文章信息
- He Yuejun, Yue Yongde, Tang Feng, Guo Xuefeng, Wang Jin
- 何跃君, 岳永德, 汤锋, 郭雪峰, 王进
- Chemical Compositions and Antioxidant Capacity of Essential Oils from Different Species of the Bamboo Leaves
- 竹叶挥发油化学成分及其抗氧化特性
- Scientia Silvae Sinicae, 2010, 46(7): 120-128.
- 林业科学, 2010, 46(7): 120-128.
-
文章历史
- Received date: 2008-10-20
- Revised date: 2009-01-12
-
作者相关文章
Reactive oxygen species(ROS) or free radicals are generated as byproducts or intermediates of aerobic metabolism and through reactions with drugs and environmental toxins. The elevated cellular levels of free radicals cause damage to nucleic acid, proteins, and membrane lipids and have associated with many aging related problems including carcinogenesis and heart diseases(Halliwell et al., 1992; Halliwell, 1996; Wang et al., 2000). The balance between the production and scavenging of ROS can therefore determine the susceptibility of the body to oxidative damage. Although almost all organisms possess antioxidant defense and repair systems, which quench or minimize the production of oxygen-derived species, thus protecting organisms against oxidative damage, these protective systems are insufficient to entirely prevent the damage(Simic, 1988)caused by endogenous or exogenous(Sun, 1990).
Moreover, ROS are predominant cause of qualitative decay of foods, which lead to rancidity, toxicity and destruction of biomolecules important in physiologic metabolism. However, with safety concerns identified for these synthetic antioxidant(Kitts, 1996; Wichi et al., 1998), considerable interest has arisen in finding alternative sources of antioxidants for use in food systems and increased in researches regarding natural antioxidants. The most widely used synthetic antioxidants used historically in the preservation of foodstuffs such as BHA(butylated hydroxyanisol), BHT(butylatedhydroxytoluene) and TBHQ(tert-butyl hydroquinone) are suspected to cause or promote negative health effects(Namiki, 1991). Indeed, they have been replaced in Japan since 1996 by the natural secondary plant metabolite ellagic acid. For this reason, there is a growing interest in replacing synthetic compounds with natural secondary plant metabolites as potential antioxidants. The use of natural antioxidants has the advantage that the consumer, considered to be safe because of no chemical contamination, readily accepts them and no safety tests are required by the legislation if the food component is Generally Recognized As Safe(GRAS)(Pokorny, 1991). A range of plants has been studied in recent years as potential sources of antioxidants. Among these many essential oils of aromatic plants and spices have been shown to be effectives in retarding the process of lipid peroxidation in oils and fatty food and have gained the interest of many research groups. Therefore, a systematic examination of antioxidant properties of various plant extracts is extremely important to validate the use of, essential oils as preservatives in both the food and pharmaceutical industries. Over the past several decades, a number of studies on the antioxidant activities of essential oils from various aromatic plants have already been shown.
Bamboo is one of the most important forest resources. More than 1 250 species belonging to 75 genera, are being reported worldwide, which are mainly distributed in the tropical and sub-tropical zone, and a few in the temperate and frigid zone. China is one of the bamboo distribution centers of the world with the most abundant bamboo resources, a high economic value and the largest bamboo area. China boasts a long history of utilizing bamboo both as edible food and medicine, but the research on chemical composition of bamboo extracts did not start until the 1950s in China. Antioxidant of bamboo leaves(AOB), a pale brown powder extracted from bamboo leaves, was capable of blocking chain reactions of lipid auto oxidation, chelating metal ions of transient state, scavenging nitrite compounds and blocking the synthetic reaction of nitrosamine reported by previous study(Lou et al., 2004). Moreover, AOB was testified to be a strong antioxidant activity and inhibitory effect on transition metal ion and free radical induced deterioration of macromolecules in vitro (Hu et al., 2000). The particular interest has focused on the potential applications of essential oil that have low toxicity and a strong antioxidant activity as alternative chemical control measures. There are some reports about studies on analysis of essential oil composition from Phyllostachys pubescens, Pleioblastus amarus, Sinocalamus affinis, Indocalamus latifolius and Indocalamus tessellatus leaves(Mao et al., 2001; Wang et al., 2001; 2002; Yang et al., 2002; Li et al., 2007). However, the studies on antioxidant capacity of essential oils from the bamboo leaves were not reported. The objectives of this study were to compare the antioxidant activity of the essential oils from the bamboo leaves, detecting the main components of the extracts by gas chromatography mass spectrometry(GC-MS), in an attempt to contribute to the use of these as alternative products for food preservation.
