文章信息
- 程彬, 付晓霞, 韩启, 张宝民, 张大明, 李兴鹏, 高长启, 孙晓玲
- Cheng Bin, Fu Xiaoxia, Han Qi, Zhang Baomin, Zhang Daming, Li Xingpeng, Gao Changqi, Sun Xiaoling
- 虫害诱导的家榆挥发物对榆紫叶甲寄主选择行为的影响
- Effects of Herbivore-Induced Ulmus pumila Volatiles on the Host Selection Process of Ambrostoma quadriimpressum
- 林业科学, 2010, 46(10): 76-82.
- Scientia Silvae Sinicae, 2010, 46(10): 76-82.
-
文章历史
- 收稿日期:2010-03-14
- 修回日期:2010-04-29
-
作者相关文章
2. 吉林省林业科学研究院 长春 130033;
3. 吉林省前郭县乌兰图嘎林场 前郭 131121;
4. 中国农业科学研究院茶叶研究所 杭州 310008
2. Jilin Provincial Academy of Forestry Sciences Changchun 130033;
3. Wulantuga Forest Farm of Qianguo County in Jilin Province Qianguo 131121;
4. Tea Research Institute, Chinese Academy of Agricultural Sciences Hangzhou 310008
植食性昆虫在复杂的生活环境中来找寻和定位寄主,必须识别其潜在寄主植物所释放的化学信号。大量研究表明:寄主植物释放的挥发物为植食性昆虫提供了识别和定位其寄主的重要信息(Halitschke et al., 2008)。寄主植物挥发物在质(Blight et al., 1995; Bartlet et al., 1997)或量上(Barata et al., 2000; Van Loon et al., 2002)的不同,或者寄主和非寄主植物挥发物在组成成分上的差异(Wright et al., 2004)体现了这种挥发性化学信息的特殊性。健康植株释放的挥发物与被植食性昆虫取食或产卵胁迫等危害后植株释放的挥发物相比,在质和量上都有很大差异(Vet et al., 1992; Dicke,1998; Dicke et al., 2000)。受害后植物挥发物的种类和释放量远远高于受害前,所以更易被植食性昆虫远距离识别(Dicke et al., 1990; Turlings et al., 1990)。叶甲科昆虫取食为害诱导的植物挥发物对同种叶甲(Peng et al.1992; Bolter et al., 1997; Landolt et al., 1999; Kalberer et al., 2001; Cossé et al., 2002; Tansey et al., 2005; Kendrick et al., 2006; Dickens,2007)或异种叶甲(Loughrin et al., 1995)具有引诱效应。
榆紫叶甲(Ambrostoma quadriimpressum)以成虫取食危害家榆(Ulmus pumila)、春榆(Ulmus japonica)和黄榆(Ulmus macrocarpa)等榆树的芽孢和叶片,连续危害造成榆树春季不发叶、整株枯死或变成“小老树”。目前的研究多集中在生物生态学(萧刚柔,1992; 安瑞军等,2005; 李彬等,2007)、化学防治(梁成杰等,1990; 史万林等,1993; 迟德富等,1995; 安丽萍等,2006)和天敌防治(钱范俊等,1981; 高长启,1987; 孟繁君等,2009)等方面。榆紫叶甲属聚集分布型(迟莉等,2007),寄主挥发物调控其成虫的聚集行为未见报道。初步的行为生测结果表明:成虫取食为害4 h后的榆树挥发物对同种叶甲的成虫具有显著的引诱作用。所以,通过深入分析虫害诱导榆树释放的挥发物的组成变化及其对成虫相应的电生理和行为生测,筛选有效的活性成分,为下一步开发植物引诱剂提供理论依据。
1 材料与方法 1.1 试验材料 1.1.1 供试榆苗试验所用的榆苗均是在室内培育的1年生扦插苗。单株移植至花盆,在自然光照条件下,室内温度保持在23~25 ℃,相对湿度保持在65%左右进行培育。试验时选取无病虫害、高度在80 cm左右、长势基本一致的榆苗。
1.1.2 供试昆虫榆紫叶甲成虫采自吉林省前郭县乌兰图嘎林场,采集后在同一养虫笼内饲养2天后,分开雌雄,并在不同养虫笼内分别饲养。在自然光照条件下,室内温度保持23~25 ℃,相对湿度65%左右。于试验前1天停止喂饲,使其饥饿24 h后用于试验。
1.1.3 采样袋和吸附剂采样袋为PET材质的保鲜袋(Bratenschlauch,Toppits,Minden,德国)。吸附剂为Super-Q (80~100目,Alltech,Deerfield,Illinois,美国),吸附管内径4 mm,每根填充35 mg吸附剂。
1.1.4 化学试剂及标准品溶剂正己烷(色谱级)由天津市光复精细化工研究所生产; 标准品包括顺-3-己烯醇、α-蒎烯、柠檬烯、顺-3-乙酸叶醇酯、芳樟醇、水杨酸甲酯、石竹烯、α-法尼烯购自美国Sigma公司,纯度大于95%。
