文章信息
- Li Changxiao, Ye Bing, Geng Yanghui, Schneider Rebcca
- 李昌晓, 叶兵, 耿养会, SchneiderRebcca
- Physiological Responses of Taxodium distichum (Baldcypress) and Taxodium ascendens (Pondcypress) Seedlings to Different Soil Water Regimes
- 落羽杉与池杉幼苗对不同土壤水分含量的生理响应
- Scientia Silvae Sinicae, 2010, 46(4): 22-30.
- 林业科学, 2010, 46(4): 22-30.
-
文章历史
- Received date: 2009-10-21
-
作者相关文章
2. Research Institute of Forestry Policy and Information, CAF Beijing 100091;
3. Chongqing Key Laboratory for the Protection and Restoration of Forest Ecology of the Three Gorges Reservoir Region Chongqing 400036;
4. Department of Natural Resources, College of Agriculture and Life Sciences, Cornell University NY 14853
2. 中国林业科学研究院科技信息研究所 北京 100091;
3. 三峡库区森林生态保护与恢复重庆市市级重点实验室 重庆 400036;
4. 美国康乃尔大学农学与生命科学院自然资源系 纽约州依萨卡市 14853
Baldcypress (Taxodium distichum) and pondcypress (Taxodium ascendens), introduced to China from North America 80 years ago, are commonly found in floodplains and bottomlands of the Yangtze River valleys of China. Both are excellent reforestation species for soil erosion control and soil stabilization. In recent years, large-scale reforestation activities are annually organized to plant baldcypress and pondcypress young seedlings or sometimes to directly sow seeds of both species into riparian zone soils. However, many project sites have to be replanted or re-sown, at least partially, due to low survival rates. Although the exact causes of poor performance are probably multifaceted, water stresses on the young plants may be one of the critical reasons according to previous studies (Pezeshki, 1991; Eclan et al., 2002; Kozlowski, 2002; Kozlowski et al., 2002; Simone et al., 2003; Jackson et al., 2005). Reforestation and/or restoration sites are subjected to dynamic hydrological conditions in riparian systems primarily imposed by human-induced changes in hydrology. The Three Gorges Dam, located in the middle reaches of the Yangtze River, has significantly altered hydrographs of the valleys. An annual 30 meters fluctuation of water level is imposed within the reservoir region that encompasses an area of about 298 km2 stretching over 2 000 km of shoreline (Diao et al., 1999). Depending on the slope and depth of base flow, both baldcypress and pondcypress seedlings may be exposed to an annual range of hydrological regimes from soil drought to flooding.
In general, plant responses to water stresses include net photosynthetic reduction, stomatal closure, and metabolic adaptation (Gimenez et al., 1992; Pezeshki, 2001; Eclan et al., 2002; Fortini et al., 2003). However, both free and bound plant water, play extremely important roles in these responses. The free water is indispensable in various metabolisms in plants and controls plant metabolic reactions to adverse environments. More free water might be able to enhance solute accumulation, leading to better osmotic adjustment and tolerance to water stress, and maintenance of the volumes of sub-cellular compartments (Singh et al., 2006). The bound water more likely plays a major role in tolerance to abiotic stresses (El-Saidi et al., 1975; Rascio et al., 1998; Misik 2000) through maintaining the structural integrity and/or cell wall extensibility of the leaves (Singh et al., 2006). Studying the free and bound water contents in plants may therefore be of significance to understanding their adaptive mechanisms. Furthermore, plant responses to water stresses in the free and bound water contents in leaves also might be indicated by some closely related indicators such as leaf electrolyte leakage, malondialdehyde concentration, proline content, and concentration of soluble sugars. Previous research on responses of baldcypress and pondcypress have focused primarily on gas exchange responses (Pezeshki et al., 1997; 1998; Anderson et al., 1999; Eclan et al., 2002), aerenchyma formation, and growth variation (Megonigal et al., 1992; Conner et al., 1997; Pezeshki et al., 1999) to soil flooding, while relatively little is known about leaf water metabolic change in adaptation to such a wide range of water gradients. Thus, the main objective of this research was to quantify the leaf water physiological functions of baldcypress and pondcypress seedlings to a series of soil water contents widely distributed in the riparian zones of the Yangtze River valleys. Such data may help to explain the mechanisms involved in responses of both species to the water regime extremes of flooding and drought.
