文章信息
- Liu Hongjun, Zhang Meng, Zhou Yonghong
- 刘红军, 张猛, 周永红
- Acid Isomer Separation of Resin Acids in Rosin and Abietic Acid Molecular Structure
- 松脂中树脂酸的酸异构分离与枞酸分子结构
- Scientia Silvae Sinicae, 2010, 46(8): 140-144.
- 林业科学, 2010, 46(8): 140-144.
-
文章历史
- Received date: 2008-08-27
- Revised date: 2009-01-10
-
作者相关文章
2. Jiangsu Qianglin Biomass Energy Co., Ltd. Liyang 213364
2. 江苏强林生物能源有限公司 溧阳 213364
Abietic acid is a levo-chiral, three rings diterpenoid compound, which naturally exists in the plant. It has biological activity, four chiral carbons and conjugated double bonds in its structure. So it can be used as chiral medicine, synthesized precursor compound(Okada et al., 1994; Ulusu et al., 2002). About 50% of resin acids of Chinese Pinus massoniana is abietic acid. Especially, the abietic type resin acid with conjugated double bonds will be apt to isomery to abietic acid by heat or acid.
Presently, the production of Chinese Pinus massoniana rosin attains 600 000 t·a-1, so abietic acid will become an important biomass resource in developing new chiral drug(Chuang et al., 2004; Shulman, 1976; Legrouyellec, 1985; Yokogawa et al., 1990a;1990b;Fernandez et al., 2001). The preparation of abietic acid in earlier period is that abietic type resin acid was rearranged with HCl or glacial acetic acid and then crystallized with used sodium or amine salt(Steele, 1922; Jin et al., 2000; Harris et al., 1948; Wang et al., 2000; Han et al., 2007; Zhou et al., 1990). Because these methods are pollutional and uneconomical for environment, there is no industrialization merchandise; it directly affected the application in the medicine field. Moreover, people studied the structure of abietic acid through isomery rearrangement reaction less, and especially, the data of MS, 1HNMR, 13CNMR, IR of abietic acid with high purity has not appeared in papers. The structure of title compound was determined by spectrals and single-crystal X-ray diffraction analysis. This research will be a bridge for the application of abietic acid.
1 Experiment 1.1 Material and instrumentPinus massoniana rosin was kindly supplied by Wuzhou city of Guangxi Province (China), first grade; other laboratory reagents were provided by various manufacturers in China. Melting point: YRT-3; HPLC: SHIMADZU LC-20AB; IR: Nicolet Magna-IR 550(American); MS: Waters TOF; NMR: BRUKER DR×500.
1.2 Analytical methods 1.2.1 Purity analysis of abietic acidHPLC (area normalization method) (Hrobonova et al., 2005): Column model ODS-C-18, 6 mm×150 mm; column temperature 40 ℃; mobile phase methyl alcohol; the flow velocity 1 mL·min-1; ultraviolet detector and UV λmax: 241 nm.
1.2.2 FTIR spectraInfrared spectrum was recorded as KBr pellets on Nicolet Magna-IR 550 spectropotometer. Typically 100 scans within the range of 4 000~400 cm-1 were done for each sample with the resolution of 2 cm-1and summed up to get the spectra.
1.2.3 The single-crystal X-ray diffraction analysisInstrument model number Bruker Smart Apex II CCD, the sample tested was choosen from the recrystal abietic acid.
1.2.4 NMR spectraNMR spectra were measured using DMSO as solvent and tetramethylsilane as internal standard.
1.3 Synthesis of abietic acid(Liu et al., 2008)100 g rosin (Pinus massoniana), 200 mL glacial acetic acid were put into a 500 mL three-necked flask, heated to 70 ℃ in the water bath. Then 30 g macropore strong acidity, cation exchange resin were introduced in until rosin was dissolved with stiring. After stiring four hours at 70 ℃, the mixture was filtered and cooled to room temperature overnight to obtain 86.7 g crude abietic acid crystal, which was recrystallized three times from aqueous ethanol by slow evaporation. 74.2 g colorless monoclinic prim crystals were obtained after drying at 50 ℃ and pressure of 20 kPa for 2 h. Mp 174.2~174.5 ℃(capillary tube method), [α] D20= -107 ° (C=5 EtOH), Data were collected at room temperature[22 ℃].