1.2 试验方法 1.2.1 动态顶空法采集植物挥发物健康寄主挥发物的采集:采样袋(1.2 m长)套住整个枝条后,用自锁扎线带绑住内衬脱脂棉的袋口一端,另一端插入一长一短2根特氟龙(Teflon)管后用同样方法封住袋口。长Teflon管依次连接玻璃管(长10 cm,内径2 cm,添装活性炭)、气体流量计和大气采样仪(北京劳动保护研究所,QC-1)的出气口; 短Teflon管依次接吸附管、气体流量计和大气采样仪的进气口。大气采样仪的出气口端气流保持在600 mL·min-1,进气口气流略低,从而使洁净空气在袋内循环流动,通过这种动态捕集方式把榆苗释放的挥发物带入吸附管内。采样前先用大气采样仪把袋内空气抽干,然后再充进经活性炭过滤的空气,反复3次。当气体充满整个取样袋后,连接上述闭路系统开始采样。
虫害诱导的植物挥发物的采集:榆苗在使用前用清水将叶片冲洗干净,然后放入取样袋中并绑住靠近主茎的一端,放置1天后开始接虫。早8时在上述榆苗上接入50头榆紫叶甲成虫(雌:雄= 1:1)。
2个健康苗和2个虫害苗同时在正午12时开始采集,采集时间为1 h,每种处理重复4次。采集的植物挥发物用500 μL二氯甲烷洗脱。
1.2.2 质谱分析采用气谱HP6890联用HP5973质谱仪,HP-5MS毛细管柱(30 m × 0.25 mm ID,膜厚0.25 μm)分析不同生理状态的植物挥发物。色谱条件为:无分流进样模式进样1 μL。程序升温为40 ℃ (保持2 min),6 ℃·min-1升至250 ℃,保持2 min。高纯氦气作载气,柱流量1.0 mL·min-1。接口温度280 ℃。质谱条件为: EI离子源,源温度230 ℃,电离能70 eV。通过谱库(NIST98)对分析物进行初步的结构鉴定,通过比较标准化合物的质谱图及色谱保留时间确定分析成分。以50 ng癸酸乙酯作外标对分析成分进行定量分析。
1.2.3 触角电位触角电位测试方法见Asaro等(2004)的文献。用刀片将触角从基部切下,并切除尖端1~2 mm。用导电胶把触角的两端连接在金属记录电极和参比电极上进行EAG测定。连续气体和刺激气体流量均设为500 mL·min-1,刺激时间为0.5 s,刺激时间间隔1~2 min。洁净湿润的空气通过直径为8 mm、长10 cm的玻璃管吹向距离端口0.5 cm处的触角上。滤纸片(1 cm2)放入200 μL的移液枪枪头内,然后滴加10 μL测试样品溶液,待溶解挥发后开始随机测试待分析样品,每个成分10次重复。测试信号经AC/DC UN-06 (Syntech,Hilversum,The Netherland)放大器放大,EAG (Syntech)软件记录数据并进行分析。为消除昆虫个体间的差异和溶剂的影响,在EAG测试开始和结束时用正己烷(1 mg·mL-1)分别测试1次作为空白对照,以平均值校正样品的EAG值。
1.2.4 嗅觉仪生物测定利用“Y”型嗅觉仪在暗室内测定榆紫叶甲对家榆挥发物的行为反应。暗室温度控制在23~25 ℃,相对湿度为65%。嗅觉仪的构造及具体生测方法参照刘芳等(2009)和娄永根等(2002)的方法并改进而成。“Y”型嗅觉仪的两臂及直管均长10 cm,内径1 cm,两臂夹角70°。“Y”型管臂分别通过Teflon管与2个作为挥发物源的玻璃管(长10 cm,直径2 cm)相连。在气流进入味源瓶之前,先经过一个活性炭和蒸馏水瓶,以净化空气和增加空气湿度。每臂的气流流量通过气体流量计控制在2.5 L·min-1。生测时,以大气采样仪的出气口作为气流源,在Y型管前上方50 cm处放一22 W的日光灯。
将榆紫叶甲成虫引入Y型嗅觉仪的直管内,观察记录3 min内榆紫叶甲对两臂气味源的选择性反应。当榆紫叶甲爬至超过某臂的5 cm处并持续1 min以上者,记录榆紫叶甲对该臂的挥发物做出了选择; 如果在榆紫叶甲引入5 min后没有做出选择的,则记为无反应。为消除各种因素的影响,在整个生测过程中,每测试5头成虫,用99%的酒精清洗嗅觉仪,并交换嗅觉仪两臂的方向; 每测试10头,将两气味源交换位置。每次只引入1头成虫,每头只用于1次测试。每个化合物做40个有效重复。植物气味源的准备同挥发物收集,接虫4 h后方可作为味源使用,每小时更换1次。待测化合物浓度为1 mg·mL-1,每次用毛细管取20 μL用于测试,毛细管放于味源瓶中,空白一端取同等量的溶剂作对照。
1.3 数据统计用Statistica软件(Statistica,SAS Institute Inc.,Cary,NC,USA)进行数据分析。健康和虫害榆苗的挥发物定量比较采用t检验。触角电位的数据采用单因素方差分析,然后进行Duncan’ s多重比较法分析榆紫叶甲雌、雄成虫各自对系列挥发物质反应的差异,雌雄之间反应的差异采用t检验。Y型嗅觉仪行为生测中所得数据采用卡方检验比较。
2 结果与分析 2.1 榆紫叶甲对健康榆苗和虫害榆苗的选择行为Y型嗅觉仪的试验结果如图 1所示。与空气对照相比,榆紫叶甲成虫明显选择健康的榆苗。当榆苗被榆紫叶甲成虫危害4 h后,榆紫叶甲成虫对被害榆苗的选择性显著高于健康榆苗。

|
图 1 榆紫叶甲雌雄成虫在Y型管中对榆苗的气味选择行为 Figure 1 Responses of walking Ambrostoma quadriimpressum to different odour sources from elm seelding in a Y-tube olfactometer * : χ2检验差异显著(P < 0.05) Significant difference by χ2 analysis (P < 0.05). |
