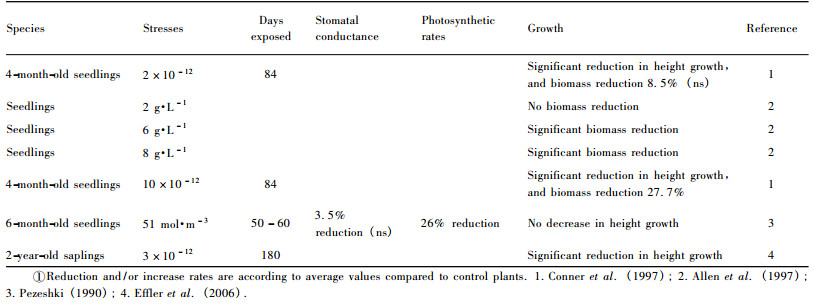
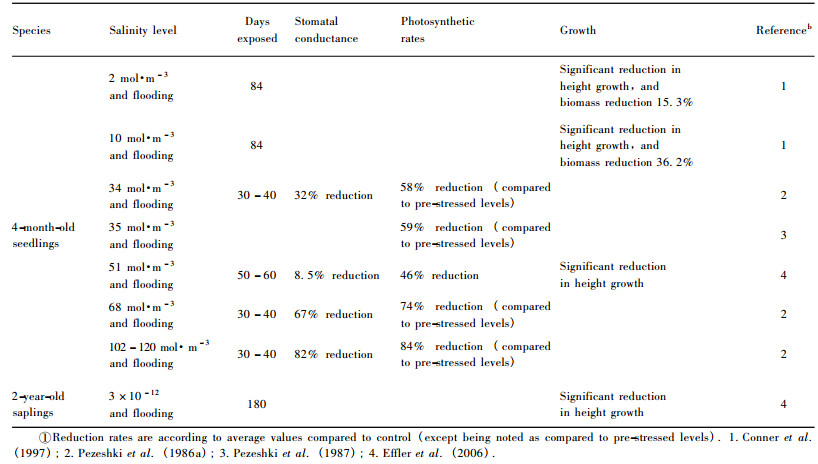
3.3 Salinity and alkalinity toleranceDenny et al.(2008) documented that there was a strong geographic component to the variation in tolerance of alkaline soils in Taxodium, with T. distichum var.mexicana (i.e., montezuma cypress) being more tolerant of an alkaline site followed by T. distichum var.distichum.Baldcypress seedlings may tolerate water at low (2 g·L-1) salinity, as shown in table 3, but reduced biomass occurs as watering salinity increased to 6 and 8 g·L-1, where biomass is reduced first in leaves, followed by roots and finally the stem (Allen et al., 1997).Pezeshki (1990) reported that baldcypress seedlings decreased 46% in carbon assimilation rates and 56% in height growth when combined stresses from flooding (5 cm above soil surface) and salinity (concentration of 51 mol·m-3) were imposed.More relevant studies related to the effects of combined flooding and salinity on growth and gas exchange of baldcypress are listed in table 4, where significant reductions in gas exchanges and growth can be found.However, variation in salt sensitivity among different populations exists (Allen et al., 1997; Pezeshki et al., 1990).Some individuals of baldcypress and pondcypress species exhibit responses characteristic of moderately tolerant species, while others demonstrate responses typical of salt-sensitive species (Allen et al., 1997).
|
|
|
|
In the riparian zones of the Three Gorges Reservoir region, karst geology accompanied with limestone soils typical in parts of the region causes difficulty in riparian eco-restoration through reforestation.The fact that more than 30% of the Reservoir region is characterized by karst geology, suggests that salt-and alkaline-tolerant species should be selected for reforestation and could play important roles in eco-restoration and rehabilitation.Although baldcypress and pondcypress may survive the acidic soils with pH = 4.7 (Shu et al., 2003), both species could not endure the soils of pH > 7.5 (Chen et al., 1996).However, Taxodium "zhongshansa 302 ", a hybrid between T.distichum and T.mucronatum, and T." zhongshansa 401 ", a hybrid between T.ascendens and T.mucronatum, exhibit such characteristics of enduring the soil with pH value of 8.0-8.5 and salinity around 0.1% (Chen et al., 1996; Zhou et al., 2000) and then could be used for reforestation.