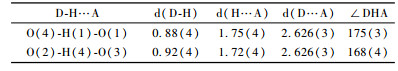
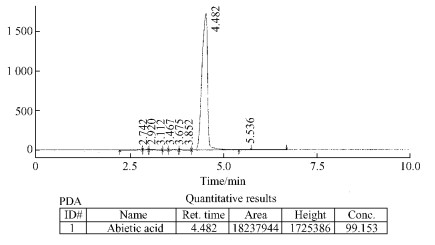
2 Result and discussion 2.1 HPLCHPLC analysis were isolated from a total of nine peaks, the main peak retention time of 4.482 min is that abietic acid content of 99.15%. The other eight component peaks for the trace shown in Fig. 1.
|
Fig.1 HPLC of abietic acid |
IR(KBr)ν, cm-1:3 427.1(m, νOH—COOH); 3 200~2 600(s, νOH, νCOOH); 2 935.1, 2 866.4, 537.2(s, νCH); 1 692.1(s, νC=O, νC=C); 1 468.6(s, νOH, νc=c), 1 395.5(s, νCH2); 1 281.7(s, νC-O); 956.7(m, νCH).
1HNMR(DMSO, 500 MHz), δ:0.768 83(S, 3H, —CH3); 0.977 67~0.981 61(S, 3H, —CH3); 0.991 36~1.014 55(S, 3H, —CH3); 1.179 69~1.132 55(S, 3H, —CH3); 12.100 00(m, 1H, —COOH); 5.349 21(s, 1H, =CH); 5.731 43(s, 1H, =CH); 2.235 19~2.181 84(m, 1H, CH); 1.705 38~1.656 32(m, 1H, CH); 1.574 20(m, 1H, CH); 2.096 29~2.027 61(m, 2H, —CH2); 2.000 32~1.934 38(m, 2H, —CH2); 1.850 89~1.798 28(m, 2H, —CH2); 1.768 13~1.721 12(m, 2H, —CH2); 1.543 79~1.524 95(m, 2H, —CH2); 1.121 27~1.061 04(m, 2H, —CH2).
13CNMR(DMSO, 500 MHz), δ: 13.781(δC19), 16.856(δC20), 17.820(δC3), 20.709(δC16), 21.296(δC17), 22.073(δC2), 25.205(δC1), 26.953(δC11), 34.033(δC5), 34.342(δC4), 36.920(δC6), 38.033(δC12), 44.590(δC9), 45.341(δC10), 50.560(δC15), 120.496(δC7), 122.505(δC14), 134.934(δC8), 144.135(δC13), 179.299(δC18).
MS(EI, 70 eV), m/z(%):302.0(83.47)(M—Me); 287.0(30.5)(M—CH3); 259.0(28.30)(M—CH(CH3)2); 241.0(71.56)(M—CH3, —COOH); 185.0(26.91)(M—CH3, —COOH, —CH3, —CH(CH3)2); 105.0(82.00)(C8H11); 91.0(100.00)(C7H8).