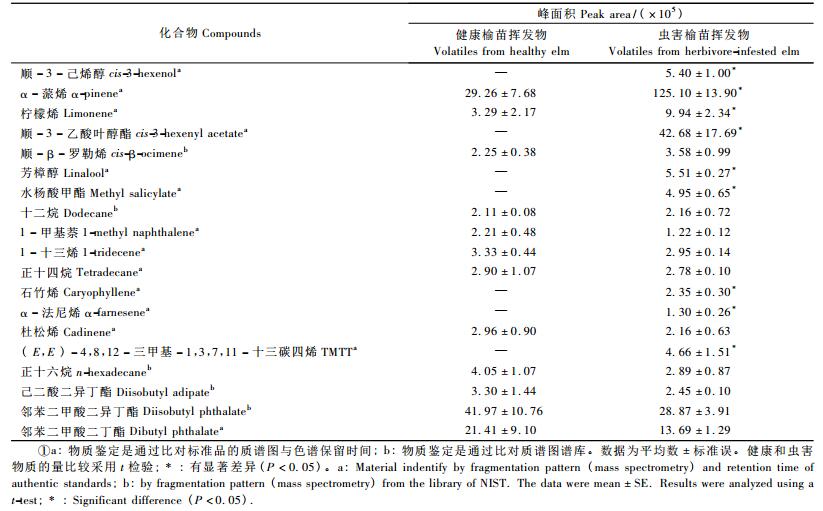
健康榆苗挥发物中含有12种化合物,榆紫叶甲成虫危害诱导的榆苗挥发物中含有19种化合物,各化合物的名称及相对定量详见表 1。由表 1可见:受榆紫叶甲成虫取食诱导之后4 h的榆苗的挥发物相比健康榆苗,不论种类还是含量都增加,其中顺-3-己烯醇、α-蒎烯、柠檬烯、顺-3-乙酸叶醇酯、芳樟醇、水杨酸甲酯、石竹烯、α-法尼烯和TMTT 9种物质的含量与健康相比有显著差异,因此将除了TMTT以外(标准品尚未商品化,无法购买)的8种物质用于生测。
|
|
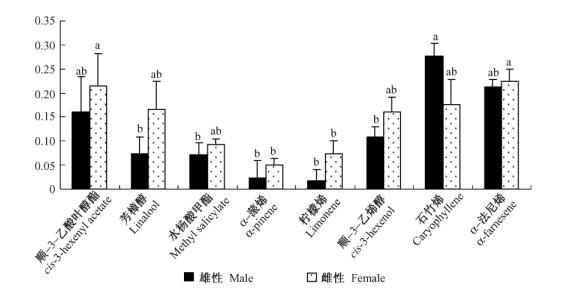
8种化学标准品用于触角电位试验,结果见图 2。雌性榆紫叶甲成虫对α-法尼烯(0.224 ± 0.060) mV、顺-3-乙酸叶醇酯(0.215 ± 0.067) mV、芳樟醇(0.167 ± 0.057) mV、顺-3-己烯醇(0.161 ± 0.046) mV、石竹烯(0.175 ± 0.067) mV、水杨酸甲酯(0.092 ± 0.053) mV 6种成分的EAG反应差异不显著,但是显著高于α-蒎烯(0.051 ± 0.025) mV和柠檬烯(0.073 ± 0.031) mV; 而雄性成虫对石竹烯(0.276 ± 0.089) mV的EAG反应强烈,但是与顺-3-乙酸叶醇酯(0.161 ± 0.0747) mV、α-法尼烯(0.214 ± 0.073) mV差异不显著,而与芳樟醇(0.073 ± 0.035) mV、顺-3-己烯醇(0.108 ± 0.047) mV、水杨酸甲酯(0.071 ± 0.027) mV、α-蒎烯(0.023 ± 0.015) mV、柠檬烯(0.017 ± 0.011) mV差异显著。雌雄二性之间对同一物质的反应没有差异。
|
图 2 榆紫叶甲对8种标准品在单一浓度(1 mg·mL-1)的相对EAG反应 Figure 2 Relative EAG responses of Ambrostoma quadriimpressum to 8 compounds at a single dose (1 mg·mL-1) 不同字母表示针对同一性别成虫,对不同化合物反应在0.05水平有显著差异。 The EAG responses of antennae to each chemical were compared by ANOVA, and if significant at P < 0.05, the differences among means were tested for significance according to the Duncan's test method. Different letters indicate the significant difference (P < 0.05) for the same sex of radult. |
根据触角电位的分析结果,榆紫叶甲雌雄二性对α-蒎烯和柠檬烯的EAG反应都很弱,因而选择顺-3-己烯醇、顺-3-乙酸叶醇酯、芳樟醇、水杨酸甲酯、石竹烯、α-法尼烯6种成分进行雌雄成虫行为测定,结果见图 3。雌性榆紫叶甲对芳樟醇和α-法尼烯的反应与空白对照比较,有极显著差异(P < 0.01);雄性榆紫叶甲对α-法尼烯、β-石竹烯、雌性榆紫叶甲对顺-3-己烯醇的反应与对照比较,有显著差异(P < 0.05)。雌雄二性榆紫叶甲对顺-3-乙酸叶醇酯和水杨酸甲酯的反应与对照相比没有差异。

|
图 3 榆紫叶甲在Y型管中的行为选择 Figure 3 Responses of walking Ambrostoma quadriimpressum to different choice situations in a Y-tube olfactometer. 结果用卡方检验分析 Results were analyzed using a χ2 analysis (*: P < 0.05, **: P < 0.01). |
大量研究结果证实虫害诱导的植物挥发物(HIPVs)具有多种生态功能。例如,通过吸引植食性昆虫的天敌前来捕食或寄生从而实现其间接防御功能,驱避同种害虫的危害而起到直接防御功能,对多种植食性昆虫的性信息素和聚集信息素可起到增效作用,以及对同种或异种植食性昆虫具有引诱作用(Turlings et al., 2004; Delphia et al., 2007; Erbilgin et al., 2007; Kalberer et al., 2001; OtáloraLuna et al., 2009)。本研究结果表明:无论是健康的榆树苗还是虫害苗均对榆紫叶甲具有引诱作用。这一现象也存在于叶甲科的其他昆虫,如马铃薯叶甲(Leptinotarsa decimlineata) (Bolter et al., 1997)、茄子叶甲(Epitrix fuscula) (Zilkowski et al., 2006)和叶甲(Oreina cacaliae) (Kalberer et al., 2001)。此外,象甲科的甘薯象甲(Sitophilus zeamais)、谷象(S.granarius)、米象(S.oryzae) (Wang et al., 2002; Wakefield et al., 2005)和茶丽纹象甲(Sun et al., 2010)也同样可以被寄主挥发物或同种象甲危害后的寄主挥发物所引诱。