1 Materials and methods 1.1 ChemicalEthyl ether, Ethanol, Hexane were obtained from Beijing Chemical Factory; Sodium sulfate, anhydrous from Beijing Yili Chemical Company; Tert-butyl hydroquinone(TBHQ) and 2, 2-diphenyl-1-picrylhydrazil(DPPH) from Sigma Chemie. All chemicals used were of analytical grade. All solutions were made up in double-distilled water.
1.2 Plant materialLeaves from adult plant of four species of the bamboo were collected during the autumn(September) from the Jiangxi Academy of Forestry in China, and sample authenticated by professor Peng Jiusheng. The dried samples were ground into fine powder. The ground samples were kept in an air-tight container and stored in a freezer(-20 ℃)until further analysis.
1.3 Steam distillationEssential oils were extracted by using extracted device of essential oil. The leaves of the bamboo species were mixture with distilled water(1: 8). The essential oils were extracted (6 h) by steam distillation using hexane as the collecting solvent. The solvent was separated throughout an auto-oil/water separator. The water fraction was extracted using ethyl ether as the collecting solvent for three times. The hexane extract and ethyl ether extract were mixed, subsequently dried over anhydrous sodium sulfate, and the vapor condensed. Each essential oil extraction was running in duplicate.
1.4 Gas chromatography coupled with mass spectrometry(GC-MS)Analyses were performed using a Agilent Technologies 5973 mass selective detector coupled to a Agilent Technologies 6890N gas chromatograph. Sample volumes of 1 μL were injected in the splitless mode into gas chromatograph. Separation of analytes was achieved using a DB-1 MS (30 m×0.25 mm×0.25 μm). Helium was used as carrier gas with velocity of 1 mL·min-1. The oven temperature program was as follows. Initial temperature 40 ℃ for 1 min, 40-100 ℃ at 5 ℃·min-1 the holding for 5 min, followed by 100-210 ℃ at 5 ℃·min-1 holding for 10 min. The GC injector temperature was 200 ℃. The mass spectrometer parameters for EI mode were ion source temperature, 200 ℃; electron energy, 70 eV; filament current, 34.6 μA; electron multiplier voltage, 1 200 V.
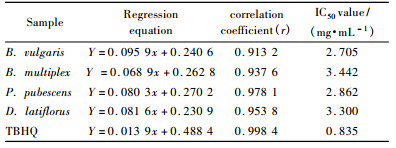
Constituents were identified by matching experimental fragmentation patterns in mass spectra with those of NIST2002, as well as comparing their spectra with those reported in the literature. The relative percentage of the oil constituents was calculated from GC peak areas.
1.5 DPPH assayThe free radical scavenging capacity of the oils was determined using the DPPH discoloration method(Silva et al., 2006). The oils was diluted in 95% ethanol giving a range of 1-6 mg·mL-1. The dilutions 0.5 mL were placed in a test tube in duplicate. The reaction was initiated by addition of 2 mL DPPH solution (51.54 mg·L-1 in 95% ethanol). The absorbance was read at 517 nm over 50 min using a UV-Vis spectrophotometer until the reading reached a plateau.
