文章信息
- Li Huiping, Huang Dazhuang, Wang Zhigang, Yan Haixia, Zheng Jianwei
- 李会平, 黄大庄, 王志刚, 闫海霞, 郑建伟
- Screening Test of Highly Virulent Strains of Entomopathogenic Fungi Beauveria bassiana against Apriona germari Larvae
- 桑天牛幼虫高毒力白僵菌菌株筛选
- Scientia Silvae Sinicae, 2007, 43(11): 66-71.
- 林业科学, 2007, 43(11): 66-71.
-
文章历史
Received date: 2006-10-21
-
作者相关文章
Apriona germari is a destructive stem-boring pest and difficult to control for numerous reasons. In China, It has one generation every 1~3 years based on the timing of adult emergence and the climate conditions. Most adults emerge from June to August, but the emergence is not synchronous and can extend well after August. After emergence, the adults feed on the phloem of twigs. And the females cut a small impression in the tree bark and lay an eggunder the bark. After hatching, Young larvae develop just under the bark but later larvae develop in the xylem to live through the winter. The fact that it has abroad hosts and can attack and kill apparently healthy trees makes this species a serious pest. Due to the enormity of problems caused by this destructive pest, it poses an enormous threat to forestry, fruit industry, mulberry and sericiculture(Li, 2005;Liu et al., 2002).
Although many scientists made great efforts to control A. germari. To date, the control of the pests is still based largely on the chemical insecticides, to which the pests have developed strong resistance. Under the increasing concern over pesticide efficacy and safety to humans and the environment, the scientists have began to seek an alternative management approaches. The biological controlmig ht provide a viable option because it has some advantages catering for the human'request. As an effective biological control agent, entomopathogenic fungi has played an important role in control of agricultural and forest insect pests. At the same time, they are relatively host specific with minimal effect on non-targe t beneficial organisms and could be compatible with other IPM program. They were known to have low mammalian toxicity. Their production is easy and cheap and does not require high input technology. All these characteristics made them ideal alternative in controlling A. germari(Godfrey et al., 2001).
Beauveria bassiana, an entomopathogenic fungi that have been studied and applied widely in the world, has a relatively high affecting rate on A. germari in the natural field(Zhou et al., 2004). There are some reports about using B. bassiana to control the stem-boring pests(Dubois, 2003; Dubois et al., 2004; He et al., 2005; Shimazu, 2004).In these reports, we found that the common method of collecting the strains was isolating from the infected and moistened insect. But th elimitation of this method, for example, hard work, time-consumed and easily in fluenced by season, restrict the controlling work using B. bassiana. Therefore, in this study, to obtain more strains to select high virulent ones, except for the traditional method of collecting the diseased insects from the field, we also employed the Tenebrio molitor Method to collect the B. bassiana from soil. Also, virulence of the isolates was evaluated and highly virulent strains were screened in the laboratory. This study also aimed to determine the exposuretime-dose-mortality relationships of virulent fungal isolates, and to confirm B. bassiana infection against A. germari under laboratory condition.
2 Materials and methods 2.1 Test insectsThe A.germari adults collected from the field were bred with branches of Morus alba in the house to lay eggs. The eggs were put in the Petri dish which has a wetted filter to hatch. The first hatching larvae were at soon inoculated to artificial groove of Populus tomentosa to breed for 20 d to be used in the bioassays.
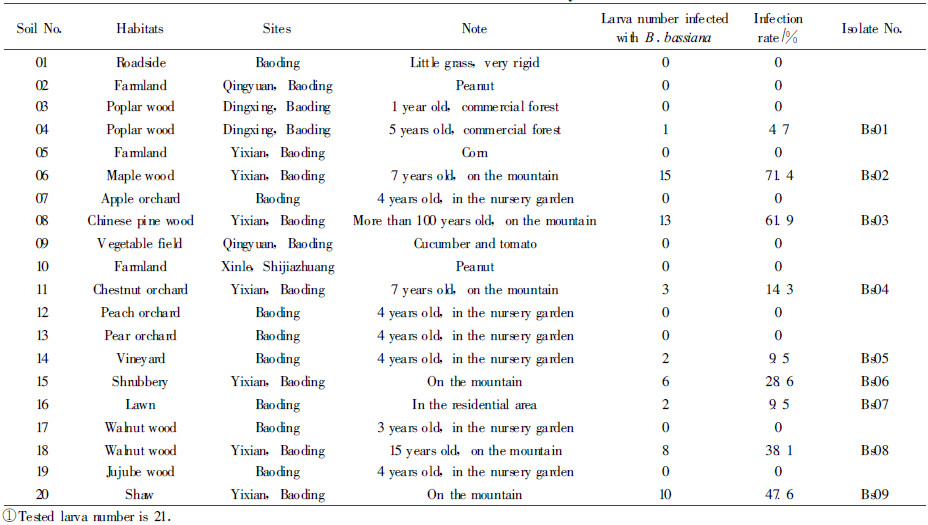
2.2 Test isolates 2.2.1 Isolates from diseased insectsThe host and locality of the isolates of 5 B. bassiana from the diseased ins ects used in this study were given in Tab. 1.