榆紫叶甲成虫在田间为聚集型分布(迟莉等,2007),是对本研究结果的佐证。榆紫叶甲成虫取食诱导的榆苗挥发物为同种叶甲成虫提供了大量的寄主适合度信息,大大缩短了榆紫叶甲成虫在定位寄主时所需的时间,从而减少了被天敌发现的可能,是植物-植食性昆虫-天敌三者间协同进化的结果。Meiners等(2000; 2005)的研究结果显示:榆叶甲(Xanthogaleruca luteola)会依据产卵诱导的寄主挥发物调整其产卵密度,当榆树枝上有少量卵着生,产卵诱导的挥发物吸引雌性甲虫继续产卵; 当枝上布满卵粒,诱导的挥发物就会驱避产卵而对其卵寄生蜂———寡节小蜂(Oomyzus gallerucae)具有引诱作用。
如前所述,很多研究均证实HIPVs对叶甲科昆虫具有引诱作用,HIPVs中挥发性化合物中活性成分的分离和鉴定亦有一些报道,如:芳樟醇、顺-3-己烯基醋酸酯以及水杨酸甲酯的混合物对马铃薯甲虫具有吸引作用(Dickens,2000),(2E,4E,6Z) -2,4,6-nonatrienal和(2E,4E,6E) -2,4,6-nonatrienal混合对茄子叶甲具有引诱活性(Zilkowski et al., 2006),但榆紫叶甲诱导榆树释放的挥发物中的活性成分还未见报道。本研究通过触角电位和Y型嗅觉仪试验证实α-法尼烯、芳樟醇、顺-3-己烯醇对榆紫叶甲雌性成虫具有显著的引诱活性,石竹烯和α-法尼烯则对雄性成虫具有显著的引诱作用。然而,这几种挥发物并非仅对榆紫叶甲具有生态功能,在其他多种植物和植食性昆虫的研究中均有报道。例如:顺-3-己烯醇可以调节植物发送信号和传递植物间的信息(Ruther et al., 2005); 法尼烯是很多蚜虫告警信息素的主要成分(Beale et al., 2006),也是榆树被榆叶甲卵寄生和取食后榆叶提取物的主要成分(Wegener et al., 2001); 石竹烯对忽布疣额蚜(Phorodon humuli)具有引诱作用(Campbell et al., 1993),还被证实是纵坑切梢小蠹(Tomicus piniperda) (Faccoli et al., 2008)聚集信息素中的一种成分(Verheggen et al., 2007)。水杨酸甲酯作为一种重要的化学信号物质,在很多天敌中都发现有生物活性,是植物组织受害后的信号级联产物(Turlings et al., 2006),并刺激昆虫的嗅觉接收神经元(Shields et al., 2001; De Bruyne et al., 2001),在田间用于引诱西部大眼长蝽(Geocoris pallens)和食蚜蝇(Hover flies) (James et al., 2004),但在行为生测中水杨酸甲酯对榆紫叶甲成虫并未显示有引诱作用。
本研究为开发引诱剂用于监测和防治榆紫叶甲提供了重要的理论基础,但是上述物质在田间对榆紫叶甲的引诱活性则尚待进一步研究。
安丽萍, 许铁军, 王威, 等. 2006. 榆紫叶甲不同发育历期药剂防治[J]. 林业勘查设计, (4): 35-38. |
安瑞军, 李秀辉, 张冬梅. 2005. 榆紫叶甲生物学特性的研究[J]. 林业科技, 30(5): 18-20. |
迟德富, 苗建才, 曲辉, 等. 1995. 灭幼脲和氟幼灵防治榆紫叶甲的研究[J]. 东北林业大学学报, 23(2): 40-47. |
迟莉, 蒲子钢, 王威. 2007. 榆紫叶甲种群空间分布型及其应用[J]. 黑龙江八一农垦大学学报, 19(4): 41-45. |
高长启. 1987. 关于榆紫叶甲(Ambrostoma quadriimpressum Motschulsky)综合防治技术的几项建议[J]. 吉林林业科技, (4): 29-30. |
李彬, 苏元吉, 李海山. 2007. 牡丹江榆树新记录害虫---榆紫叶甲的生物学特性与防治[J]. 中国林副特产, 87(2): 59. |
梁成杰, 舍楞, 王建文, 等. 1990. 两种菊酯农药防治榆紫叶甲试验[J]. 农药, 29(2): 52-53. |
刘芳, 包善微, 卢海燕, 等. 2009. 稻虱缨小蜂对吡虫啉处理的水稻植株挥发物的行为反应[J]. 植物保护学报, 36(1): 70-76. |
娄永根, 程家安, 平霄飞, 等. 2002. 稻虱缨小蜂对褐飞虱和白背飞虱卵的识别机制[J]. 昆虫学报, 45(6): 770-776. |
史万林, 童进义, 梁春秀. 1993. 榆紫叶甲的化学防治试验[J]. 林业科技, 18(1): 24-25. |
孟繁君, 张大明, 宋丽文, 等. 2009. 榆紫叶甲生物学特性及其防治技术[J]. 林业科技, 34(3): 33-34. |
钱范俊, 郑孝如, 裴玉芹. 1981. 榆紫叶甲卵的天敌初步观察[J]. 昆虫天敌, (21): 79-82. |
萧刚柔. 1992. 中国森林昆虫[M]. 2版(增订本). 北京: 中国林业出版社: 521-523.
|