3.4 Tolerance to pollution and diseaseBaldcypress has not been observed to accumulate cobalt under natural conditions, and cobalt uptake by 2-year seedlings can be negligible under a cobalt treatment of 100 mg per pot (McLeod et al., 2007). Guthrie et al.(1979) reported that cobalt concentration in baldcypress growing in an ash basin was 0.1 mg·kg-1 on a wet weight basis.Interestingly, McLeod et al.(2003) found that leaf manganese concentration increased drastically under manganeseenriched, water-saturated soil conditions, while no manganese accumulation occurred under natural conditions.A previous study (McDonald et al., 2008) showed that Taxodium seedlings from high-rainfall, high-humidity provenance areas had less defoliation associated with the presence of a leaf blight fungus (Cercosporidium sequoiae), even though defoliation varied among provenances within all geographical regions.
Baldcypress and pondcypress are expected to grow well even when subjected to pollutants and diseases. But, much more research is needed to better understand the responses of both species to different kinds of pollution and diseases.In the Three Gorges Reservoir region, environment pollution and tree diseases are generally severity (Xie et al., 2004), despite great efforts to mitigate and/or control these problems.The significance of screening adequate tree species for reforestation cannot be overstated in ecological restoration of the riparian zones.
4 Management implicationsWe conclude that baldcypress and pondcypress seedlings are susceptible to multiple stresses such as drought, salinity, alkalinity, air pollution, and diseases, although the two species are widely recognized adaptable and/or tolerant to flooding. Thus, care should be taken to mitigate and/or get rid of the stresses that negatively influence the growth and development of baldcypress and pondcypress seedlings. In addition, selecting the highly resistant individuals for reforestation is very necessary and also feasible as variations in capability of tolerance to multiple stresses exist among individuals and provenances of baldcypress and pondcypress.Thus, a reliable and efficient screening technique to select genotypes from regions shown to yield tolerant individuals is of significance. Besides traditional means, some modern molecular and genetic technologies could also be used to screen and/or cultivate the outperformed individuals for tolerance to multiple stresses.
In riparian zones of the Three Gorges Reservoir region, 3-year-old baldcypress and pondcypress saplings, even older and bigger ones, may be selected for reforestation.In case of a need in reforestation under high salinity and alkalinity environments such as in karst geological zones, the hybrids of Taxodium "zhongshansa 302"and T."zhongshansa 401"could be used, instead of baldcypress and pondcypress.
In conclusion, baldcypress and pondcypress are promising candidates for reforestation in the Three Gorges Reservoir region.However, much more research on the performances of baldcypress and pondcypress under deep submergence and under a combination of two or more stresses still needs to be done.
Allen J A, Chambers J L, McKinney D. 1994. Intraspecific variation in the response of Taxodium distichum seedlings to salinity[J]. Forest Ecology and Management, 70(1/3): 203-214. |
Allen J A, Chambers J L, Pezeshki S R. 1997. Effects of salinity on baldcypress seedlings: Physiological responses and their relation to salinity tolerance[J]. Wetlands, 17(2): 310-320. DOI:10.1007/BF03161419 |
Anderson P H, Pezeshki S R. 1999. The effects of intermittent flooding on seedlings of three forest species[J]. Photosynthetica, 37(4): 543-552. |
Arnold M A. 2002. Landscape plants for Texas and environments[M]. 2nd edition. Champaign, IL: Stipes Publishing L L C.
|
Brown C A, Montz G N. 1986. Baldcypress: the tree unique, the wood eternal[M]. Baton Rouge, LA: Claitor's Publishing Division.
|
Brown S. 1981. A comparison of the structure, primary productivity, and transpiration of cypress ecosystems in Florida[J]. Ecological Monographs, 51(4): 403-427. DOI:10.2307/2937322 |
Chen Y H(陈永辉), Wu S P(吴寿彭), Yin Y L(殷云龙), et al. 1996. Plantation experiment of Taxodium hybrids on the saline and alkaline soils in the coastal area in Jiangsu province. Journal of Jiangsu Forestry Science & Technology(江苏林业科技), 23(4), 18-22. |
Christensen N L. 1988. Vegetation of the southeastern coastal plain// Barbour M G, Billings W D. North American terrestrial vegetation. New York: Cambridge University Press, 317-363.
|
Conner W H, McLeod K W, McCarron J K. 1997. Flooding and salinity effects on growth and survival of four common forested wetland species[J]. Wetlands Ecology and Management, 5(2): 99-109. DOI:10.1023/A:1008251127131 |
Cox P W, Leslie P. 1988. Texas trees: A friendly guide[M]. San Antonio, TX: Corona Publishing Co.