Cadavers of insects those seemed to be fungal diseases collected in the field were kept at room temperature to allow outer mycerial growth and sporulation. When the presence of a fungus was observed, conidia were removed from the insect surface with a sterile loop and streaked onto PDA plates and incubated at 25 ℃. The fungi were identified by microscopically inspecting of the sporulating structures and conidial morphology. A fungus recovered from an infected insect was considered to be an isolate.
|
|
To extract B. bassiana existed in soil, the bate method was employed. The bate insects for this study were the old larvae of Tenebrio molitor bought from the market. Several larvae were placed in humidified Petri dish prior to the baiting experiments to ensure that they were healthy. Soil samples were collected at the various sites and habitats shown in Tab. 2.
|
|
On each occasion, four approximately 0.5 kg of soil samples(from a depth of 10 cm and area of 3 m2)were taken in each field. The four samples were combined, mixed thoroughly and coarse debris removed. They were brought back to the laboratory, and kept in a refrigerator before use. Each soil sample was placed in 3 pet ridishes of 12 cm in diameter and a small amount of sterilized water was added to the dish. Seven bate larvae were placed in each dish and the dishes were kept at room temperature. The larvae were inspected once every two days for a period of two weeks.The dead larvae were removed and surface-sterilized in 1% sodium hypochlorite for 3 min, then washed three times with sterile distilled water. Excess water was removed with a piece of sterilized paper. Then the dead, intact larvae were transferred to a Petri dish with a sterilized filter paper and incubated at 25 ℃, and were checked for fungal outgrowth every 2 d. The fungi on the surface of T. molitor was identified and cultured as described above. A fungus recovered from a soil sample was considered to be an isolate.
2.3 Preparation of conidial suspensionAll isolates were grown on PDA at 25 ℃.Conidia were harvested from 2 weeks old cultures surface by scraping. Spore suspensions were made by placing harvested spores in 20 mL sterile distilled water in little beaker containing 0.05% Tween -80.Beakers were agitated on a vibrant shaker for 10 min to produce a homogenous conidial suspension. The spore concentrations were then quantified with the Neu bauer hemocytometer and adjusted to 1×108 conidia·mL-1.Viability of the conid ia was checked by a germination test prior to the experiment and assure to be >90% for all isolates.
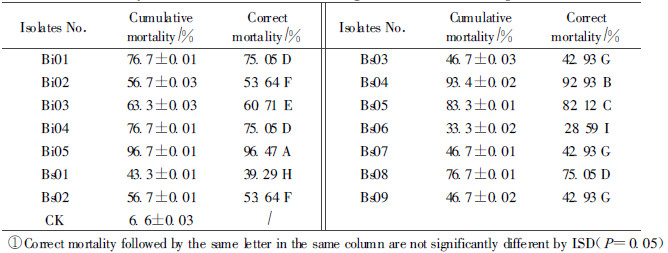
2.4 Screening bioassaysA discriminatory single concentration bioassay was conducted to determine the motality and LT50 values for each of the isolates to select the highly virulent strains for further studies. The larvae were dipped into the conidial suspensionf or approximately 30 s, drained of excess suspension by filter paper, and reared in the artificial groove as described above. Controls were treated withst erile distilled water containing 0.05% Tween-80.Mortality was recorded daily for 10 d. Dead insects were surface sterilized and transferred into a Petri dish lined with moistened filter paper and mortality due to B.bassiana was confir med by microscopic examination of hyphae and spores on the surface of the dead insect. The experiment consisted of 30 insects for one isolate. Cumulative mortality data was corrected for control mortality by Abbott's formula and subjected to aone-way analysis of variance. Treatment effects were tested by the Fisher protected least significant difference (LSD) test if significant differences were dete cted. LT50 values were calculated by linear interpolation.
