文章信息
- Zhang Qiwei, Wang Guixian, Wang Muhua
- 张启伟, 王桂仙, 王慕华
- Thermodynamic and Kinetic Parameters for Adsorption of Nitrobenzene by Bamboo-Charcoal
- 竹炭对硝基苯吸附的热力学与动力学参数
- Scientia Silvae Sinicae, 2009, 45(8): 113-116.
- 林业科学, 2009, 45(8): 113-116.
-
文章历史
- 收稿日期:2008-04-28
-
作者相关文章
Nitrobenzene is an important industrial material and was widely used in manufacturing of aniline, dyes, TNT, soap and spices, etc. Because of its highly toxicity, it was listed in the forefront in the list of priority control toxic organic pollutants of world environment and it is necessary to reduce its spread in the environment. The adsorption is one of the easiest and most effective methods to remove deleterious substances from wastewater. And the effective, economical adsorbents will be used to remove deleterious substances from wastewater.
The bamboo-charcoal was the pyrogenation product of bamboo with wide surface area and strong adsorption ability. As a novel adsorbent, the bamboo charcoal is widely applied in waste water treating (Zhang et al., 2001; Jiang, 2002; Hu, 2002). The bamboo charcoal is an economical adsorbent for removing organic substances from wastewater because of its abundance and effectiveness. The adsorption properties of organic substances on bamboo-charcoal have been reported in recent years(Xu et al., 2002; Zhang et al., 2006; Wang et al., 2008).
In this article, the adsorption properties, thermodynamic and kinetic parameters of bamboo-charcoal for nitrobenzene were investigated. The optimal pH, contact time, adsorption isotherm equation, apparent rate constant, activation energy and thermodynamic parameters were included. Theoretical basis is supplied for adsorption treating of nitrobenzene in wastewater.
1 Materials and methods 1.1 Instruments723 spectrophotometer, Sartorius PB-20 pH meter, SHZ-B temperature constant shaking machine, WD900SL23-2 gelanshi household microwave oven.
1.2 MaterialsA standard solution of 2.690 mg·mL-1 nitrobenzene was prepared with nitrobenzene(AR) and 0.01 mol·L-1 H2SO4 and ethanol. Buffer solution with pH 3.2~6.4 was prepared by HAc-NaAc. buffer solution with pH 5.8~7.4 was prepared by KH2PO4-NaOH, other reagents were AR grade.
The machine-kiln bamboo charcoal was provided by Zhejiang Forest Bamboo Charcoal Limited Company, the experiments adopted bamboo-charcoal that average diameters were 0.60~0.90 and 0.18~0.22 mm. The surface area were 905 and 977 m2·g-1 respective. They were soaked in dilute NaOH overnight and then washed thoroughly with deionized water until the pH became neutral. After the pretreatment, bamboo charcoal was then dried at 250℃ in an electric oven until weight invariableness.
1.3 Analytical methodThe concentrations of nitrobenzene were analyzed with reduction-azo spectrophotometric method(Li, 1997).
1.4 Sorption equilibrium experimentA desired amount of treated bamboo charcoal was weighed and added into a conical flask, then a desired volume of buffer solution and a required amount of standard solution of nitrobenzene were added. The flask was shaken in a shaker at constant temperature. The upper layer of clear solution was taken out for analysis until adsorption equilibrium was reached. The adsorption amounts[Q/(mg·g-1)] and adsorption rate(E/%) was calculated as following:
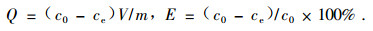
|
c0: initial concentration of nitrobenzene in solution(mg·mL-1); ce:equilibrium concentration of nitrobenzene in solution(mg·mL-1); V:total volume of solution(mL); m:mass of bamboo charcoal(g).
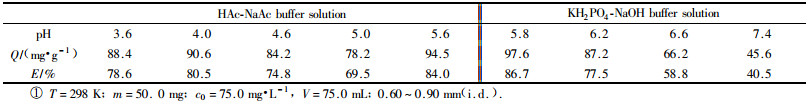
2 Results and discussion 2.1 Influence of pH on adsorptionThe test was carried out according to the above mentioned method. The influence of pH on the sorption behavior of bamboo charcoal for nitrobenzene was shown in Tab. 1.
|
|
The results shows that the bamboo-charcoal has high ability of adsorption for nitrobenzene at a wide pH range(pH 3.6~6.2). The adsorption amount was highest at pH 5.6 in HAc-NaAc medium. The adsorption amount was highest at pH 5.8 in KH2PO4-NaOH medium. All following experiments were carried out at pH 5.8 in KH2PO4-NaOH medium.
2.2 Determination of equilibrium time and the apparent adsorption rate constants and activated energyAccording to the experimental condition as shown in Fig. 1 and the method mentioned above, 100.0 mg of bamboo charcoal was weighed accurately, then the upper layer solution was taken out at intervals and the remains concentrations were determined. After all of the volumes were collected, a series of data was obtained as shown in Fig. 1.
