文章信息
- Zhang Min, Li Rongjin, Huang Libin, Ji Yonghua, Dou Quanqin, Qian Meng, Fang Yanming
- 张敏, 李荣锦, 黄利斌, 季永华, 窦全琴, 钱猛, 方炎明
- Physiological and Biochemical Response of Tonoplast Vesicles Isolated from Broussonetia papyrifera to NaCl Stress
- NaCl胁迫下构树幼苗液泡膜生理生化响应
- Scientia Silvae Sinicae, 2009, 45(8): 50-55.
- 林业科学, 2009, 45(8): 50-55.
-
文章历史
- 收稿日期:2009-03-12
-
作者相关文章
2. 江苏省林业科学研究院 南京 211153;
3. 南京农业大学生命科学学院生命科学实验中心 南京 210095
2. Jiangsu Academy of Forestry Nanjing 211153;
3. Life Science Laboratory Center, Nanjing Agricultural University Nanjing 210095
Salinity is one of the major abiotic stresses, which has a major impact on plant production and productivity. Excess amount of salt in the soil adversely affects plant growth and development (Sairam et al., 2004). It is now generally accepted that the cell membrane is the primary site of injury when plants are subjected to salt stress (Surjus et al., 1996). Membranes play an important role in many cellular activities including ion transport, proton-pumping ATPase, signal transduction, etc (Huang, 1996). It is not until recently that the significance of membrane lipids in plant salinity tolerance has been appreciated. In the previous studies some researchers have investigated the changes in fatty acids, phospholipids and sterols (Surjus et al., 1996; Wu et al., 2005; Liang et al., 2005) in order to establish a relationship between lipid alteration and salt stress in plants. However, the results are often controversial (Surjus et al., 1996).
The ability of plants to grow in high NaCl concentrations is associated with the ability of the plants to transport, compartmentalize or extrude Na+ (Apse et al., 2007). The pumping of Na+ into the vacuole is catalyzed by vascular Na+/H+ antiporter (Tester et al., 2003). H+-electrochemical potential gradient, which provides the driving force, was initially established by the H+-ATPase and H+-pyrophosphatase on the tonoplast (Tester et al., 2003). Vacuolar H+-ATPases (V-H+-ATPase) are multi-subunit enzymes that pump protons into intracellular compartments (Coker et al., 2003). V-H+-ATPase plays important roles in pH homeostasis and various stress responses (Coker et al., 2003; Sze et al., 2002). The V-H+-ATPase has been reported to be involved in salt tolerance in the previous studies (Liang et al., 2005; Zhang et al., 2002; Yu et al., 2005).
Membrane structural and functional stability is crucial in plant adaptation to abiotic stresses (Liang et al., 2005). The maintains of membrane fluidity is a prerequisite for the function, viability, growth and reproduction of cells (Lu et al., 2006). Changes in membrane fluidity may affect the membrane micro-environment surrounding proteins, which in turn influences membrane functions such as carrier-mediated transport and the activity of membrane bound enzymes including ATPase activity (Surjus et al., 1996).
Until now, there is few studies have been carried out to analyze the changes of lipid composition and membrane fluidity of woody plants under salt stress. In this study, the tonoplast vesicles from the leaves and roots of Broussonetia papyrifera grew under NaCl stress were isolated and the effect of NaCl on tonoplast H+-ATPase activity and membrane fatty acid composition were investigated. The membrane fluidity of tonoplast vesicles was also studied. The aim of our study is to gain better understanding of the mechanisms of salt tolerance in woody plant.
1 Materials and methods 1.1 Plant materialB. papyrifera used in this study was introduced from Japanes and was cultivated in the nursey of Jiangsu Academy of Forestry. Uniform-sized B. papyrifera in vitro regenerated rooting plantlets were planted into plastic pots filled with 500 mL of half-strength MS solutions in a growth chamber. The nutrient solution was renewed every other day. The experimental design consisted of a control (no NaCl) and three treatments (50, 100 and 150 mmol·L-1 NaCl) and was established in a randomized design with three replicates. After five days treatment the roots and leaves were harvested for isolation of tonoplast vesicles.