Asaro C, Sullivan B T, Dalusky M J, et al. 2004. Volatiles associated with preferred and nonpreferred hosts of the Nantucket pine tip moth, Rhyacionia frustrana[J]. Journal of Chemical Ecology, 30(5): 977-990. DOI:10.1023/B:JOEC.0000028462.05927.fa |
Barata E N, Pickett J A, Wadhams L J, et al. 2000. Identification of host and nonhost semiochemicals of eucalyptus woodborer Phoracantha semipunctata by gas chromatographyelectroantennography[J]. Journal of Chemical Ecology, 26(8): 1877-1895. DOI:10.1023/A:1005548824429 |
Bartlet E, Blight M M, Lane P, et al. 1997. The responses of the cabbage seed weevil Ceutorhynchus assimilis to volatile compounds from oilseed rape in a linear track olfactometer[J]. Entomologia Experimentalis et Applicata, 85(3): 257-262. DOI:10.1046/j.1570-7458.1997.00256.x |
Beale M H, Birkett M A, Bruce T J A, et al. 2006. Aphid alarm pheromone produced by transgenic plants affects aphid and parasitoid behavior[J]. Proceeding of the National Academy of Sciences, 103(27): 10509-10513. DOI:10.1073/pnas.0603998103 |
Blight M M, Pickett J A, Wadhams L J, et al. 1995. Antennal perception of oilseed rape, Brassica napus (Brassicaceae), volatiles by the cabbage seed weevil Ceutorhynchus assimilis (Coleoptera: Curculionidae)[J]. Journal of Chemical Ecology, 21(11): 1649-1664. DOI:10.1007/BF02033667 |
Bolter C J, Dicke M, Van Loon J J A, et al. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination[J]. Journal of Chemical Ecology, 23(4): 1003-1023. DOI:10.1023/B:JOEC.0000006385.70652.5e |
Campbell C A M, Pettersson J, Pickett J A, et al. 1993. Spring migration of Damson-Hop aphid, Phorodon humuli (Homoptera: Aphididae), and summer host plant-derived semiochemicals released on feeding[J]. Journal of Chemical Ecology, 19(7): 1569-1576. DOI:10.1007/BF00984897 |
Cossé A A, Bartelt R J, Zilkowski B W. 2002. Identification and electrophysiological activity of a novel hydroxy ketone emitted by male cereal leaf beetles[J]. Journal of Natural Products, 65(8): 1156-1160. DOI:10.1021/np020063q |
De Bruyne M, Foster K, Carlson J R. 2001. Odor coding in the Drosophila antenna[J]. Neuron, 30(2): 537-552. DOI:10.1016/S0896-6273(01)00289-6 |
Delphia C M, Mescher M C, De Moraes C M. 2007. Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips[J]. Journal of Chemical Ecology, 33(5): 997-1012. DOI:10.1007/s10886-007-9273-6 |