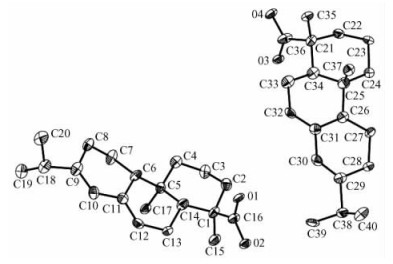
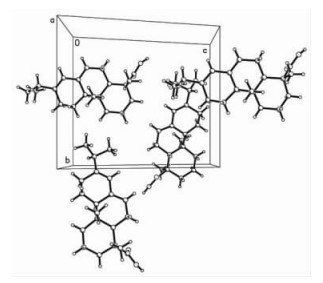
2.2 Singal-crystal X-ray diffraction analysisThe crystal and molecular structure of the abietic acid have determined by singal-crystal X-ray diffraction analysis: The molecular formula C20H30O2, M=302.44, Crystals are monoclinic (in Fig. 2); space group P21(in Fig. 3). a=11.755 7(14), b=11.909 7(14), c=14.101 2(17) Å, β =112.077(2)°, Dx =1.098 g·cm-3, Z=4.
|
Fig.2 The asymmetric unit elipsoid diagram of the object compound (probability 30% of elipsoid) |
|
Fig.3 View of the crystal packing down the c axis for the title compound |
Fig. 2 and 3 show the molecular structure and perspective view of the crystal packing in the unit cell of the title compound, respectively. The selected bond lengths, bond angles and hydrogen-bonding geometry are listed in Tab. 1 and 2, respectively. In the title compound, the bond lengths and bond angles are all normal, and those in the hydrophenanthrene ring are also in good agreement with the values reported previously. As shown in Fig. 3, the packing structure of the title compound shows a three-dimensional supramolecular network formed via intermolecular O—H…O hydrogen bonds and weak π-π stacking interactions. Torsion angles show that ring (C(5), C(6), C(11) ~C(14)) and ring (C(6) ~C(11)) exhibit chair and half-chair configurations, respectively. The carboxy group is directly attached to the chiral atom of hydrophenanthrene structure with trans configuration.Two methyl groups(C(19) and C(20)) are disordered in the structure. The title compound has four chiral centers. The ORTEP diagram reveals the R—, R—, S— and R— absolute stereo-configurations for C(1), C(5), C(6) and C(14)in turn. The bond lengths of C(9)-C(10) and C(11)-C(12) are1.339(5) and 1.347(5) Å, respectively, which are indicative of double bond.
|
|
|
|
In this research, the isomery reaction of abietic type resin acids in rosin was catalyzed by cation exchange resin of macropore strong acidity. The reaction was shown in Fig. 4. First levo-pimaric acid, abietic acid and palustric acid in the presence of H+ formed their own relatively stable allyl-type carbon cation, then rearranged to 5. The hydrogen is adjacent to the B ring of 5 to leave as a form of H+ proton, the remaining electron delocalization are occupied by two P tracks of adjacent carbons to form π bond. So, abietic acid was obtained.
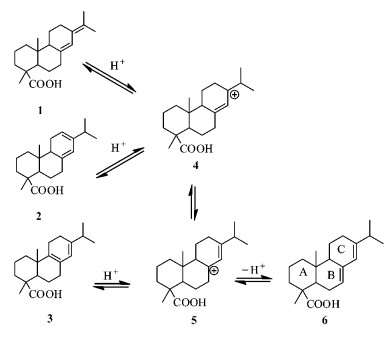
|
Fig.4 Resin in the resin acid composition of the resin acids abietic acid heterogeneous reaction mechanism 1. Neoabietic acid; 2. Levopimaric acid; 3. Palustric acid; 4, 5.Carbocation intermediate; 6. Abietic acid. |
In the crystal structure of abietic acid, it generated two hydrogen bonds connection, which is the main reason for high-purity crystal.The polarity of hydrogen bonds between molecules led to larger affinity enhanced, to make molecules' long-range and short-range orientation of an increase of impetus.So, the abietic acid crystallized faster.
Hydrogen bonds were presented between carboxyls of abietic acid molecules in crystal cells, which formed far and near range order crystal lattice. So the recrystallization of abietic acid did not break this lattice. Under the mass transfer force, it separated from the surface of macropore strong acidity, cation exchange resin, when the concentration of abietic acid reached certain degree.