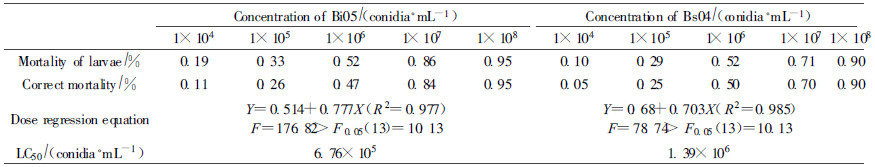
2.5 Dose-response testIsolates Bi05 and Bs04 were selected for further study on the basis of screening bioassays. To determine the LC50, Conidia of fungi were suspended in a 20 mL aqueous solution of Tween 80, and diluted to make concentrations of 1×104, 1×105, 1×106, 1×107, and 1×108 conidia·mL-1.The control consisted of same soluti on without the fungus. The insects were inoculated and reared as described in 2. 4. For one conidial concentration of each fungus, 21 larvae were used. Dead insects were treated as described above to confirm the cause of death. The LC50 was calculated using the probit procedure of the SAS statistical package.
3 Results 3.1 Isolation and identification of B.bassiana baited from the soilThe occurrence of B.bassiana in different soil samples was showed in tab. 2. 9 isolates of B.bassiana were obtained from 20 samples of soil(45% occurence). Among all the soils, the occurrence of B.bassiana was more frequent in maple wood(71.4% occurence) and Chinese pine wood(61.9% occurence) and this indicated that a considerably high density of B. bassiana was contained in these soils.
3.2 Screening bioassaysApproximately 2 d after inoculation, some response of treated insects were observed. In contrast to the control, the treated insects showed infested symptom, often appearing to inappetency, weaken activity and sometimes being unresponsive to external stimuli. Later, somelarvae died and rigidified.
Mortalities of larvae inoculated with all the isolates were shown in Tab. 3.All fungal isolates were significantly different in their virulence against A. germari after 10 d. Among the tested isolates, Isolates Bi05 and Bs04 were the more virulent ones towards A. germari, causing 96.47% and 92.93% mortality respectively 10 d post-infection.These 2 isolates were followed by isolate Bs05(82.12%), Bi01(75.05%), Bi04(75.05%) and Bs08 (75.05%), their isolates caused 28.59%~60.71% mortality. For the control, cumulative larval mortality was 6.6% after 10 d, and no fungi were observed on the dead larvae.
|
|
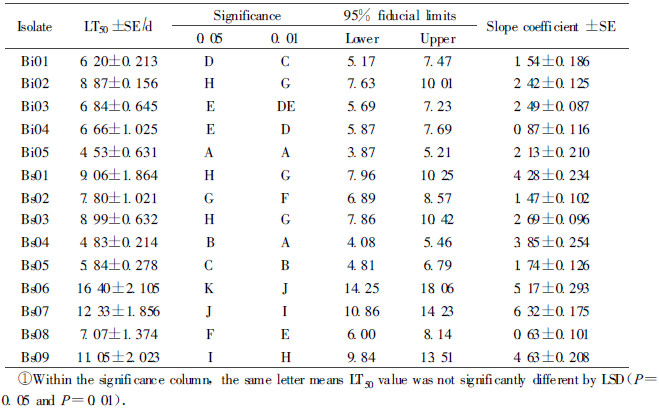
The lethal speed was noted by the use of the time taken to kill 50% of the larvae(LT50). In this study, LT50 values of all isolations were shown in tab. 4.
|
|
LT50 values differed significantly among the fungal isolates when applied at a concentration of 1×108 conidia·mL-1 in larvae. Isolates Bi05 and Bs04 had signi ficantly lower LT50 values (4.53 d and 4.83 d) for larvae at 10 d after treat ment compared to the other isolates resulted in 5.84~16.40 d. Taking the correct mortality and lethal time LT50 into accounted, the isolate Bi05 and Bs04 we re selected for the further experiments.