Dicke M, Van Beek T A, Posthumus M A, et al. 1990. Isolation and identification of volatile kairomone that affects acarine predator-prey interactions[J]. Journal of Chemical Ecology, 16(2): 381-396. DOI:10.1007/BF01021772 |
Dicke M. 1998. Induced indirect plant defence: communication and exploitation in multitrophic context[J]. Mitt Disch Ges Allg Angew Ent, 11: 453-464. |
Dicke M, Van Loon J J A. 2000. Multitrophic effects of herbivoreinduced plant volatiles in an evolutionary context[J]. Entomologia Experimentalis et Applicata, 97(3): 237-249. DOI:10.1046/j.1570-7458.2000.00736.x |
Dickens J C. 2000. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants[J]. Agricultural and Forest Entomology, 2(3): 167-172. DOI:10.1046/j.1461-9563.2000.00065.x |
Dickens J C. 2007. Sexual contact influences orientation to plant attractant in Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae)[J]. Naturwissenschaften, 94(10): 847-852. DOI:10.1007/s00114-007-0261-z |
Erbilgin N, Krokene P, Kvamme T, et al. 2007. A host monoterpene influences Ips typographus (Coleoptera: Curculionidae, Scolytinae) responses to its aggregation pheromone[J]. Agricultural and Forest Entomology, 9(2): 135-140. DOI:10.1111/afe.2007.9.issue-2 |
Faccoli M, Anfora G, Tasin M. 2008. Responses of the mediterranean pine shoot beetle Tomicus destruens (Wollaston) to pine shoot and bark volatiles[J]. Journal of Chemical Ecology, 34(9): 1162-1169. DOI:10.1007/s10886-008-9503-6 |
Halitschke R, Stenberg J A, Kessler D, et al. 2008. Shared signals 'a larm calls'from plants increase apparency to herbivores and their enemies in nature[J]. Ecology Letters, 11(1): 24-34. |
James D G, Price T S. 2004. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops[J]. Journal of Chemical Ecology, 30(8): 1613-1628. DOI:10.1023/B:JOEC.0000042072.18151.6f |
Kalberer N M, Turlings T C J, Rahier M. 2001. Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants[J]. Journal of Chemical Ecology, 27(4): 647-661. DOI:10.1023/A:1010389500009 |
Kendrick A P, Raffa K F. 2006. Sources of insect and plant volatiles attractive to cottonwood leaf beetles feeding on hybrid poplar[J]. Journal of Chemical Ecology, 32(12): 2585-2594. DOI:10.1007/s10886-006-9184-y |