1.2 Isolation of tonoplast-enriched vesiclesTonoplast vesicles were isolated according to the previously reported method (Liang et al., 2005) with minor modifications. Roots and leaves were homogenized with an ice-cold mortar and pestle in the homogenization buffer (50 mmol·L-1 HEPES-Tris, pH 7.6, 250 mmol·L-1 sorbitol, 125 mmol·L-1 KCl, 5 mmol·L-1 EGTA, 2.5 mmol·L-1 K2S2O5, 2 mmol·L-1 PMSF, 1.5% PVP, 0.1% BSA, 2 mmol·L-1 DTT). The homogenates were filtered through four layers of cheesecloth and subjected to differential centrifugation at 10 000×g for 15 min and subsequently at 50 000×g for 30 min at 4 ℃. The 10 000×g to 50 000×g pellets were suspended in 6 mL of buffer A (300 mmol·L-1 sucrose, 10 mmol·L-1 KCl, 1 mmol·L-1 EGTA, and 2 mmol·L -1 DTT, pH 7.8). Then 4 mL of buffer B (250 mmol·L-1 sorbitol, 1 mmol·L-1 EGTA, 5 mmol·L-1 HEPES, pH 7.3) were layered over the buffer A gradient. The gradients were then subjected to ultracentrifugation (100 000×g, 2 h, 4 ℃) with a Ti90 rotor in an Optima L-80XP ultracentrifuge (Beckman Coulter Inc., Fullerton, CA, USA). Vesicles banding at the buffer A/B interface were collected and stored at -80 ℃ until use.
1.3 Determination of H+-ATPase activity in tonoplastActivity of V-H+-ATPase was measured according to Shi et al. (2007) with minor modification. The reaction was carried out in a 0.5 mL mixture (30 mmol·L-1 HEPES-Tris, pH7.5, 3 mmol·L-1 MgSO4, 100 mmol·L-1 KCl, 0.5 mmol·L-1 NaN3; 0.1 mmol·L-1 Na3VO4, 0.1 mmol·L-1 (NH4)4MoO4, 0.02% Triton X-100, 3 mmol·L-1 ATPNa2). Reaction was started by adding 50 μL tonoplast vesicles. The reaction was terminated by the addition of 0.1 mL of 10% SDS after incubation at 37 ℃ for 30 min. The release of Pi from ATP was determined by the Fiske-Subbarow reagent (Fiske et al., 1925), using NaH2PO4 as the standard.
1.4 Tonoplast fatty acid analysisFatty acids of tonoplast vesicles were analyzed using the method described by Liang et al. (2005) with some modifications. Fatty acids were methylated with boron trifluoride-ethyl ether/methanol (1:3, v/v) including 10 μg of heptadecanoic acid served as internal standard. The fatty acid methyl esters were then analyzed by Thermo Trace GC Ultra gas chromatograph equiped with a flame ionization detector (FID) and an Sp-2560 quartz capillary column (100 m×0.25 mm×0.2 μm). Highly pure N2 was used as the carrier gas. The injection and detection temperature were both set at 220 ℃. The following temperature program was used: The initiation temperature was set at 140 ℃ and maintained for 5 min, followed by 4 ℃·min-1 to 220 ℃ for 25 min. Quantitation was achieved by normalization with an internal standard of heptadecanoic methyl ester. The fatty acid composition was expressed as mol %.
1.5 Determination of tonoplast fluidityDPH (1, 6-diphenyl-1, 3, 5-hexatriene) was used as a fluorescent probe to determine the fluidity of tonoplast vesicles in this study. A stock solution of DPH (2 mmol·L-1) was obtained by dissolving DPH in tetrahydrofuran. For lebeling of tonoplast vesicles, DPH was used at a concentration of 2 μmol·L-1. The mixture was incubated at 37 ℃ for 1 h in the dark. The fluorescence polarization of DPH was determined on a Hitachi 850 fluorescence spectrometer fitted with a polarization attachment as described by Pang et al. (2005). The samples were excited at 360 nm, and the emissions at 429 nm were recorded. Both excitation and emission slits were set at 5 nm. The degree of fluorescence polarization (P) was calculated according to the following formula: P=(I//-I⊥)/(I//+GI⊥), in which I// and I⊥ are the fluorescence intensities measured with parallel and perpendicular oriented polarizers, respectively, and G is the calibration factor. Here, G = I⊥/ I//.
1.6 Statistical analysisStatistical analysis was carried out by one-way ANOVA using SPSS 10.0 software to determine the different significance. When the ANOVA was significant at P < 0.05, the Duncan multiple range test was used for mean comparison. Data presented were mean ±SD of three experiments.