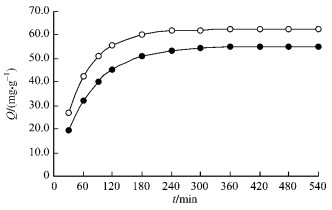
|
Fig.1 Determination of equilibrium time T=298 K; m=100.0 mg; c0=75.0 mg·L-1, V=75.0 mL; ● 0.60~0.90 mm(i.d.); ○ 0.18~0.22 mm(i.d.). |
The result shows that equilibrium time of different size bamboo charcoal is equal to 540 min. The adsorption amount increases with the decrease of size of bamboo charcoal. According to Brykina method, the sorption apparent rate constant k can be calculated from -ln(1-F)=kt, where F=Qt/Q∞, Qt and Q∞ are the adsorption amounts at certain time and at equilibrium, respectively. If it has good linear relationship of -ln(1-F) versus t, the adsorption kinetics can be described with pseudo first-order kinetics.
Studying of kinetics showed that adsorption of the different size bamboo charcoal(0.45~0.60 mm and 0.18~022 mm) for nitrobenzene can be described with pseudo first-order reaction (Fig. 2), the correlation coefficients were 0.995 and above.
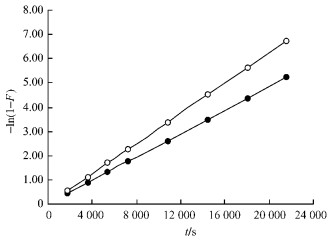
|
Fig.2 Determination of apparent rate constants ● 0.60~0.90 mm -ln(1-F)=2.42×10-4t, ○ 0.18~0.22 mm -ln(1-F)=3.13×10-4t. |
Similarly, the apparent rate constants were determined in different temperature, the adsorption rate constant k at the temperature of 298, 308, 318 K were obtained (among them k298=2.42×10-4 s-1, k308=2.77×10-4 s-1, equilibrium time was 420 min and k318=3.14×10-4 s-1, equilibrium time was 360 min), respectively. Plot lnk against 1/T, the slope of straight line kslope=-1.23×103. According to Arrhenius equation lnk=-Ea/2.303RT+constant, the apparent activation energy can be obtained (Ea =23.5 kJ·mol-1).
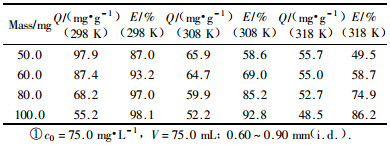
2.3 Isotherm adsorption curve and determination of thermodynamics parametersThe different mass of bamboo charcoal was weighed accurately, according to the experimental condition shown in Tab. 2 and the above mentioned. After equilibrium, the upper layer clear solution was taken out for the determination of remains concentrations and a series of adsorption amounts was calculated and shown in Tab. 2.
|
|
The result shows that the adsorption amounts decrease with the increases of mass of bamboo charcoal, and the adsorption efficiency increases with the increases of mass of bamboo charcoal.
The experiment data were treated by Langmuir and Freundlich adsorption isotherm equation which are most widely used (Wang et al., 2005; Wei et al., 2004; Zhang et al., 2008). They can be expressed as following:
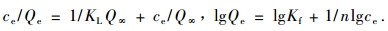
|
KL: adsorptive coefficient; Q∞: saturated adsorptive capacity; Kf: adsorptive coefficient; n: measurement adsorptive strength constant. All of above are adsorptive characteristic parameters.
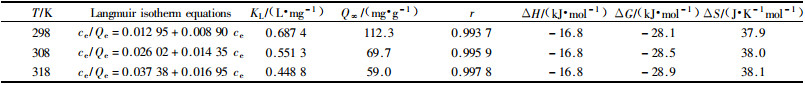
The experimental result shows:it is better to describe adsorption isotherm with Langmuir adsorption isotherm equation than Freundlich adsorption isotherm equation. The interrelated coefficient were 0.988 7 and 0.942 5 respective. The Langmuir adsorption isotherm equation and the adsorptive coefficients in different temperature are shown in Tab. 3.
|
|
The KL of Langmuir adsorption isotherm equation was changed with temperature and heat of adsorption. The relationship can be described with following equation:lnKL=lnK0-ΔH/RT. The adsorptive enthalpy change can be obtained by this equation and the ΔH is isometrical enthalpy change. The result shows that lnKL versus 1/T has good linear relationship. And the ΔH is equal to -16.8 kJ·mol-1 by calculating with the slope of straight line. ΔG=-RTlnK can be obtained by Gibbs equations and K is adsorptive equilibrium constant. K is adsorption coefficient KL in this experiment. Accordingto ΔS=(ΔH-ΔG)/T, free energy change and entropy change can be calculated in different temperature. The thermodynamic parameters were also shown in Tab. 3.
The adsorption amounts decrease with the increase of adsorption temperature and the isometrical enthalpy change is a negative value. It means that the sorption process is an exothermic process. ΔH<40 kJ·mol-1 shows that the process is also a physical adsorption process. It is concordance with literature(Chen, 2004) that the main adsorption of organic substance with bamboo-charcoal is a physical adsorption. According to the data, the change of temperature has little influence on ΔG. The adsorption free energy change is a negative value shows that the process of adsorbate from solution to adsorbent surface is a spontaneous process.