1 Materials and methods 1.1 Plant material and growing conditionsThe baldcypress and pondcypress seeds used in this experiment were provided by Yunnan Provincial Tree Seeds Center and sown in Geleshan seedling nursery of Chongqing Forestry Institute (106°12′ E, 29°37′ N) on March 12. On June 15, 120 seedlings of each species with a mean height of 14.7 and 14.6 cm for baldcypress and pondcypress, respectively, were transplanted into containers (13 cm width, 12 cm depth, one plant per pot) in which purple soil (Regosols in FAO Taxonomy or Entisols in USDA Taxonomy) was previously filled. Purple soil is a typical soil type in the region, with a pH value of 6.5. In consideration of the natural status of the riparian habitat, treatments were not fertilized. Then, potted seedlings were moved into Southwest University's Experimental Zone of Ecology (106°30' E, 29°49' N, 249 m in altitude, about 30 km distance to the Yangtze River), to acclimate under ambient conditions for about six weeks. Water treatments began on July 25 in an experimental booth, open on four sides and covered by removable transparent plastic film only used during rain events.
1.2 Experimental designA completely randomized block design was adopted in the experiment. One-hundred-and-twenty seedlings of each species were randomly divided into four groups with 30 seedlings for each different group. The four water treatment groups included a control (C), mild drought (MD), wet soil (WS) and flooding (FL). The C was maintained at a soil water content of 60%~63% of field capacity (soil water content measured by weight), a regime where the seedlings showed normal growth and no wilting during sunny days. The MD had a soil water content of 47%~50% of field capacity, a regime where fresh leaves of the seedlings typically wilted around 13: 00 and recovered around 17: 00. The experimental design for C and MD was based on field investigations in the riparian zones of the Three Gorges Reservoir region, where tree seedlings grew normally at soil water content of about 60%~63% of field capacity, while experiencing mild drought stress at 47%~50%. In the WS treatment, a high level of soil moisture, near saturation, was maintained by watering the pots every 2 h from 6: 00 to 24: 00. Flooding treatments (FL) were continuously flooded 1 cm above the soil surface by infusing tap water into the basins. Flood depth was limited to 1 cm to prevent immersion of the foliage of the small seedlings. Plastic basins 68 cm in diameter and 22 cm in depth were used to hold the potted seedlings in the FL treatment.
Previous literature (Eclan et al., 2002) showed that physiological parameters of baldcypress seedlings can be significantly affected by soil water treatment in a time period of six days. Thus, a 6 d interval for our data collection was chosen. Measurements were made on the growing seedlings on five separate dates: July 31, August 6, 12, 18 and 24. Leaf samples collected before dawn (between 5: 30 and 6: 30 h) were used for various measurements in this experiment, with five replicates in each measurement period for each treatment x species combination. Sampled leaves were contained in sealed plastic bags, covered with a moistened paper towel and ice in an insulated box for transport to the laboratory for immediate measurement. The experiment lasted 30 days and ended on August 25.
1.3 Measurement of physiological responses 1.3.1 Measurement of leaf water contentsLeaf water contents (%) were measured according to Zhang (1990). Total water content was calculated by dry weight divided by fresh weight. Dry weight was obtained by oven-dry at 105 ℃ for 24 h. Free water content was measured by Abbel refractometer according to Zhang (1990). Bound water content was calculated by total water content minus free water content.
1.3.2 Electrolyte leakageA conductivity meter HI 8733 (Hanna Instruments, Woonsocket, R.I., USA) was used to measure electrolyte leakage as described by Zwiazek et al. (1990) and Renault et al. (1998). About 0.5 g leaves were taken from each of five seedlings per species× treatment combination, washed with deionized water three times and placed in separate tubes, each holding 20 ml of deionized water. After 5 h of incubation on an orbital shaker at 50 r·min-1, initial electrical conductivity was measured. To obtain total electrolytes, the samples were autoclaved at 121 ℃ for 15 min followed by freezing overnight at -85 ℃. Then, the samples were thawed for about 5 h in a water bath at room temperature. Total electrolytes of the sample solution were measured and the percentage of initial conductivity to the total electrolytes was calculated as electrolyte leakage.
1.3.3 MalondialdehydeLeaves of the seedlings were used to measure content of the malondialdehyde (MDA). The MDA content was determined according to Kramer et al. (1991). The absorbance of the supernatant was determined at 450, 532 and 600 nm using an Ultrascope Ⅲ spectrophotometer (Pharmacia LKB, UK). The MDA content was calculated using the following formula: C=6.45(OD532-OD600)-0.56OD450.