|
Denny G C, Arnold M A. 2007. Taxonomy and nomenclature of baldcypress, pondcypress, and montezuma cypress: one, two or three species?[J]. HortTechnology, 17: 125-127. |
Denny G C, Arnold M A, Mackay W A. 2008. Alkalinity tolerance of selected provenances of Taxodium Rich[J]. HortScience, 43(7): 1987-1990. |
Dickson R E, Broyer T C. 1972. Effects of aeration, water supply, and nitrogen source on growth and development of tupelo gum and baldcypress[J]. Ecology, 53(4): 626-634. DOI:10.2307/1934776 |
Eclan J M, Pezeshki S R. 2002. Effects of flooding on susceptibility of Taxodium distichum L. seedlings to drought[J]. Photosynthetica, 40(2): 177-182. DOI:10.1023/A:1021381204684 |
Effler R S, Goyer R A. 2006. Baldcypress and water tupelo sapling response to multiple stress agents and reforestation implications for Louisiana swamps[J]. Forest Ecology and Management, 226(1/3): 330-340. |
Faulkner P L. 1985. Genetic variation among half-sib families of baldcypress seedlings planted on two different sites[M]. Baton Rouge, LA: Louisiana State University.
|
Fisher H M, Stone E L. 1990. Air-conducting porosity in slash pine roots from saturated soils[J]. Forest Science, 36(1): 18-33. |
Godfrey R K. 1988. Trees, shrubs, and woody vines of Northern Florida and adjacent Georgia and Alabama[M]. Athens, GA: University of Georgia Press.
|
Griffiths M. 1994. The new royal horticultural society dictionary: Index of garden plants[M]. Portland, Oregon: Timber Press.
|
Grosse W, Frye J, Lattermann S. 1992. Root aeration in wetland trees by pressurized gas transport[J]. Tree Physiology, 10(3): 285-295. DOI:10.1093/treephys/10.3.285 |
Guthrie R K, Cherry D S. 1979. The uptake of chemical elements from coal ash and settling basin effluent by primary producers: I. Relative concentrations in predominant plants[J]. Science of the Total Environment, 12(3): 217-222. DOI:10.1016/0048-9697(79)90087-1 |
Hardin J W. 1971. Studies of the Southeastern United States flora: Ⅱ.The gymnosperms[J]. Journal of the Elisa Mitchell Scientific Society, 87: 43-50. |
Harper R M. 1902. Taxodium distichum and related species, with notes on some geological factors influencing their distribution[J]. Bull Torrey Botany Club, 29: 383-399. DOI:10.2307/2478602 |
Hook D D, Scholtens J R. 1978. Adaptation and flood tolerance of tree species// Hook D D, Crawford R M M. Plant life in Anaerobic environments. MI, USA: Ann Arbor Science Publications, 299-331.
|
Huang J H(黄家洪), Ding M T(丁明堂). 1994. Reforestation techniques on Taxodium ascendens trees in Lixiahe region and the agri-forestry complex management. Development of Forestry Science and Technology(林业科技开发), (1), 14-16. |
Islam M A, Macdonald S E, Zwiazek J J. 2003. Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene treatments[J]. Tree Physiol, 23(8): 545-552. DOI:10.1093/treephys/23.8.545 |
Islam M A, Macdonald S E. 2004. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding[J]. Tree Physiol, 18(1): 35-42. DOI:10.1007/s00468-003-0276-9 |
Kozlowski T T. 1984. Plant responses to flooding of soil[J]. Bio-Science, 34(3): 162-167. |
Krussmann G. 1985. Manual of cultivated conifers[M]. Warda H, Epp M (trans.). Portland, Ore: Timber Press.
|
Langdon O G. 1965. Baldcypress (Taxodium distichum[L. ] Rich. )// Fowells H A. Silvics of forest trees of the United States. USDA Handbook No. 271, 671-677.