3.4 The lethal concentration of Bi05 and Bs04LC50 value means the concentration taken to kill 50% of the larvae. It not only can be used to evaluate the virulence of isolates, but also to guide the applyi ng dose in the actual use when controlling in the forestry. By dose-mortality analyses, the mortality of A.germari larvae differed significantly when inocul ated with different concentration of isolates Bi05 and Bs04.The corrective mortality was only 11% and 5% when the concentration was 1×104 conidia·mL-1, and amount to 95% and 90% when 1×108 conidia·mL-1 for Bi05 and Bs04.By the dose-regression equation, the LC50 of Bi05 and Bs04 were 6.76 ×105 conidia·mL-1 and 1.39×106 conidia·mL-1 respectively.
|
|
1) Bedding et al. (1975)and latterly Zimmerman (1986)recommended the use of the Galleria Bait method for the detection in soil of naturally occurring entomopathogenic fungi. To date, many scientists have succ essly obtained entomopathogenic fungi using different baits from soils(Shimazu et al., 2002; Jia et al., 2005).In this study, we also obtained 9 strains of B.bassiana from 20 samples of soil using T. molitor as the bait insect, furthermore among which we screened a virulent isolate to A.germari. This indicated that using T. molitor, a cheap and easily available insect, to bait the B.bassiana is a feasible method.
2) The isolate rate of B.bassiana differed with the different soil samples. But most isolates were obtained from the soils collected from the mountain, and there is very little in the soils from the farmland, orchards and vegetable field wh ere people's frequent working affect the soil characteristic and other insects lived in soils. Among the soils tested in this study, the occurrence of B.bassiana was higher in maple wood(71.4% occurence) and Chinese pine wood(61.9% occurence)than in other soils. The maple wood has many ground cover and no evidence of human activity. Chinese pine wood, which has luxuriate branches and leaves, abundant ground cover in which there are many insects, can provide a better nutrition and environment conditions for the growth of B.bassiana and many opportunity of serial infections. Analysed the relationship between the isolaterate and the soil characteristic, we draw a conclusion that B.bassiana was recovered more frequently in natural or undisturbed habitats in which has a high content of organic matter content and more other insects lived in soils.This phenomenon has also been observed in other origins of the world(Ali-shtayeh et al., 2003; Mietkiewski et al., 1997; Rath et al., 1992; Bidochka et al., 1998; Vanninen, 1995). This result can be used to to guide the future isolate selection programs.
3) The results of the bioassays indicated that all the fungal isolates were pathogenic to A.germari. Based on time-dose-mortality relationships, B. bassiana Bi05 and Bs04 were superior among the 14 isolates tested. Bi05 was from the diseased A.germari and the Bs04 was from the soil. Most authors agreed that strains of entomopathogenic fungi were more virulent to the species from which they were is olated(Ekesi, 1999). However, Bs04, isolated from the soil also showed high level of pathogenicity to the A.germari. Maniania reported high pathogenic acti vity of isolates of B. bassiana and M. anisopliae isolated from the soil to the stem borers, Chilo partellus and Busseola fusca(Maniania, 1992). Ekesi et al.(1998) also reported high virulence of one isolate of B. bassiana and two isolates of M. anisopliae isolated from the soil to the legume flower thrips. This suggests that although B. bassiana could be relatively host-specific as pathotypes, isolates from other environment such as the soil also offers potential for highly virulent isolates that warrants further exploration. This also advo cates the need for careful selection of fungal isolates for biological control purposes.
4) The results presented in this study demonstrated a pathogenic effect of B. bassiana on A.germari larvae and the excellent potential of isolate Bi05 and Bs04 for biological control of A. germari under laboratory conditions.This only the first step of selecting virulent strains in using B. bassiana to control the pests. To our knowledge, the field conditions is much different from the laboratory conditions. Climatic conditions such as temperature, moisture, and solar radiatio nare critical to the development of fungal infection in insects and in some cases can completely inhibit infection.So further research is necessary to determine the effectiveness of virulent isolates under field conditions and to examine its potential impact on nontarget species.