3 ConclusionThe result shows that adsorption equilibrium time is 540 min and the bamboo-charcoal shows high adsorption ability for nitrobenzene in water phase at pH 5.6. The adsorption obeys general adsorption law. The adsorption amounts increased with the decrease of size of babmoo-charcal and the increase of surface area. As the supplied adsorbent amounts increased, the adsorption amounts per gram adsorbent decreased and adsorption efficiency increased.
The adsorption decreased with the adsorption temperature increases. And it has been proved that the adsorption process is an exothermic process. So it is disadvantage for adsorption behavior to increase temperature.
The adsorption behavior of bamboo-charcoal for nitrobenzene obeys the Langmuir isotherm model.
The studying of kinetics shows:the adsorption kinetics of bamboo-charcoal for nitrobenzene can be described by pseudo first-order kinetics. The adsorption apparent rate constant k298 is 2.42×10-4 s-1 and activation energy Ea is 23.5 kJ·mol-1 at the temperature of 298 K.
The studying thermodynamic shows that the adsorption process of bamboo-charcoal on nitrobenzene is an exothermic process. The heat of adsorption (ΔH) is equal to -16.8 kJ·mol-1, entropy change (ΔS) is equal to 37.9 J·K-1 mol-1 and free energy change (ΔG)is equal to 28.1 kJ·mol-1. The ΔG is a negative value shows that the adsorption process of adsorbate from solution to adsorbent surface is a spontaneous process.
Chen Wenyuan(陈文渊). 2004. Reasearch on the bamboo charcoal adsorbs the organic pollutants in the water. Dissertation for Master's Degree of Fujian Agriculture and Forestry University(福建农林大学硕士学位论文), 1-8.
|
Hu Yonghuang(胡永煌).2002. Progress on production technology and applications of bamboo carbon and bamboo distillate. Chemistry and Industry of Forest Products(林产化学与工业), 22(3): 79-83. http://en.cnki.com.cn/Article_en/CJFDTOTAL-LCHX200203019.htm
|
Jiang Zehui(江泽慧). 2002. Bamboo and rattan in the world. Shenyang: Science and Technology Publishing House (沈阳: 沈阳科技出版社), 3-19.
|
Li Li(李丽). 1997. Improvement of nitrobenzene determination by reducti on a zo-photometry. Arid Environmental Monitoring (干旱环境监测), 11(3): 149-151. http://en.cnki.com.cn/Article_en/CJFDTOTAL-GHJC199703005.htm
|
Wang Guixian(王桂仙), Wang Muhua(王慕华), Zhang Qiwei (张启伟).2008. Adsorption proper of aniline on bamboo-charcoal. Scientia Silvae Sinicae(林业科学), 44(3): 135-139. http://www.linyekexue.net/CN/abstract/abstract4926.shtml
|
Wang Xiufang(王秀芳), Zhang Huiping(张会平), Xiao Xinyan(肖新颜), et al. 2005. Adsorption equilibrium and dynamics of phenol on bamboo charcoals. Function Material (功能材料), 36(5): 746-749. http://en.cnki.com.cn/Article_en/CJFDTOTAL-GNCL200505032.htm
|
Wei Ruixia(魏瑞霞), Chen Jinlong(陈金龙), Chen Lianlong(陈连龙), et al. 2004. Study of adsorption of 2-thiopheneacetic acid on three ads orbent resins. Acta Polymerica Sinica(高分子学报), (4): 471-477.
|
Xu Yigang(徐亦钢), Shi Lili(石利利).2002. Characteristics of 2, 4-dichlorophenol adsorption by bamboo carbon and affecting factors. Rural Eco-Environment(农村生态环境), 18(1): 35-37. http://en.cnki.com.cn/Article_en/CJFDTOTAL-NCST200201007.htm
|
Zhang Qisheng(张齐生), Jiang Shuhai(姜树海), Huang Heliang(黄禾良). 2001. Research adsorption properties and relative factors of bamboo charcoal on 2, 4-di-chloro hydroxylbenzene editorial committee. Mechanism and science of bamboo charcoal and bamboo vinegar. Beijing: China Forestry Publishing House(北京: 中国林业出版社), 168-173.
|
Zhang Qiwei(张启伟), Wang Guixian(王桂仙).2006. Adsorption behavior of the bamboo-charcoal for mercury(Ⅱ) in aqueous solution. Scientia Silvae Sinicae (林业科学), 42(9): 102-105. http://www.linyekexue.net/CN/abstract/abstract5651.shtml
|
Zhang Qiwei(张启伟), Wang Guixian(王桂仙). 2008. Study on the kinetic and the thermodynamic parameters for adsorption of the phenol by bamboo-charcoal. Biomass Chemical Engineering(生物质化学工程), 42(2): 19-22.
|
 2009, Vol. 45
2009, Vol. 45