IC50 value was determined from the plotted graph of scavenging activity versus the concentration of essential oils, which was defined as the total antioxidant necessary to decrease the initial DPPH radical concentration by 50%. Triplicate measurements were carried out and their activity was calculated by the percentage of DPPH scavenged.
1.6 StatisticsThe volume of essential of oil producing 50%(IC50) inhibition of oxidation or reduction in the DPPH assays were determined using the Table curve program. The standard errors at each concentration used lower than 2% and are therefore not shown in either the tables or figures. A significant difference was considered at the level of P < 0.05.
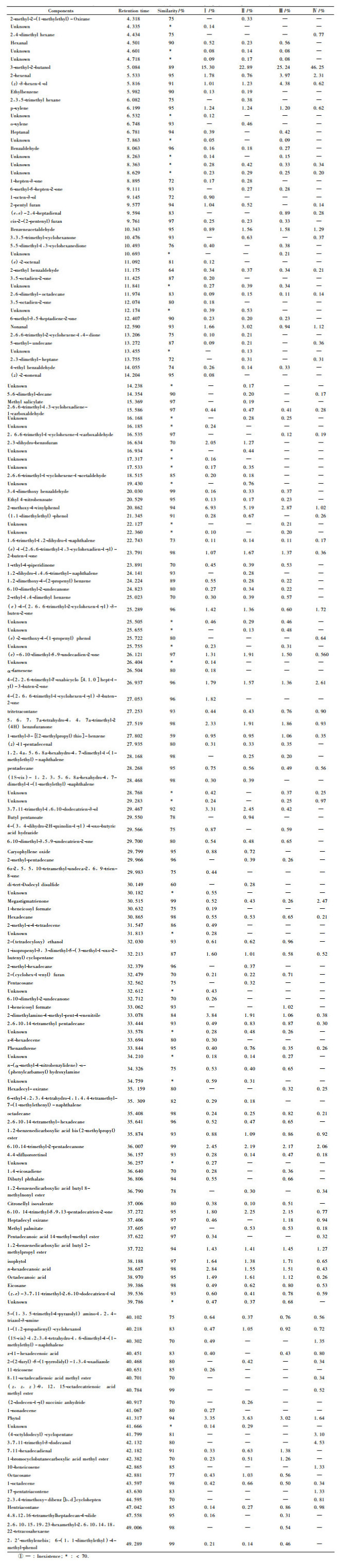
2 Results and discussion 2.1 Chemical compositions of the oilsThe amount of essential oils obtained from the bamboo species was variable (Tab. 1). The greater yield was from Bambusa vulgaris (0.827%) and the least from Phyllostachys pubescens (0.391%). The chemical compositions in bamboo leaves were analyzed by GC-MS. The results showed that 168 chromatographic humps were gained, and 132 kinds of composition were identified. The major volatile components detected and identified by GC-MS was also variable(Tab. 2). A major volatile was 3-methyl-2-butanol, detected in four bamboo species (maximum in Dendrocalamus latiflorus at 44.838%). Other major components detected were 2-methoxy-4-vinylphenol, 2-hexenal, 3, 7, 11-trimethyl-1, 6, 10-dodecatrien-3-ol, benzeneacetaldehyde, nonanal, phytol, 6, 10, 14-trimethyl-2-pentadecanone, 5, 6, 7, 7a-tetrahydro-4, 4, 7a-trimethyl-2(4H)benzofuranone and isophytol.
|
|
|
|
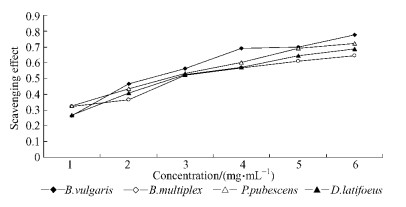
The proton radical scavenging action is known to be one of the various mechanisms for measuring antioxidant activity. DPPH is one of the compounds that possess a proton free radical and shows a maximum absorption at 517 nm for essential oil from bamboo leaf. There was a correlation between radical scavenging rate and the concentration of essential oil from bamboo leaves. The concentration-dependent scavenging of reactive oxygen species by the oil was depicted in Fig. 1.