1.3.4 Proline contentTo determine the free proline content, we modified the method adopted by Bates et al. (1973). A 5.0 mL aliquot of 3% (weight/volume) sulfosalicylic acid was added to 0.5 g of finely ground leaf powder, and a water bath at 100 ℃ was adopted for 30 min in glass tubes with the top covered. The mixture was centrifuged at 2 000 g for 5 min at 25 ℃. A 200 μL aliquot of the abstract was mixed with 400 μL deionized water and 2.0 mL of the reagent mixture (30 mL glacial acetic acid, 20 mL deionized water and 0.5 g of ninhydrin), and boiled at 100 ℃ for 1 h. After cooling the reaction mixture, 6.0 mL toluene were added. The chromophore containing toluene was separated and read at A520, using toluene as a blank. Proline content was calculated in mg·g-1 DW, using L-proline for the standard curve.
1.3.5 Soluble sugarsSoluble sugars were extracted from leaves of baldcypress and pondcypress seedlings (n=5 per species×treatment combination each time) three times with hot 85% ethanol at 95 ℃. The concentrations were determined colorimetrically using phenolsulphuric acid as described by Smith et al. (1964). Absorbance readings were determined with an Ultrascope Ⅲ spectrophotometer (Pharmacia LKB, UK) and the concentration was calculated on a dry weight basis.
1.4 Data AnalysisAnalyses of the data employed GLM Procedures (SPSS 13.0 Version) to determine any significant overall differences among treatment groups. Multiple pair-wise comparisons (Duncan's method) were used to determine significant differences at the 0.05 level between individual treatment groups.
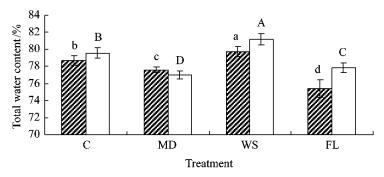
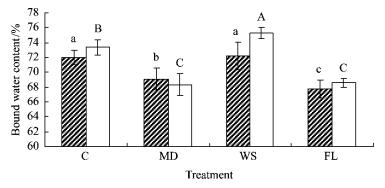
2 ResultsBoth species were significantly affected by different water treatments in water contents, malondialdehyde concentration, proline content and soluble sugar concentration in contrast to no significant impact on electrolyte leakage (Tab. 1). During the experiment total water content in both species significantly (P < 0.001) increased in WS while decreasing in MD and FL as compared to respective controls (C) (Fig. 1). In contrast, free water content in MD and FL showed significant increases compared to that in C in either species (Fig. 2). Moreover, free water content in WS was 12.1% higher than in C in baldcypress seedlings, in contrast to no significant difference between WS and C in pondcypress. Interestingly, bound water contents in MD and FL were significantly lower than in C in baldcypress and pondcypress seedlings, respectively (Fig. 3). Bound water content in WS was not significantly changed in baldcypress, but was significantly elevated in pondcypress seedlings as compared to C. There were no significant effects by species×time in total water content and by species in free water content, although significant effects were found in time, species×treatment, treatment×time and treatment×species×time in total, free and bound water contents (Tab. 1).
|
|
|
Fig.1 Effects of water treatment on total water content in leaves of baldcypress and pondcypress
 Baldcypress, □ Pondcypress, The same below Seedlings with ditterent treatment. Vertical bars indicate standard error. Each column stands for the mean of 25 samples. According to Duncan multiple range test, the values of each species with different letters are significantly different at the 0.05 level. The lower-case letters are for T. distichum seedlings while upper-case letters are for T. ascendens seedlings.control (C), mild drought (MD), wet soil (WS) and flooding (FL).The same below. Baldcypress, □ Pondcypress, The same below Seedlings with ditterent treatment. Vertical bars indicate standard error. Each column stands for the mean of 25 samples. According to Duncan multiple range test, the values of each species with different letters are significantly different at the 0.05 level. The lower-case letters are for T. distichum seedlings while upper-case letters are for T. ascendens seedlings.control (C), mild drought (MD), wet soil (WS) and flooding (FL).The same below.
