文章信息
- 朱道弘, 贺一原, 赵吕权, 黄向东, 阳艳萍.
- Zhu Daohong, He Yiyuan, Zhao Lüquan, Huang Xiangdong, Yang Yanping.
- 栗瘿蜂体内Wolbachia的感染及其wsp基因序列分析
- Sequencing and Phylogenetic Analysis of the wsp Gene of Wolbachia in a Gallwasp Species, Dryocosmus kuriphilus (Hymenoptera: Cynipidae)
- 林业科学, 2007, 43(3): 133-137.
- Scientia Silvae Sinicae, 2007, 43(3): 133-137.
-
文章历史
- 收稿日期:2006-07-21
-
作者相关文章
Wolbachia是一类以节肢动物及线虫为宿主的细胞质共生细菌,属于Proteobacteria的α亚门。其传播方式主要为垂直传播,即母代感染的细菌经卵细胞的细胞质传播至子代(Stouthamer et al., 1999)。Wolbachia能对被感染昆虫等节肢动物的生殖方式进行调控,如引起宿主胞质不亲和(cytoplasmic incompatibility)、诱导孤雌生殖(parthenogenesis-inducing)、雌性化(feminizing)及雄性致死(male-killing)(Werren, 1997; Stouthamer et al., 1999)。根据近年的研究,感染Wolbachia的昆虫种类估计超过17%(Werren et al., 1995; 2000;West et al., 1998),一些学者甚至推测感染昆虫的种类可能达到76%(Jeyaprakash et al., 2000)。Wolbachia有可能是自然界分布最广、丰度最大的共生菌。在膜翅目昆虫中,许多寄生蜂种类的产雌孤雌生殖(thelytoky)被证实与Wolbachia的共生有关(Stouthamer et al., 1993; Chen et al., 1992;Arakaki et al., 2001; Huigens et al., 2004),还发现Wolbachia能引起一些种类寄生蜂的胞质不亲和(Breeuwer et al, 1993; Perrot-Minnot et al., 1996; Vavre et al., 2000)。
瘿蜂科昆虫主要危害壳斗科植物,亦有一些种类危害蔷薇科、菊科等,一般于危害部位形成虫瘿。瘿蜂科可划分为6个族,即Aylacini族(危害草本植物)、Eschatocerini族[危害金合欢属(Acacia)和牧豆树属(Prosopis)植物]、Diplolepidini族[危害蔷薇属(Rosa)植物]、Synergini族(本身不形成虫瘿,寄居于其他瘿蜂形成的虫瘿内)、Pediaspidili族[危害槭属(Acer)植物]及Cynipini族[危害栎(Quercus)属、栗属(Castanea)及其近缘属](Lijeblad et al., 1998; Ronquist, 1999)。Aylacini族、Eschatocerini族、Diplolepidini族和Synergini族主要进行产雄孤雌生殖(arrhenotoky)(孤雌生殖中所产生的后代都是雄性的生殖方式),在Aylacini族和Diplolepidini族中亦有一些种类进行产雌孤雌生殖(Askew, 1984; Plantard et al., 1998;1999)。Pediaspidili族和Cynipini族进行周期性的孤雌生殖,即孤雌生殖与两性生殖周期性发生的生殖方式(Askew, 1984; Atkinson et al., 2002; Stone et al., 2002)。Cynipini族种类最多,已知有44属974种,多数种类进行周期性孤雌生殖,但也有一些种类营产雌孤雌生殖(Lijeblad et al., 1998; Ronquist, 1999)。这些种类的产雌孤雌生殖,很有可能与Wolbachia的共生有关,因Wolbachia的影响导致产雄孤雌生殖世代的缺失(Abe, 1986; Stone et al., 2002)。
栗瘿蜂(Dryocosmus kuriphilus属瘿蜂科Cynipini族,主要危害板栗(C. mollissima),也危害茅栗(C. sequinii)和锥栗(C. henryi)。受害芽春季形成瘤状虫瘿。国内分布于陕西、河北、山东、河南、湖北、湖南、福建等省,国外分布于日本、朝鲜。1年发生1代,营孤雌生殖(靳杏蕊等,1995;吴晖等,2004;丁玉洲等,2004)。本文通过PCR法对栗瘿蜂的株洲种群共生Wolbachia进行了检测, 并对其wsp基因序列进行了测定,通过与已知瘿蜂科中其他种类Wolbachia的wsp基因序列对比, 确定了株洲种群栗瘿蜂共生Wolbachia的进化位置,为进一步探讨Wolbachia对其孤雌生殖的诱导提供了基础。