|
Fig.1 Scavenging effect of essential oil of bamboo leaves on DPPH radicals Values are expressed as mean±standard deviation(n=3). |
The antioxidant capacity of the oils correlated positively (r=0.91, P < 0.05) with the concentration of essential oils. Radical scavenging rate was enhanced with increasing concentration of essential oils. B. vulgaris showed the highest scavenging effect, whereas B. multiplex exhibited the lowest activity among the bamboo species. However there was no significant difference between these bamboo species. The scavenging activity of essential oils on DPPH radicals rapidly increased from 1 to 6 mg·mL-1. Results showed that scavenging activity was increased as the concentration of essential oils increased until a mild ascend state was reached after 4 mg·mL-1. At a concentration of 3 mg·mL-1, the essential oils showed higher scavenging activity than a concentration of 1 mg·mL-1.
IC50 value was determined from the plotted graph of scavenging activity against the concentration of essential oils, which is defined as the amount of antioxidant necessary to decrease the initial DPPH radical concentration by 50%(Tab. 3). The lowest IC50 indicates the strongest ability of the essential oils to act as DPPH scavengers. The IC50 value of B. vulgaris was 2.705, which was slightly lower than B. multiplex. However, no significant difference existed between these bamboo species. B. vulgaris exhibited a significant higher scavenging effect compared to B. multiplex. The scavenging activity of essential oils was in the order of B. vulgaris > P. pubescens > D. latiflorus > B. multiplex. Given that the production of secondary plant metabolites is mainly related to the preservation of the organism, it was of interest to determine whether the production of antioxidant volatile compounds showed any temporal variation in bamboo species.
|
|
A previous study had reported that antioxidant activity and the yield of phenolic content was influenced by different extracting solvents(Sun et al., 2005). For example, a water extract of Terminalia chebuta showed good antioxidant activity, compared to methanolic extracts of Lycopersicon esculentum (Cai et al., 2004). Moreover, from a toxicological point of view, ethanol and water are safer than acetone, methanol and other organic solvents(Oktay et al., 2003).The essential oils obtained by steam distillation from plant leaves may be safe for using. In the present study, the essential oils were obtained by steam distillation from four bamboo species of the B. vulgaris, B. multiplex, P. pubescens, and D. latiflorus. The yield of essential oils from bamboo species was variable, with B. vulgaris providing over two times more than P. pubescens. The steam distillation is an effective method for obtaining essential oils from bamboo leaves.
By adopting distillation of P. pubescens leaves and GC-MS analysis, the volatile composition of P. pubescens was extracted and identified in which 67 chromatographic humps were gained. 53 kinds of composition were identified. 3-hexen-1-ol and 2-hexenal were major components(Mao et al., 2001). However, the results of the present study showed that 168 chromatographic humps were gained, and 132 kinds of composition were identified. The major volatile components were 3-methyl-2-butanol, 2-methoxy-4-vinylphenol, 2-hexenal, nonanal, phytol, 6, 10, 14-trimethyl-2-pentadecanone, and isophytol. The difficulty of GC-MS analysis arises due to the complexity of the volatile compositions, this is particularly due to presence of natural essential oils and other ingredients consisting of complex chemical mixtures.The variety of extract solvents and different analysis method by GC-MS may be exactly the reasons for different results between two studies on essential oil from bamboo leaves.