|

|
Fig.2 Effects of water treatment on free water content in leaves of baldcypress and pondcypress |
|
Fig.3 Effects of water treatment on bound water content in leaves of baldcypress and pondcypress seedings with different treatment |
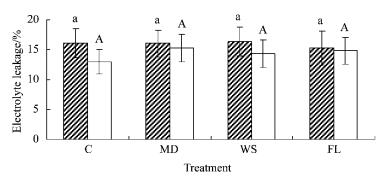
Overall there were no significant (P=0.154>0.05) treatment effects in electrolyte leakage in baldcypress or pondcypress seedlings (Tab. 1). Throughout the time period of the experiment, electrolyte leakage gradually declined in both species, especially after 18 d of treatment. However, mean values of electrolyte leakage during the experiment in baldcypress seedlings exhibited 23.8, 5.1, 14.4 and 2.6% higher than in pondcypress in C, MD, WS and FL, respectively (Fig. 4), reflecting significant (P=0.028 < 0.05) species×treatment effect in electrolyte leakage. There were also significant species, time, treatment×time, species×time and treatment×species×time effects.
|
Fig.4 Effects of water treatment on electrolyte leakage of leaves of baldcypress and pondcypress seedings with different treatment |
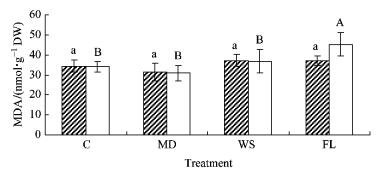
There were significant treatment, species and time effects in malondialdehyde concentration in both species (P < 0.001) (Tab. 1). No significant differences in MDA were found in either treatment in baldcypress seedlings (Fig. 5). On the contrary, MDA in FL was significantly higher than C, MD and WS in pondcypress seedlings in which the latter three groups demonstrated no significant difference. Although species×treatment interaction was not significant, the treatment×time, species×time and treatment×species×time interactions were significant.
|
Fig.5 Effects of water treatment on malondialdehyde concentration in leaves of baldcypress and pondcypress seedlings with different treatment |
Water treatment, species, time and their interactions significantly (P < 0.001) affected proline content of both baldcypress and pondcypress seedlings (Tab. 1). Flooding (FL) significantly (P < 0.001) reduced proline content of both baldcypress and pondcypress seedlings during the experiment (Fig. 6). By contrast, proline contents were significantly (P < 0.001) increased in baldcypress seedlings while decreasing in pondcypress seedlings under mild drought (MD) and wet soil (WS) water stresses as compared to their respective controls.

|
Fig.6 Effects of water treatment on proline content in in leaves of baldcypress and pondcypress seedlings with different treatment |
Overall soluble sugar concentration in flooded (FL) seedlings of baldcypress and pondcypress increased significantly, with 16.6% and 24.9% higher than in C, respectively (baldcypress: P < 0.01; pondcypress: P < 0.001) (Fig. 7). However, soluble sugar concentration in MD and WS seedlings was maintained comparable to C in both species. The treatment×time, species×time and treatment×species×time interactions were significant, but species×treatment was not (Tab. 1).

|
Fig.7 Effects of water treatment on soluble sugar content in leaves of baldcypress and pondcypress seedlings with different treatment |
The results of our study suggest that baldcypress and pondcypress species had some similar traits in water contents in leaves when counteracting water stresses, as we found significant reductions in bound water and total water in contrast to increases in free water under MD and FL conditions in both species (Tab. 1, Fig. 1, 2 and 3). Such adaptive responses in water contents in leaves of both species might partially reflect their taxonomic proximity, as they originated from the same genus, even the same species as argued by Denny et al. (2007). Although controversies have existed on whether or not baldcypress and pondcypress should be classified as two distinct botanical species, some physiological differences in response to adverse environments can be easily detected, as shown in our studies. In fact, the two species revealed significant differences in electrolyte leakage, MDA, proline and soluble sugar content (Tab. 1).
Overall water treatment in MD and FL can significantly (P < 0.001) elevate free water content in leaves of both species as compared to C (Tab. 1; Fig. 1), indicating that seedlings of baldcypress and pondcypress held more water sources available for metabolism at water regime extremes. However, water treatment in WS maintained free water content in the leaves of pondcypress seedlings identical to C, reflecting that water sources subject to metabolic use remained almost unchanged. The relatively stable free water content in pondcypress was contrasted with more water sources available in baldcypress when WS was imposed as compared to C. Free water content in the plant participates into various metabolic activities, hence limiting the strength of plant metabolism. Although higher free water content can lead to more vigorous plant metabolic activities, plants usually need to have more bound water to counteract some environmental stresses. Bound water content in pondcypress seedlings showed significant increase in WS indicating that leaf osmotic adjustment may have played an important role in adapting to wet soil environment. Interestingly, both MD and FL reduced bound water content in leaves of both species as compared to C, implying that more leaf water was probably oriented to free water instead of bound water use so as to strengthen the metabolic activities. This inference was further verified by significantly (P < 0.001) lower ratios of bound water to free water in groups of MD and FL in both species as compared to C.