|
Li C X(李昌晓), Zhong Z C(钟章成). 2007. Influences of mimic soil water regime on the contents of malic acid and shikimic acid and biomasses in the roots of Taxodium distichum seedlings in the hydrofluctuation belt of the Three Gorges Reservoir Area. Acta Ecologica Sinica(生态学报), 27(11), 4394-4402. |
Li C X(李昌晓), Zhong Z C(钟章成). 2005. Comparative study on photosynthetic characteristics of Taxodium distichum and Taxodium ascendens seedlings under simulated soil water changes in the hydrofluctuation belt of Three Gorges Reservoir Area. Scientia Silvae Sinicae(林业科学), 41(6), 28-34. |
Li C X(李昌晓), Zhong Z C(钟章成), Tao J P(陶建平). 2008. Malic acid, shikimic acid, and biomass accumulation in the roots of Taxodium ascendens seedlings under different soil water conditions. Scientia Silvae Sinicae(林业科学), 44(10), 1-7. |
Lickey E B, Walker G L. 2002a. Population genetic structure of baldcypress (Taxodium distichum[LL. ] Rich. var. distichum) and pondcypress (T. distichum var. imbricarium[nuttall ] Croom) : Biogeographic and taxonomic implications[J]. Southeastern Naturalist, 1(2): 131-148. DOI:10.1656/1528-7092(2002)001[0131:PGSOBT]2.0.CO;2 |
Lickey E B, Watson F D, Walker G L. 2002b. Differences in bark thickness among populations of baldcypress (Taxodium distichum [L. ] Rich. var. distichum) and pondcypress (Taxodium distichum var. imbricarium[Nuttall] Croom)[J]. Castanea, 67(1): 33-41. |
Liu E H, Iglich E M, Sharitz R R, et al. 1990. Population genetic structure of baldcypress (Taxodium distichum) in a thermally effected swamp forest[J]. Silvae Genetica, 39(3/4): 129-133. |
Mattoon W R. 1915. The southern cypress[M]. Washington, D C: USDA Bulletin No. 272.
|
McDonald G V, Denny G C, Arnold M A, et al. 2008. Comparative canopy damage among provenances of baldcypress associated with the presence of Cercosporidium sequoiae (Ellis and Everth.) W. A. Baker and Partridge[J]. HortScience, 43(6): 1703-1705. |
McLeod K W, Ciravolo T G. 2003. Sensitivity of water tupelo (Nyssa aquatica) and (Taxodium distichum) seedlings to manganeseenrichment under water saturated conditions[J]. Environmental Toxicology and Chemistry, 22(12): 2948-2951. DOI:10.1897/02-457 |
McLeod K W, Ciravolo T G. 2007. Cobalt uptake by Nyssa aquatica, N. sylvatica var. biflora, and Taxodium distichum seedlings[J]. Wetlands, 27(1): 40-43. DOI:10.1672/0277-5212(2007)27[40:CUBNAN]2.0.CO;2 |
Megonigal P J, Day F P. 1992. Effects of flooding on root and shoot production of baldcypress in large experimental enclosures[J]. Ecology, 73(4): 1182-1193. DOI:10.2307/1940668 |
Murphy J B. 1974. Further germination studies//Cypress wetlands for water management, recycling and conservation. First annual report to NSF-RANN and the Rockfeller Foundation, Center for Wetlands, University of Florida, 395-397.
|
Murphy J B, Stanley R G. 1975. Increased germination rates of baldcypress and pondcypress seed following treatments affecting the seed coat[J]. Physiologia Plantarum, 35(2): 135-139. DOI:10.1111/ppl.1975.35.issue-2 |
Neufeld H S. 1983. Effects of light on growth, morphology, and photosynthesis in baldcypress (Taxodium distichum (L.) Rich.) and pondcypress (T. ascendens Brongn.) seedlings[J]. Bulletin of the Torrey Botanical Club, 110(1): 43-54. DOI:10.2307/2996516 |
Neufeld H S. 1986. Ecophysiological implications of tree architecture for two cypress taxa, Taxodium distichum (L.) Rich. and T. ascendens Brongn[J]. Bulletin of the Torrey Botanical Club, 113(2): 118-124. DOI:10.2307/2995934 |
Pezeshki S R. 1990. A comparative study of the response of Taxodium distichum and Nysssa aquatica seedlings to soil anaerobiosis and salinity[J]. Forest Ecology and Management, 33/34: 531-541. DOI:10.1016/0378-1127(90)90216-X |
Pezeshki S R. 1993. Differences in patterns of photosynthetic responses to hypoxia in flood-tolerant and flood-sensitive tree species[J]. Photosynthetica, 28: 423-430. |
Pezeshki S R. 1994. Responses of baldcypress (Taxodium distichum) seedlings to hypoxia: leaf protein content, ribulose-1, 5-bisphosphate carboxylase/oxygenase activity and photosynthesis[J]. Photosynthetica, 30(1): 59-68. |
Pezeshki S R, Chambers J L. 1986a. Variation in flood-induced stomatal and photosynthetic responses of three bottomland tree species[J]. Forest Science, 32(4): 914-923. |
Pezeshki S R, DeLaune R D, Patrick W H Jr. 1990. Flooding and saltwater intrusion: potential effects on survival and productivity of wetlands forests along the US gulf coast[J]. Forest Ecology and Management, 33/34: 287-301. DOI:10.1016/0378-1127(90)90199-L |
Pezeshki S R, DeLaune R D, Patrick W H Jr. 1986b. Gas exchange characteristics of baldcypress (Taxodium distichum L.) : Evaluation of responses to leaf aging, flooding and salinity[J]. Canadian Journal of Forest Research, 16: 1394-1397. DOI:10.1139/x86-250 |
Pezeshki S R, DeLaune R D, Patrick W H Jr. 1987. Physiological response of baldcypress to increases in flooding salinity in Louisiana's Mississippi River Deltaic Plain[J]. Wetlands, 7: 1-10. DOI:10.1007/BF03160798 |
Schopmeyer C S. 1974. Seeds of woody plants in the United States.USDA handbook no. 45[M]. Washington D C: Forest Service, USDA.