DPPH is a free radical donor, which has been widely used to evaluate the free radical scavenging effect of natural antioxidants(Matsukawa et al., 1997; Jao et al., 2002). The IC50 values measured in the DPPH assay for essential oil of each bamboo species was extremely low, especially that of B. vulgaris, even in comparison with TBHQ, which was only over three times than TBHQ. Six major components detected were 2-methoxy-4-vinylphenol, 3, 7, 11-trimethyl-1, 6, 10-dodecatrien-3-ol, phytol, 6, 10, 14-trimethyl-2-pentadecanone, 5, 6, 7, 7a-tetrahydro-4, 4, 7a-trimethyl-2(4H) benzofuranone and isophytol in four bamboo leaves, which may contribute to enhance antioxidant capacity of essential oils from bamboo leaves. The antioxidant capacity of the oils, however, is not clearly related to the proportion and profile of secondary plant compounds(Maria et al., 2006).
Other factors that also influence the antioxidants activity are antioxidants concentration, extraction medium, temperature, pH of medium(Gazzani et al., 1998)chemical structures and position in the molecule(Prior et al., 2005). A high antioxidant activity could also be due to other compounds besides phenolics which are soluble in water. In the present study, a part of volatile components in essential oils were extract from water fraction using ethyl ether as extract solvent, which may be also positively correlated with antioxidant activity of essential oils from bamboo leaves.
Food such as fruits, vegetables and grains are reported to contain a wide variety of antioxidant components, including phenolic compounds. These compounds are found to be well correlated with antioxidant potential(Katalinic et al., 2004). Furthermore, an increase in the horticulure of bamboo leaves, such as B. vulgaris, would appear to be advantageous in terms of future application for incorporation into functional foods and pharmaceutical products. Also, benefits may accrue by the utilization of technologic processes or genetic manipulation to increase the yield of oil from this species. Furthermore, the oils are currently undergoing a battery of further in-vitro tests(Gerhauser et al., 2003)to clarify their preservation of pharmaceutical products and validity as food additives.
Cai Y, Qiong L, Mei S, et al. 2004. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer[J]. Life Science, 74: 2157-2184. DOI:10.1016/j.lfs.2003.09.047 |
Gazzani G, Papetti A, Massolini G, et al. 1998. Anti-and prooxidant activity of water soluble components of so common diet vegetables and the effect of thermal treatment[J]. J Agric Food Chem, 46: 4112-4118. |
Gerhauser C, Klimo K, Heiss E, et al. 2003. Mechanism-based in vitro screening of potential cancer chemopreventive agents[J]. Mut Res, 523-524: 163-172. DOI:10.1016/S0027-5107(02)00332-9 |
Halliwell B, Gutteridge J M C, Cross C E. 1992. Free radicals, antioxidants, and human disease. Where are we now?[J]. J Lab Clin Med, 119: 598-620. |
Halliwell B. 1996. Antioxidant in human health and disease[J]. Ann Rev Nutr, 16: 33-50. DOI:10.1146/annurev.nu.16.070196.000341 |
Hu C, Zhang Y, Kitts D D. 2000. Evaluation of antioxidant and prooxidant activities of bamboo Phyllostachys nigra var. henonis leaf extract in vitro[J]. J Agric Food Chem, 48: 3170-3176. DOI:10.1021/jf0001637 |
Jao C H, Ko W C. 2002. 1, 1-Diphenyl-2-picrylhydrazyl(DPPH)radical scavenging by protein hydrolyzates from tuna cooking juice[J]. Fish Sci, 68: 430-435. DOI:10.1046/j.1444-2906.2002.00442.x |
Katalinic V, Milos M, Modun D, et al. 2004. Antioxidant effectiveness of selected wines in comparison with(+)catechin[J]. Food Chemistry, 86: 593-600. DOI:10.1016/j.foodchem.2003.10.007 |
Kitts D D. 1996. Toxicity and safety of fats and oils//Hui Y H. Baileys Industrial Oil and Fat Products. Wiley Interscience, New York, 215-280.
|
Li Shuifang(李水芳), Wen Ruizhi(文瑞芝), Zeng Dong(曾栋), et al.2007.Extraction and determination of essential oils in Indocalamus latifolius leaves and Indocalamus tessellatus leaves. Chinese Journal of Chromatography(色谱), 25(1): 53-57.