Our results were different from previous studies by Ma et al. (2008), who demonstrated that lower free water content, higher bound water content and a greater ratio of bound water to free water were important characteristics of desert Caragana species in adapting to an arid environment. Such a different result in drought adaptation might be due to species difference and/or experimental conditions as Caragana species are dominant species in the desert region. Different adaptive strategies found in leaf water contents in baldcypress and pondcypress seedlings may suggest that metabolic adaptation was more active than cell osmotic regulation in leaves of both species to counteract stresses of MD and FL. Thus, leaf water contents in baldcypress and pondcypress seedlings may be ideal indicators to explain their high tolerances to flooding and drought.
Increased electrolyte leakage can indicate higher membrane damage in the leaves. Different water treatments had no significant effect in electrolyte leakage in this study demonstrating that membrane damage was not directly caused by water stresses of drought or flooding. Given the fact that pondcypress seedlings showed lower electrolyte leakage than baldcypress throughout the study period, better performance in water stress tolerances may be found in pondcypress when compared to baldcypress. This is in agreement with our previous studies that pondcypress seedlings may be more resilient than baldcypress in dealing with adverse soil water environment in responses of leaf gas exchange and root biochemical adaptation (Li et al., 2005; 2007; 2008). Furthermore, when the experimental treatment progressed, electrolyte leakage was significantly decreased in all four treatment groups in both species (data not shown). This time-dependent reduction in electrolyte leakage implies that growth of seedlings could significantly (P < 0.001) mitigate or reduce membrane damage of the leaves. Previous studies showed that older and larger seedlings take on higher capabilities to prevent or escape cell injuries of leaves imposed by flooding and/or drought (Islam et al., 2003; 2004).
T-tests found that seedlings of baldcypress and pondcypress exhibited parallel (P=0.936, 0.894, 0.951 and 0.173 >0.05 in C, MD, WS and FL, respectively) responses in levels of MDA within each treatment illustrating that membrane lipid peroxidation had no significant difference in seedling leaves in each treatment, and highlighting no significant influence given by species x treatment interaction (Tab. 1). As a marker for membrane lipid peroxidation (Masia, 2003), MDA can also react with DNA to form adducts to deoxyguanosine and deoxyadenosine (Marnett, 1999). Thus, MDA may directly cause cell deterioration in leaves of both species, probably further leading to inactivation of membrane-bound proteins and the increase of membrane permeability (Smirnoff, 1993; Asada, 1999). However, overall a decreasing trend in MDA content in leaves of seedlings occurred as the experiment treatment continued, showing that seedling growth may enhance the plant's anti-stress capacities, otherwise such a gradual decrease may be attributed to the gradual adaptation of the seedlings to adverse soil environment.
Proline content was commonly understood as a biochemical marker of drought-stress level in many plants (Larcher et al., 1981; Ranney et al., 1991; Schultz et al., 1993; Wang et al., 1995; Ain-Lhout et al., 2001; Sofo et al., 2004). In our present study, proline content in leaves of baldcypress seedlings was significantly increased under MD conditions implying that osmotic adjustment of lowering osmotic potential was functioning. However, pondcypress seedlings exhibited significant lower proline content in leaves throughout the experiment indicating either a different strategy or otherwise a higher capacity to counteract drought-stress-induced damage. Our previous study found that under mild drought stress conditions pondcypress seedlings could effectively maintain leaf water potential and stomatal conductance by continuous water supply through significant improvement of root growth, especially lateral roots (Li et al., 2008). Such an advantage in pondcypress to deal with soil water deficit may well explain its lower proline content in the leaves found in this present study. Interestingly, flooding can significantly reduce proline content during the entire study period as compared to the controls, either in baldcypress or in pondcypress. Because baldcypress grows better in flooded conditions (Dickson et al., 1972), a low proline content may suggest less plant stress and increased plant fitness for the seedlings of both species under flooding. Thus, we may assume that osmotic adjustment may still be in existence in flooding conditions. Although such an inference needs to be studied further for baldcypress and pondcypress, Carpenter et al. (2008) documented that osmotic adjustment was found in roots and shoots of Salix nigra during flooding stress.