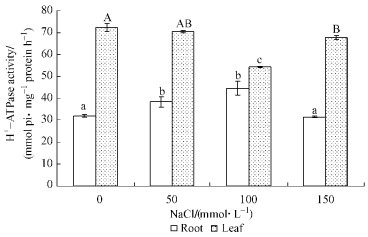
2 Results 2.1 Effect of NaCl stress on V-H+-ATPase activity from roots and leaves of B.papyriferaTo assess the influence of NaCl on the activity V-H+-ATPase, B. papyrifera were treated with different concentrations of NaCl and tonoplast vesicles were isolated from the roots and leaves. The results showed that the activity of V-H+-ATPase in the roots increased with the increase of NaCl concentration. And then V-H+-ATPase activity decreased when NaCl concentration reached 150 mmol·L-1, but similar with the control (Fig. 1). However, the changes of V-H+-ATPase activity in the leaves were not consistent with the roots. In leaves, V-H+-ATPase had no significant difference to control when treated with low NaCl concentration. At salinity of 100 mmol·L-1 the activity decreased significantly and reached the lowest level. After then, the activity recovered at highest concentration of NaCl (150 mmol·L-1) and it almost close to the level as control.
|
Fig.1 Changes in V-H+-ATPase activity from roots and leaves of Broussonetia papyrifera Each measurement was conducted with three replicates, and data were mean ±SD. Different letters indicate significant difference by Duncan's multiple range test at 0.05 level. |
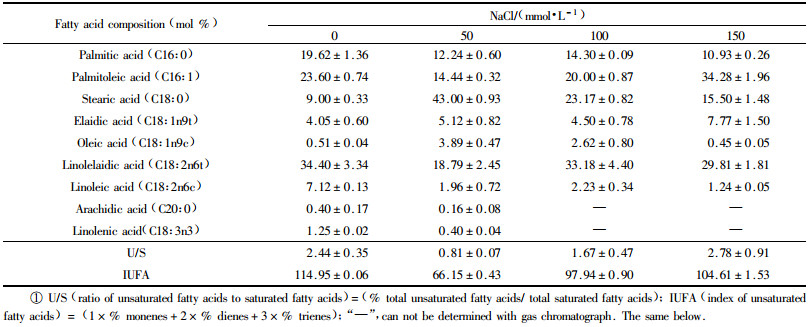
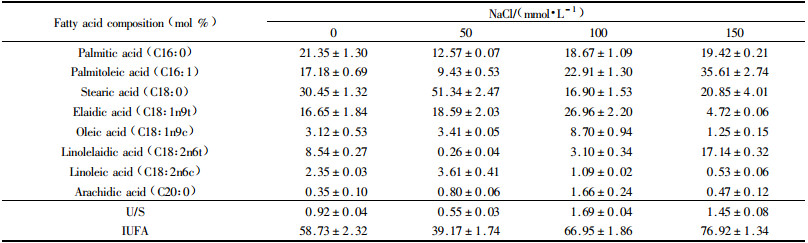
The analysis of fatty acid composition of the tonoplast vesicles lipid extract revealed that the fatty acids in tonoplast of B. papyrifera roots and leaves mainly consisted of C16:0, C16:1, C18:0, C18:1, C18:2 and C20:0, whereas C18:3 was found in the roots rather than in the leaves (Tab. 1 and 2). The ratio of unsaturated fatty acids to saturated fatty acids (U/S) decreased in the tonoplasts of roots and leaves at lower levels of NaCl. While the U/S of roots increased and was slightly higher than control under 150 mmol·L-1 NaCl. In leaves, the U/S increased gradually at 100 and 150 mmol·L-1 NaCl treatment (Tab. 1). Moreover, the index of unsaturated fatty acids (IUFA) decreased in the roots after NaCl treatmen. In contrast, the IUFA decreased in the leaves under 50 mmol·L-1 NaCl, and then increased under 100 and 150 mmol·L-1 NaCl (Tab. 2).
|
|
|
|
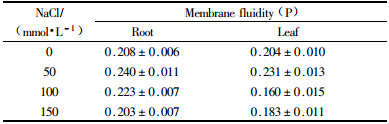
The tonoplast vesicles were labeled with the fluorescent reagent DPH to evaluate the changes in the fluidity of tonoplast vesicles under NaCl stress. The fluorescence labeled tonoplast vesicles were scanned with a fluorescence spectrometer, and the peak value of excitation and emission was found at 360 and 429 nm, respectively (Fig. 2 A and B). The membrane fluidity was detected by the steady-state fluorescence polarization measurements. The results in Tab. 3 demonstrated that in the roots the P value increased under 50 and 100 mmol·L-1 NaCl, which indicated the decrease in tonoplast fluidity. And tonoplast fluidity increased when NaCl concentration reached 150 mmol·L-1. Whereas, in the leaves tonoplast fluidity decreased under 50 mmol·L-1 NaCl, and then it increased with the increase of NaCl concentration.