3 ConclusionsThe (-)-(4R, 10R, 9S, 5R)-7, 13-abietadien-18-oic acid with 99.15% high purity was gained in 74.2% yield after using the rosin acid isomers, isolated by recrystallization. Its molecular configuration was determined by single crystal X-ray diffraction. Hydrogen bonds were presented between carboxyls of abietic acid molecules in crystal cells, which formed far and near range order crystal lattice. The polarity of hydrogen bonds between molecules led to larger affinity enhanced, to make molecules' long-range and short-range orientation of an increase of impetus. The result is that abietic acid crystallization can be easily generated.
Acknowledgment
The single-crystal X-ray diffraction analysis of (-)-(4R, 10R, 9S, 5R)-7, 13-abietadien-18-oic acid was deteminated and calculated by professor Li Yizhi, cordination chemistry state key laboratory of Nanjing University. It is great thankful for his works and helpful discussions.
Chuang H S, Lin C H. 2004. Use of abietic acid or derivative thereof for modulation permeability of plasma membrane[J]. US: 7015248. |
Fernandez M A, Tornos M P, Garcia M D. 2001. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var.grissea[J]. Journal of Pharmacy and Pharmacology, 53(6): 867-872. DOI:10.1211/0022357011776027 |
Han Chunrui(韩春蕊), Song Zhanqian(宋湛谦), Shang Shibin(商士斌). 2007. A new method of purifying abietic acid. Chemistry and Industry of Forstry Products(林产化学与工业), 8(4):42-46.
|
Harris G C, Sanderson T F. 1948. Resin acids I. An improved method of method of isolation of resin acids: the isolation of a new abietic-type acid, neoabietic acid[J]. Journal American Chemical Society, 70: 334-339. DOI:10.1021/ja01181a104 |
Hrobonova K, lehotay J, Skacani I. 2005. HPLC determiuation and MS identification of dehydroabietic acid and abietic acid in propolis[J]. Journal of Liquid Chromatography & Related Technologies, 28: 1725-1735. |
Jin Z M, Pan Y J, Liu J G. 2000. Separation of rosin acids by molecular recognition: crystal structure of the complex of neoabietic acid with 2-amino-6.Methyl-pyridine[J]. Journal of Chemical Crystal lography, 30(3). |
Legrouyellec A. 1985. Use of abietic acid as a film-forming product to be used on wounds and burns[J]. FR 8400611A. |
Liu Hongjun(刘红军), Zhou Yonghong(周永红). 2008. A preparation method of abietic acid. CN, 101219949A.
|
Okada K, Takekuma S. 1994. Crystal structure and conformational analysis of 7, 13-abietadien-18 oic acid[J]. Bull Chem Soc Jpn, 67(3): 807-815. DOI:10.1246/bcsj.67.807 |
Shulman M. 1976. Methods of treating burns using colophony containing preparations. US 3943248.
|
Steele L. 1922. Abietic acid and certain metal abietates[J]. J Am Chem Soc, 44: 1333-1338. DOI:10.1021/ja01427a016 |
Ulusu N N, Ercil D, Sakar M K, et al. 2002. Abietic acid inhitis lipoxygenase activitry[J]. Phytother Res, 16: 88-90. DOI:10.1002/(ISSN)1099-1573 |
Wang Wenjun(王文军), Dai Qianhuan(戴乾圜). 2000. Extract, purification and structure identification of 7, 13-Abietadien-18-oic. Journal Industry University of Beijing(北京工业大学学报), 26(4):77-79.
|
Yokogawa Y, Tsutsumi Y. 1990a. Composition for oral cavity. JP 02138116.
|
Yokogawa Y, Tsutsumi Y. 1990b. Skin drug for external use. JP 02188513.
|
Zinlel D F, Rusell J. 1989. Naval stores production[J]. Chemistry·Utilization, NY:Pulp Chemical Association, 289. |
 2010, Vol. 46
2010, Vol. 46