Content and storage of carbohydrates in plant tissues play critical roles for plant responses to adverse environmental conditions (Huang et al., 1995). Overall the significantly high accumulation of soluble sugars in leaves of both species in FL suggests that more carbohydrates were produced by carbon assimilation activities, with mean content values 16.6% and 24.9% higher in baldcypress and pondcypress seedlings in FL as compared to the controls, respectively. As a consequence, more carbohydrates can be stored, allocated and/or translocated in plant tissues. More assimilated carbohydrates may be one of the critical strategies to hypoxia tolerance in both baldcypress and pondcypress seedlings. Bacchus et al. (2000) documented that greater concentrations of soluble sugars were detected in pondcypress branch tips due to anthropogenic perturbations. Higher total ethanol-soluble carbohydrates were also found in Salix nigra roots and shoots during soil moisture stress in periodic flooding and drought treatments (Carpenter et al., 2008). As our present study focused on leaf water responses, future experimental tests on carbohydrates in roots and shoots of baldcypress and pondcypress seedlings will be necessary in order to understand their adaptive mechanisms more in depth.
In conclusion, seedlings of baldcypress and pondcypress increase leaf free water content while lowering leaf bound water content to counteract water deficit (MD) and flooding (FL) conditions. The free or bound water content in WS never showed less than that in C in both species. No significant changes were demonstrated in electrolyte leakage and concentration of MDA in baldcypress or pondcypress seedlings under MD, WS and FL, except the MDA concentration in FL in pondcypress. Flooding significantly increased soluble sugar concentration and decreased proline content in both species. Moreover, proline content was enhanced in baldcypress seedlings, but reduced in pondcypress seedlings in MD and WS. Thus, baldcypress and pondcypress seedlings had some different traits in responses to water stresses although many similar responses occurred in these two congeneric species.
Ain-Lhout F, Zunzunegui F A, Diaz Barradas M C, et al. 2001. Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit[J]. Plant Soil, 230: 175-183. DOI:10.1023/A:1010387610098 |
Anderson P H, Pezeshki S R. 1999. The effects of intermittent flooding on seedlings of three forest species[J]. Photosynthetica, 37: 543-552. |
Asada K. 1999. The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons[J]. Annu Rev Plant Physiol Plant Mol Biol, 50: 601-639. DOI:10.1146/annurev.arplant.50.1.601 |
Bacchus S T, Hamazaki T, Britton K O, et al. 2000. Soluble sugar composition of pond-cypress: a potential hydroecological indicator of ground water perturbations[J]. J Am Water Resour As, 36: 55-65. DOI:10.1111/jawr.2000.36.issue-1 |
Bates L S, Waldren R P, Teare I K. 1973. Rapid determination of free proline for water stress studies[J]. Plant Soil, 39: 205-208. DOI:10.1007/BF00018060 |
Carpenter L T, Pezeshki S R, Shields Jr F D. 2008. Responses of nonstructural carbohydrates to shoot removal and soil moisture treatments in Salix nigra[J]. Trees, 22: 737-748. DOI:10.1007/s00468-008-0234-7 |
Conner W H, McLeod K W, McCarron J K. 1997. Flooding and salinity effects on growth and survival of four common forested wetland species[J]. Wetlands Ecol Manage, 5: 99-109. DOI:10.1023/A:1008251127131 |
Denny G C, Arnold M A. 2007. Taxonomy and nomenclature of baldcypress, pondcypress, and montezuma cypress: one, two or three species?[J]. HortTechnology, 17: 125-127. |
Diao C T, Huang J H. 1999. A preliminary study on land resources of the water-level-fluctuating zone in the Three Gorges Reservoir[J]. Resources and Environment in the Yangtze Basin, 8(1): 75-80. |