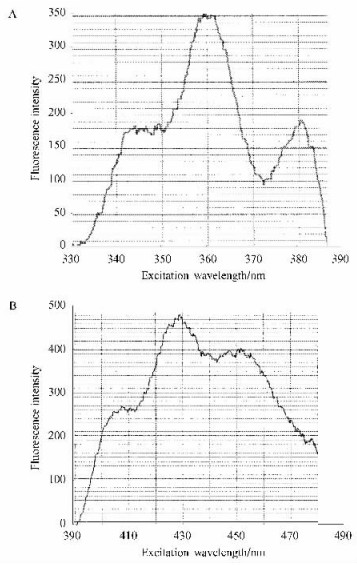
|
Fig.2 Excitation (A) and emission (B) spectrum of fluorescence labeled tonoplast vesicle |
|
|
The fundamental basis of the adaptation of plants to salinity stress is the control of transport of salt across membranes. Two major membranes of plant cells, the plasmalemma and the tonoplast, are particularly involved in the process of compartmentation of the NaCl taken up (Lüttge, 1993). The tonoplasts of plant cells contain proteins catalyzing primary-and secondary-active processes of ion transport, which are essential in the salt compartmentation involved in adaptation to salinity (Lüttge, 1993). V-H+-ATPase plays a fundamental role in energizing Na+/H+ antiport activity in cells accumulating significant quantities of NaCl (Zhang et al., 2002). It has been shown that the activity of the V-H+-ATPase increases in the tonoplast vesicles isolated from barly roots under treatment with NaCl (Zhang et al., 2002). In this study, we found that at low concentrations of NaCl treatment the activity of V-H+-ATPase isolated from the roots of B. papyrifera was stimulated. And the activity decreased slightly under 150 mmol·L-1 NaCl, but not significant compared with the control. However, the changes in the activity of V-H+-ATPase isolated from the leaves were different from the roots. The V-H+-ATPase activity in the leaves decreased in response to NaCl stress. These differences observed may be because that differentiated cells in various plant tissues and organs may function differently in coordination with neighboring cells to achieve salinity tolerance (Wu et al., 2005).
Membranes have been shown to play an important role in the ability of plants to cope with salinity (Mansour et al., 2005). Various kinds of environmental stress, such as temperature stress and osmotic stress, cause alterations in the physical properties of the membrane lipids in living cells (Los et al., 2004). The physical state of membrane lipids also acts directly to regulate the activity of membrane-bound proteins, such as the ion channels (Sukharev, 1999), sensor proteins (Sugiura et al., 1994), and proton-pumping ATPase (Surjus et al., 1996). Determining how salinity affects the membrane composition of the woody plant B. papyrifera will increase our understanding of plant salt tolerance. We observed that in the roots, NaCl concentration at which H+-ATPase activity increased was identical with that at which tonoplast fluidity decreased. This phenomenon was also reported in the tonoplast isolated from barley roots under salinity (Zhang et al., 2002). The previous study has suggested that proper fluidity of membrane was important for optimal structure and high activity of H+-ATPase (Zhang et al., 2002). Moreover, we found that changes in the activity of V-H+-ATPase in the leaves was not consistent with the roots. V-H+-ATPase activity declined with the increase in NaCl concentration.
Traditionally, alterations in fatty acid unsaturation degree are related to changes in membrane fluidity, and it is known that a decrease in fatty acid unsaturation results in a decrease in membrane fluidity (Navari-Izzo et al., 2000; Quartacci et al., 2002). In our study, the observed decline in tonoplast fluidity might due to the decrease in the ratio of unsaturated fatty acids to saturated fatty acids (U/S).
In a previous review, Munns (1993) has concluded that "Advances in salt tolerance at the molecular level will be in manipulating the expression and structure of proteins that control the transport of salt across membranes". Therefore, it is of great important to investigate the changes of the V-H+-ATPase under salinity, especially in woody plants.
In conclusion, the detailed analysis of the isolated tonoplast vesicle component may provide valuable information with respect to the physiological responses of plants to salt stress.