|
Shanklin J J, Kozlowski T T. 1985. Effects of flooding of soil on growth and subsequent responses of Taxodium distichum seedlings to SO2[J]. Environmental Pollution, 38(3): 199-212. DOI:10.1016/0143-1471(85)90126-6 |
Sharma G. K., Madsen L. 1978. Variation in baldcypress from different habitats[J]. Journal of the Tennessee Academy of Science, 53: 115-116. |
Shu D L(舒东膂), Huang C Q(黄程前), Zeng L Z(曾玲珍), et al. 2003. Screening techniques on highly water-tolerant Taxodium ascendens trees grown in water fluctuation zones of the reservoir. Hunan Forestry & Technology(湖南林业科技), 30(2), 23-25. |
Souther R F, Shaffer G P. 2000. The effects of submergence and light on two age classes of baldcypress (Taxodium distichum (L.) Richard) seedlings[J]. Wetlands, 20: 697-706. DOI:10.1672/0277-5212(2000)020[0697:TEOSAL]2.0.CO;2 |
St Hilaire R. 2001. Seed coat treatments influence germination of Taxodium mucronatum[J]. Desert Plants, 17(1): 15-18. |
Tsumura Y, Yoshimura K, Tomaru N, et al. 1995. Molecular phylogeny of conifers using RFLP analysis of PCR-amplified specific chloroplast genes[J]. Theoretical and Applied Genetics, 91(8): 1222-1236. |
Tsumura Y, Tomaru N, Suyama Y, et al. 1999. Genetic diversity and differentiation of Taxodium in the south-eastern United States using cleaved amplified polymorphic sequences[J]. Heredity, 83: 229-238. DOI:10.1038/sj.hdy.6885810 |
Turner R G Jr., Watson F D. 1999. Botanica[M]. New York: Barnes & Noble.
|
Wang X D(王小德), Ren H F(任海芳), Zhang W R(张万荣), et al. 2002. Protection and development of wetland landscape in area of Qingshan lake. Journal of Zhejiang Forestry College(浙江林学院学报), 19(4), 408-411. |
Wasowski S, Wasowski A. 1997. Native Texas plants: Landscaping region by region[M]. Houston, TX: Gulf Publishing Co.
|
Watson F D. 1983. A taxonomic study of pondcypress and baldcypress[M]. Raleigh, NC: North Carolina State University Press.
|
Watson F D. 1986. The nomenclature of pondcypress and baldcypress (Taxodiaceae)[J]. Taxon, 34: 506-509. |
Watson F D. 1993. Taxodium Richard∥Flora of North America Editorial Committee. Flora of North America north of Mexico. New York: Oxford University Press, 403-404.
|
Xie H Y(谢红勇), Hu Z H(扈志洪). 2004. Principles and models for eco-restoration of the water-fluctuation zone of the Three Gorges Reservoir region. Research on Development(开发研究), (3), 36-39. |
Yamamoto F. 1989. Effects of waterlogging on morphology and anatomy of Taxodium distichum[J]. IAWA Bulletin, 10(3): 350-355. |
Zhou K(周康), Jia C(贾春), Chen Y H(陈永辉), et al. 2000. Analysis on growth of Taxodium distichum and T. "zhongshansa 302 " on the alkaline low land. Journal of Jiangsu Forestry Science & Technology(江苏林业科技), 24(5), 13-17. |
Wang Q M(汪企明), Jiang Z P(江泽平), Lü X S(吕祥生), et al. 1995. Studies on the variation of provenances and families in genus Taxodium: Introduction to the genus. Journal of Jiangsu Forestry Science & Technology(江苏林业科技), 22(2), 14-18. |
 2010, Vol. 46
2010, Vol. 46