Dickson R E, Broyer T C. 1972. Effects of aeration, water supply, and nitrogen source on growth and development of tupelo gum and bald cypress[J]. Ecology, 53: 626-634. DOI:10.2307/1934776 |
Eclan J M, Pezeshki S R. 2002. Effects of flooding on susceptibility of Taxodium distichum L. seedlings to drought[J]. Photosynthetica, 40: 177-182. DOI:10.1023/A:1021381204684 |
El-Saidi M T, Gabr A I, El-Kadi M, et al. 1975. The effect of certain pre-sowing treatments and early phosphorus supplement on cell sap concentration and water fractions in leaves of maize (Zea mays L.) plants grown under soil moisture stress conditions[J]. Biol Plant, 17: 281-291. DOI:10.1007/BF02921221 |
Fortini L B, Mulkey S S, Zarin D J, et al. 2003. Drought constraints on leaf gas exchange by Miconia ciliate (Melastomataceae) in the understory of an eastern Amazonian regrowth forest stand[J]. Amer J Bot, 90: 1064-1070. DOI:10.3732/ajb.90.7.1064 |
Gimenez C, Mitchell V J, Ferris D M. 1992. Regulation of photosynthetic rate of two sunflower hybrids under water stress[J]. Plant Physiol, 96: 635-643. |
Huang B, Johnson J W. 1995. Root respiration and carbohydrate status of two wheat genotypes in response to hypoxia[J]. Ann Bot, 75: 427-432. DOI:10.1006/anbo.1995.1041 |
Islam M A, Macdonald S E, Zwiazek J J. 2003. Responses of black spruce (Picea mariana) and tamarack (Larix laricina) to flooding and ethylene treatments[J]. Tree Physiol, 23: 545-552. DOI:10.1093/treephys/23.8.545 |
Islam M A, Macdonald S E. 2004. Ecophysiological adaptations of black spruce (Picea mariana) and tamarack (Larix laricina) seedlings to flooding[J]. Tree Physiol, 18: 35-42. DOI:10.1007/s00468-003-0276-9 |
Jackson M B, Colmer T D. 2005. Response and adaptation by plants to flooding stress[J]. Ann Bot, 96: 501-505. DOI:10.1093/aob/mci205 |
Kozlowski T T, Pallardy S G. 2002. Acclimation and adaptive responses of woody plants to environmental stresses[J]. Bot Rev, 68: 270-334. DOI:10.1663/0006-8101(2002)068[0270:AAAROW]2.0.CO;2 |
Kozlowski T T. 2002. Physiological-ecological impacts of flooding on riparian forest ecosystems[J]. Wetlands, 22: 550-561. DOI:10.1672/0277-5212(2002)022[0550:PEIOFO]2.0.CO;2 |
Kramer G F, Norman H A, Krizek D T, et al. 1991. Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipid in cucumber[J]. Phytochemistry, 30: 2101-2108. DOI:10.1016/0031-9422(91)83595-C |
Larcher W, Moroes J A P V, Bauer H. 1981. Adaptive responses of leaf water potential, CO2 gas exchange and water use efficiency of Olea europaea during drying and rewatering//Margaris N S, Mooney H A, eds. Components and Productivity of Mediterranean-Climate Regions: Basic and Applied Aspects. Dr W Junk Publisher, The Hague, 77-84.
|
Li C X, Zhong Z C. 2005. Comparative study on photosynthetic characteristics of Taxodium distichum and Taxodium ascendens seedlings under simulated soil water changes in the hydro-fluctuation belt of Three Gorges Reservoir Area[J]. Scientia Silvae Sinicae, 41(6): 28-34. |
Li C X, Zhong Z C. 2007. Influences of mimic soil water regime on the contents of malic acid and shikimic acid and biomasses in the roots of Taxodium distichum seedlings in the hydro-fluctuation belt of the Three Gorges Reservoir Area[J]. Acta Ecologica Sinica, 27(11): 4394-4402. |
Li C X, Zhong Z C, Tao J P. 2008. Malic acid, shikimic acid, and biomass accumulation in the roots of Taxodium ascendens seedlings under different soil water conditions[J]. Scientia Silvae Sinicae, 44(10): 1-7. |
Ma C C, Gao Y B, Guo H Y, et al. 2008. Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau[J]. J Arid Environ, 72: 247-254. DOI:10.1016/j.jaridenv.2007.05.009 |
Marnett L J. 1999. Lipid peroxidation-DNA damage by malondialdehyde[J]. Mutat Res, 424: 83-95. DOI:10.1016/S0027-5107(99)00010-X |
Masia A. 2003. Physiological effects of oxidative stress in relation to ethylene in postharvest produce//Hodges D M. Postharvest Oxidative Stress in Horticultural Crops. Food Products Press, New York, 165-197.