Apse M P, Blumward E. 2007. Na+ transport in plants. FEBS Letter, 581: 2247-2254. DOI:10.1016/j.febslet.2007.04.014 |
Coker J S, Jones D, Davies E. 2003. Identification, conservation, and relative expression of V-ATPase cDNAs in tomato plants. Plant Molecular Biology Reporter, 21: 145-158. DOI:10.1007/BF02774241 |
Fiske C H, Subbarow Y. 1925. The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66: 375-400. |
Huang C Y. 1996. Salt-stress induces lipid degradation and lipid phase transition in plasma membrane of soybean plants. Taiwania, 41: 96-104. |
Liang Y C, Zhang W H, Chen Q, et al. 2005. Effects of silicon on H+-ATPase and H+-APase activity, fatty acid composition and fluidity of tonoplast vesicles from roots of salt-stressed barley (Hordeum vulgare L.). Environmental and Experimental Botany, 53: 29-37. DOI:10.1016/j.envexpbot.2004.02.010 |
Los D A, Murata N. 2004. Membrane fluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta, 1666: 142-157. DOI:10.1016/j.bbamem.2004.08.002 |
Lu Y N, Qiu Q Y, Wang Y, et al. 2006. Effects of phellodendron and its main components on the cell membrane fluidity. Chinese Journal of pathophisiology, 22: 156-159. |
Lüttge U. 1993. Plant cell membranes and salinity: structural, biochemical and biophysical changes. R Bras Fisiol Veg, 5: 217-224. |
Mansour M M F, Salama K H A, Al-Mutawa M M, et al. 2002. Effect of NaCl and polyamines on plasma membrane lipids of wheat roots. Biologia Plantarum, 45: 235-239. DOI:10.1023/A:1015144607333 |
Munns R. 1993. Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant, Cell & Environment, 16: 15-24. |
Navari-Izzo F, Quartacci M F, Pinzino C, et al. 2000. Protein dynamics in thylakoids of the desiccation-tolerant plant Boea hygroscopica during dehydration and rehydration. Plant Physiology, 124: 1427-1436. DOI:10.1104/pp.124.3.1427 |
Pang Y H, Zhu H, Wu P, et al. 2005. The characterization of plasma membrane Ca2+-ATPase in rich sphingomyelin-cholesterol domains. FEBS Letters, 579: 2397-2403. DOI:10.1016/j.febslet.2005.03.038 |
Quartacci M F, Glišić O, Stevanović B, et al. 2002. Plasma membrane lipids in the resurrection plant Ramonda serbica following de hydration and rehydration. Journal of Experimental Botany, 53: 2159-2166. DOI:10.1093/jxb/erf076 |
Sairam R K, Tyagi A. 2004. Physiology and molecular biology of salinity stress tolerance in plants. Current Science, 86: 407-421. |
Shi Q H, Ding F, Wang X F, et al. 2007. Exogenous nitric oxide protect cucumber roots against oxidative stress induced by salt stress. Plant Physiology and Biochemistry, 45: 542-550. DOI:10.1016/j.plaphy.2007.05.005 |
Sugiura A, Hirokawa K, Nakashima K, et al. 1994. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Molecular Microbiology, 14: 929-938. DOI:10.1111/mmi.1994.14.issue-5 |
Sukharev S. 1999. Mechanosensitive channels in bacteria as membrane tension reporters. FASEB Journal, 13(Suppl.): S55-S61. |
Surjus A, Durand M. 1996. Lipid changes in soybean root membranes in response to salt treatment. Journal of Experimental Botany, 47: 17-23. DOI:10.1093/jxb/47.1.17 |
Sze H, Schumacher K, Müller M L, et al. 2002. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends in Plant Science, 7: 157-161. DOI:10.1016/S1360-1385(02)02240-9 |
Tester M, Davenport R. 2003. Na+ tolerance and Na+ transport in higher plants. Annals of Botany, 91: 503-527. DOI:10.1093/aob/mcg058 |
Wu J L, Seliskar D M, Gallagher J L. 2005. The response of plasma membrane lipid composition in callus of the halophyte Spartina patens (Poaceae) to salinity stress. American Journal of Botany, 92: 852-858. DOI:10.3732/ajb.92.5.852 |
Yu B J, Lam H M, Shao G H, et al. 2005. Effects of salinity on activities of H+-ATPase, H+-PPase and membrane lipid composition in plasma membrane and tonoplast vesicles isolated from soybean (Glycine max L.) seedlings. Journal of Environmental Sciences, 17: 259-262. |
Zhang W H, Liu Y L. 2002. Relationship between tonoplast H+-ATPase activity, ion uptake and Calcium in barley roots under NaCl stress. Acta Botanica Sinica, 44: 667-672. |
 2009, Vol. 45
2009, Vol. 45