Landolt P J, Tumlinson J H, Alborn D H. 1999. Attraction of Colorado potato beetle (Coleoptera: Chrysomelidae) to damaged and chemically induced potato plants[J]. Environmental Entomology, 28(6): 973-978. DOI:10.1093/ee/28.6.973 |
Loughrin J H, Manukian A, Heath R R, et al. 1995. Volatiles emitted by different cotton varieties damaged by feeding beet armyworm larvae[J]. Journal of Chemical Ecology, 21(8): 1217-1227. DOI:10.1007/BF02228321 |
Meiners T, Hilker M. 2000. Induction of plant synomones by oviposition of a phytophagous insect[J]. Journal of Chemical Ecology, 26(1): 221-232. DOI:10.1023/A:1005453830961 |
Meiners T, Hacker N K, Anderson P, et al. 2005. Response of the elm leaf beetle to host plants induced by oviposition and feeding: the infestation rate matters[J]. Entomologia Experimentalis et Applicata, 115(1): 171-177. DOI:10.1111/eea.2005.115.issue-1 |
Otálora-Luna F, Hammock J A, Alessandro R T, et al. 2009. Discovery and characterization of chemical signals for citrus root weevil, Diaprepes abbreviatus[J]. Arthropod-Plant Interactions, 3(2): 63-73. DOI:10.1007/s11829-009-9058-7 |
Peng C, Weiss M J. 1992. Evidence of an aggregation pheromone in the flea beetle, Phyllotreta cruciferae (Goeze) (Coleoptera, Chrysomelidae)[J]. Journal of Chemical Ecology, 18(6): 875-884. DOI:10.1007/BF00988328 |
Ruther J, Kleier S. 2005. Plant-plant signaling: ethylene synergizes volatile emission in Zea mays induced by exposure to (Z) -3-hexen-1-ol[J]. Journal of Chemical Ecology, 31(9): 2217-2222. DOI:10.1007/s10886-005-6413-8 |
Shields V D C, Hildebrand J G. 2001. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds[J]. Journal of Comparative Physiology, 186(12): 1135-1151. DOI:10.1007/s003590000165 |
Sun X L, Wang G C, Cai X M, et al. 2010. The tea weevil, Myllocerinus aurolineatus, is attracted to volatiles induced by conspecifics[J]. Journal of Chemical Ecology, 36(4): 388-395. DOI:10.1007/s10886-010-9771-9 |
Tansey J A, McClay A S, Cole D E, et al. 2005. Evidence for the influence of conspeciflc chemical cues on Aphthona nigriscutis (Coleoptera: Chrysomelidae) behaviour and distribution[J]. Bio Control, 50(2): 343-358. |
Turlings T C J, Tumlinson J H, Lewis W J. 1990. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps[J]. Science, 250: 1251-1253. DOI:10.1126/science.250.4985.1251 |