Bedding R A, Akhurst R J. 1975. A simple technique for the detection of insect parasitic rhabditid nematodes in soil. Nematol, 21: 109-116. DOI:10.1163/187529275X00419 |
Bidochka M J, Kasperski J E, Wild GA.M. 1998. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from tempe rature and near-northern habitats. Can J Botany, 7: 1198-1204. |
Dubois T.2003.Biological control of the Asian longhorned beetle, Anoplophora labripennis, with entomopathogenic fungi.Ph.D.dissertation.Cor nell University https://www.researchgate.net/publication/35473189_Biological_control_of_the_Asian_longhorned_beetle_Anoplophora_glabripennis_with_entomopathogenic_fungi
|
Dubois T, Li Z, Hu J, et al. 2004. Efficacy of fiber bands impregnated with Beauveria brongniartii cultures against the Asian longhorned beetle, Anoplophora glabripennis (Coleoptera:Cerambycidae). Biol Control, 31: 320-32. DOI:10.1016/j.biocontrol.2004.03.011 |
Ekesi S. 1999. Selection of virulent isolates of entomopathogenic hyphomycetes a gainst Clavigralla tomentosicollis St l.and evaluation in cage experiment using three cowpea varieties. Mycopathol, 148: 131-139. DOI:10.1023/A:1007122622650 |
Ekesi S, Maniania N K, Onu I, et al. 1998. Pathogenicity of entomopathogenic, fungi (Hyphomycetes) to the legume flower thrips, Egalurothrips sjostedti(Trybom)(Thysan., Thripidae). J Appl Entomol, 22: 629-634. |
Godfrey P C, Susanne V. 2001. Potential effects of Beauveria bassiana(Balsmo) Vuillemin on Neochetina bruchi Hustache (Coleoptera:Curculionidae), a biological control agent of water hyacinth. Biol Control, 21: 105-110. DOI:10.1006/bcon.2001.0920 |
He Xueyou (何学友), Chen Shunli (陈顺立), Huang Jinshui (黄金水).2005.Preliminary screening of virulent strains of Metarhizium anisopliae against Monocham us alternatu. Acta Entomol Sinica (昆虫学报), 48(6): 975-981
|
Jia Chunsheng (贾春生), You Shijiang (由士江), Song Xiaohui (宋晓辉), et al.2005.Investigate on entomopathogenic fungi in forest soil of Jilin.J Shaoguan Uni (NaturalScience)(韶关学院学报), 26(3): 86-89 http://en.cnki.com.cn/Article_en/CJFDTOTAL-SSCG200503022.htm
|
Li Chengde (李成德).2005.Forest Entomology.Chinese Forest Press, China
|
Liu Huimei (刘会梅), Sun Xugen (孙绪艮), Wang Xiangjun (王向军).2002.Advances in the research on Apriona germari Hope.For Pest and Dis (中国森林病虫), 5: 30-33 http://en.cnki.com.cn/Article_en/CJFDTOTAL-SLBC200205012.htm
|
Maniania N K. 1992. Pathogenicity of entomogenous fungi (Hyphomycetes) to larvae of stemborers, Chilo partellus(Swinhoe) and Busseola fusca Fuller. Insect Sci Applic, 13: 691-696. |
Mietkiewski R T, Pell J K, Clark S J. 1997. Influence of pesticide use on the natural occurrence of entomopathogenic fungi in arable soils in the UK:field and laboratory comparisons. Biol Sci and Technolo, 7: 565-575. |
Ali-Shtayeh M S, Mara'I Abdel-Basit B M, Jamous R M. 2003. Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian area. Mycopathol, 156: 235-244. DOI:10.1023/A:1023339103522 |
Rath A C, Koen T B, Yip H Y. 1992. The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae in Tasmanian pasture soils. Mycol Res, 96: 378-384. DOI:10.1016/S0953-7562(09)80956-8 |
Shimazu M. 2004. A novel technique to inoculate conidia of entomopathogenic fungi and its application for investigation of susceptibility of the Japanese pine sawyer, Monochamus alternatus, to Beauveria bassiana. Appl Entomol Zool, 3: 485-4901. |
Shimazu M, Zhang B, Liu Y. 2002. Fungal pathogens of Anoplophora glabripennis (Coleoptera:Cerambycidae) and their virulences. Bulletin of FFPRI, 1: 123-130. |
Vanninen I. 1995. Distribution and occurrence of four entomopathogenic fungi in Finland:effect of geographical location, habitat type and soil type. Mycol Res, 100: 93-101. |
Zhou Qiuju (周秋菊), Pan Xianli (潘贤丽).2004.Advances in biological control of Cerambycidae beetles (Coleoptera).Plant Prot (植物保护), 30(l): 12-16 http://en.cnki.com.cn/Article_en/CJFDTotal-ZWBH200401003.htm
|
Zimmerman G. 1986. The"Galleria bait method"for detection of entomopathogenic fungi in soil. Z Angew Entomol, 102: 213-215. |
 2007, Vol. 43
2007, Vol. 43