|
Lou D D, Zhang Y, Wu X Q, et al. 2004. Application of antioxidant of bamboo leaves(AOB) in Weixin western sausages[J]. Chin Food Fermentat Ind, 30: 13-17. |
Mao Yan(毛燕), Liu Zhikun(刘志坤). 2001.Extraction and GC-MS analysis of volatile Composition of Phyllostachys pubescens leaves. Journal of Fujian College of Forestry(福建林学院学报), 21(3): 265-267.
|
Maria T S T, Maria G V S, Beate P, et al. 2006. Characterization of the volatile pattern and antioxidant capacity of essential oils from different species of the Genus Ocimum[J]. J Agric Food Chem, 54: 4378-4382. DOI:10.1021/jf060181+ |
Matsukawa R, Dubinsky Z, Kishimoto E, et al. 1997. A comparison of screening methods for antioxidant activity in seaweeds[J]. J Appl Phycol, 9: 29-35. DOI:10.1023/A:1007935218120 |
Namiki M. 1991. Antioxidants/antimutagents in food[J]. Crit Rev Food Sci Nutr, 29: 273-300. |
Oktay M, Gulcin I, Kufrevioglu O I. 2003. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts[J]. Lebensmittel Wissenschaft Und Technologie, 36: 263-271. DOI:10.1016/S0023-6438(02)00226-8 |
Pokorny J. 1991. Natural antioxidants for food use[J]. Trends Food Sci Technol, 2: 223-227. DOI:10.1016/0924-2244(91)90695-F |
Prior R L, Wu X, Schaich K. 2005. Standardized methods for the determination of antioxidants capacity and phenolics in foods and dietary supplements[J]. J Agric Food Chem, 43: 401-403. |
Silva J P, Areias F M, Proenca F M, et al. 2006. Oxidative stress protection by newly synthesized nitrogen compounds with pharmacological potential[J]. Life Sci, 78: 1256-1257. DOI:10.1016/j.lfs.2005.06.033 |
Simic M G. 1988. Mechanisms of Inhibition of Free-Radical[J]. Processed In Mutagenesis and Carcinogenesis. Mutation Res, 202: 377-386. |
Sun T, Ho C T. 2005. Antioxidant activities of buckwheat extracts[J]. Food Chemistry, 90: 743-749. DOI:10.1016/j.foodchem.2004.04.035 |
Sun Y. 1990. Free radicals, antioxidant enzymes and carcinogenesis[J]. Free Radical Biol Med, 8: 583-599. DOI:10.1016/0891-5849(90)90156-D |
Wang S Y, Jiao H. 2000. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen[J]. J Agri Food Chem, 48: 677-684. |
Wang Xueli(王学利), Lü Jianquan(吕健全), Zhang Yide(章一德). 2002.Analysis of volatile oil composition of Pleioblastus amarus. Journal of Zhejiang Forestry College(浙江林学院学报), 19(4): 387-390.
|
Wang Xueli(王学利), Mao Yano(毛燕). 2001. GC-MS analysis of volatile composition of Indicalamus leaves. Journal of Bamboo Research(竹子研究汇刊), 20(2): 36-38.
|
Wichi C M, Kendall C W C, Stamp D, et al. 1998. hermally oxidized dietary fat on colon carcinogenesis in rodents[J]. Nutr Cancer, 30: 69-73. DOI:10.1080/01635589809514643 |
Yang Jianjia(杨弿嘉), Liu Jianhua(刘建华), Gao Yuqiong(高玉琼), et al. 2002.Studies on the chemical constituents of volatile oil from Sinocalamus affinis (rendle) mc-clure. Natural Product Research and Development(天然产物研究与开发), 14(6): 31-32.
|
 2010, Vol. 46
2010, Vol. 46