Turlings T C J, Wäckers F L. 2004. Recruitment of predators and parasitoids by herbivore damaged plants//Cardé R T, Millar J G. Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, UK, 21-75.
|
Turlings T C J, Ton J. 2006. Exploiting scents of distress: the prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests[J]. Current Opinion in Plant Biology, 9(4): 421-427. DOI:10.1016/j.pbi.2006.05.010 |
Van Loon J J A, Wang C Z, Nielsen J K, et al. 2002. Flavonoids from cabbage are feeding stimulants for diamondback moth larvae additional to glucosinolates: chemoreception and behaviour[J]. Entomologia Experimentalis et Applicata, 104(1): 27-34. DOI:10.1046/j.1570-7458.2002.00987.x |
Verheggen F J, Fagel Q, Heuskin S, et al. 2007. Electrophysiological and behavioral responses of the multicolored Asian lady Beetle, Harmonia axyridis Pallas, to sesquiterpene semiochemicals[J]. Journal of Chemical Ecology, 33(11): 2148-2155. DOI:10.1007/s10886-007-9370-6 |
Vet L E M, Dicke M. 1992. Ecology of infochemical use by natural enemies in a tritrophic context[J]. Annual Review of Entomology, 37: 141-172. DOI:10.1146/annurev.en.37.010192.001041 |
Wakefield M E, Bryning G P, Chambers J. 2005. Progress towards a lure to attract three stored product weevils, Sitophilus zeamais Motschulsky, S. oryzae (L.) and S. granarius (L.) (Coleoptera: Curculionidae)[J]. Journal of Stored Products Research, 41(2): 145-161. DOI:10.1016/j.jspr.2004.01.001 |
Wang Y, Kays S J. 2002. Sweetpotato volatile chemistry in relation to sweetpotato weevil (Cylas formicarius) behavior[J]. Journal of the American Society for Horticultural Science, 127(4): 656-662. |
Wegener R, Schulz S, Meiners T, et al. 2001. Analysis of volatiles induced by oviposition of elm leaf beetle Xanthogaleruca luteola on Ulmus minor[J]. Journal of Chemical Ecology, 27(3): 499-515. DOI:10.1023/A:1010397107740 |
Wright G A, Smith B H. 2004. Variation in complex olfactory stimuli and its influence on odour recognition[J]. Proceedings of the Royal Society London, 271(1535): 147-152. DOI:10.1098/rspb.2003.2590 |
Zilkowski B W, Bartelt R J, Cossé A A, et al. 2006. Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula):identification, synthesis, and field biossays[J]. Journal of Chemical Ecology, 32(11): 2543-2558. DOI:10.1007/s10886-006-9163-3 |
 2010, Vol. 46
2010, Vol. 46