|
Megonigal P J, Day F P. 1992. Effects of flooding on root and shoot production of baldcypress in large experimental enclosures[J]. Ecology, 73: 1182-1193. DOI:10.2307/1940668 |
Misik S. 2000. Bound water in vine cane studied by microwave method[J]. Acta Hort, 526: 177-182. |
Pezeshki S R, Anderson P H. 1997. Responses of three bottomland species with different flood tolerance capabilities to various flooding regimes[J]. Wetlands Ecol Manage, 4: 245-256. |
Pezeshki S R, DeLaune R D, Anderson P H. 1999. Effect of flooding on elemental uptake and biomass allocation in seedlings of three bottomland tree species[J]. J Plant Nutr, 22: 1481-1494. DOI:10.1080/01904169909365729 |
Pezeshki S R, Santos M I. 1998. Relationships among rhizosphere oxygen deficiency, root restriction, photosynthesis, and growth in baldcypress (Taxodium distichum L.) seedlings[J]. Photosynthetica, 35: 381-390. DOI:10.1023/A:1006912318352 |
Pezeshki S R. 1991. Root responses of flood-tolerant and flood-sensitive tree species to soil redox conditions[J]. Trees, 5: 180-186. |
Pezeshki S R. 2001. Wetland plant responses to soil flooding[J]. Environ Exp Bot, 46: 299-312. DOI:10.1016/S0098-8472(01)00107-1 |
Ranney T G, Bassuk N L, Whitlow T H. 1991. Osmotic adjustment and solute constituents in leaves and roots of water-stressed cherry (Prunus) trees[J]. J Am Soc Hort Sci, 116: 684-688. |
Rascio A, Russo M, Platani C, et al. 1998. Drought intensity effects on genotypic differences in tissue affinity for strongly bound water[J]. Plant Sci, 132: 121-126. DOI:10.1016/S0168-9452(98)00006-5 |
Renault S, Lait C, Zwiazek J J, et al. 1998. Effect of high salinity tailings waters produced from gypsum treatment of oil sands tailings on plants of the boreal forest[J]. Environ Pollut, 102: 177-178. DOI:10.1016/S0269-7491(98)00099-2 |
Schultz H R, Matthews M A. 1993. Growth, osmotic adjustment, and cell-wall mechanics of expanding grape leaves during water deficits[J]. Crop Sci, 33: 287-294. DOI:10.2135/cropsci1993.0011183X003300020015x |
Simone O D, Junk W J, Schmidt W. 2003. Central Amazon floodplain forests: Root adaptations to prolonged flooding[J]. Russ J Plant Physl, 50: 848-855. DOI:10.1023/B:RUPP.0000003285.70058.4c |
Singh V, Pallaghy C K, Singh D. 2006. Phosphorus nutrition and tolerance of cotton to water stress Ⅱ: Water relations, free and bound water and leaf expansion rate[J]. Field Crops Res, 96: 199-206. DOI:10.1016/j.fcr.2005.06.011 |
Smiroff N. 1993. The role of active oxygen in the response to water deficit and desiccation[J]. New Phytol, 125: 27-28. DOI:10.1111/nph.1993.125.issue-1 |
Smith P, Paulsen G M, Raguse C A. 1964. Extraction of total available carbohydrate from grass and legume tissue[J]. Plant Physiol, 39: 960-962. DOI:10.1104/pp.39.6.960 |
Sofo A, Dichio B, Xiloyannis C, et al. 2004. Lipoxygenase activity and proline accumulation in leaves and roots of olive trees in response to drought stress[J]. Physiol Plant, 121: 58-65. DOI:10.1111/ppl.2004.121.issue-1 |
Wang Z, Quebedeaux B, Stutte G W. 1995. Osmotic adjustment: effect of water stress on carbohydrates in leaves, stems and roots of apple[J]. Aust J Plant Physiol, 22: 747-754. DOI:10.1071/PP9950747 |
Zhang Z L. 1990. Experimental guide to plant physiology[M]. Beijing: Higher Education Press of China.
|
Zwiazek J J, Blake T J. 1990. Effects of preconditioning on electrolyte leakage and lipid composition in black spruce (Picea mariana) stressed with polyethylene glycol[J]. Physiol Plant, 79: 71-77. DOI:10.1111/ppl.1990.79.issue-1 |
 2010, Vol. 46
2010, Vol. 46