1 材料与方法 1.1 材料栗瘿蜂的虫瘿于2004年5月采自湖南省株洲市中南林业科技大学株洲校区标本园,采集的带虫瘿的板栗枝条基部以吸水的脱脂棉包被置于(25±1) ℃的人工气候室内(宁波江南仪器设备厂),羽化成虫以无水乙醇浸泡,于-20 ℃的冷冻柜保存备用。
1.2 总DNA的提取每次进行DNA提取时,以超纯水洗涤后的栗瘿蜂成虫1个体置于装有50 μL STE缓冲液(100 mmol·L-1 NaCl, 10 mmol·L-1 Tris-HCl, 1 mmol·L-1 EDTA, pH 8.0)的1.5 mL离心管内, 将其充分捣碎。加SDS至终浓度为1%,加蛋白质分解酶K(20 mg·mL-1), 37 ℃水浴加温30 min。加等量的PCL (苯酚:氯仿:异戊醇= 25:24:1)抽提,5 000 r·min-1离心10 min,取上清液,再加等量PCL抽提,5 000 r·min-1离心10 min,取上清液,然后,加1/10体积3 mol·L-1醋酸钠,加2.5倍体积无水乙醇-20 ℃过夜,经低温离心,去上清液,用75%冷乙醇洗涤, 低温离心去上清液、干燥后, 获纯净DNA。加TE缓冲液溶解DNA备用。
1.3 Wolbachia的wsp基因片段的PCR扩增使用的wsp基因特异性引物为wsp81F(5′-TGGTCCAATAAGT GATGAAGAAAC-3′)和wsp 691R(5′-AAAAATTAAACGCTACTCCA-3′。PCR扩增体积为25 μL, 包括2 μL模板DNA, 17.1 μL H2O, 2.5 μL 10×buffer, 1 μL dNTPs (10 mmol·L-1), 上游和下游引物(10 μmol·L-1)各1 μL以及0.4 μL Taq DNA聚合酶(2.5 U·μL-1)。PCR扩增循环是: 95 ℃预变性3 min, 95 ℃ 30 s, 52 ℃ 30 s, 72 ℃ 1 min, 共35个循环, 循环结束后72 ℃处理7 min。
PCR扩增产物用1.0 %的琼脂糖凝胶于0.5×TBE缓冲液中电泳, 电压70 V, 电泳时间约1 h。电泳后以溴化乙锭染色, 凝胶成像系统检测并摄影。DNA分子质量采用Fermentas公司的DNA MW Maker标记。以水为阴性对照。
1.4 Wolbachia的wsp基因片段序列测定与分析采用克隆测序方法,回收后的PCR产物与pMD18-T载体(大连宝生物公司)连接,再转化到感受态大肠杆菌中,筛选阳性克隆, 随机挑选3个克隆产物由上海英俊生物技术有限公司进行序列测定,以它们的一致序列为准。DNA序列检索和同源性比较利用BLAST工具(NCBI)。利用Clustal X软件对所测得的Wolbachia的wsp基因序列以及GenBank中已知的其他瘿蜂Wolbachia的wsp基因序列进行序列完全排列,用支序系统学PHYLIP 3.6a软件包进行1 000次的自导复制(bootstrap replication), 用DNADIST程序根据Kimura's 2-parameter模型计算遗传距离, 在NEIGHBOR程序中用邻位相连法(neighbour-joining analysis)获得聚类图, 最后在CONSENSE程序中形成系统进化树。
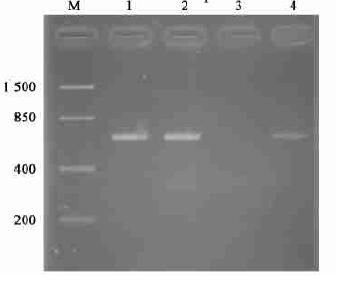
2 结果与分析 2.1 栗瘿蜂共生Wolbachia的PCR检测利用Wolbachia的wsp基因的一对通用引物(81F, 691R),成功地从栗瘿蜂总DNA中扩增到一条600 bp左右的wsp基因片段(图 1), 证实了Wolbachia在我国栗瘿蜂体内的感染。采用克隆测序方法,对该基因片段进行了序列测定。栗瘿蜂Wolbachia的wsp基因片段为617 bp,已提交GenBank注册(注册号为DQ493720)。
|
图 1 栗瘿蜂株洲种群Wolbachia的wsp基因片段的PCR扩增 Fig. 1 The PCR amplification of the wsp gene fragment of Wolbachia in Zhuzhou population of D. kuriphilus M:核酸分子量参照Molecular mass marker;1、2、4:栗瘿蜂D. kuriphilus;3:阴性对照(水)Negative control(water). |
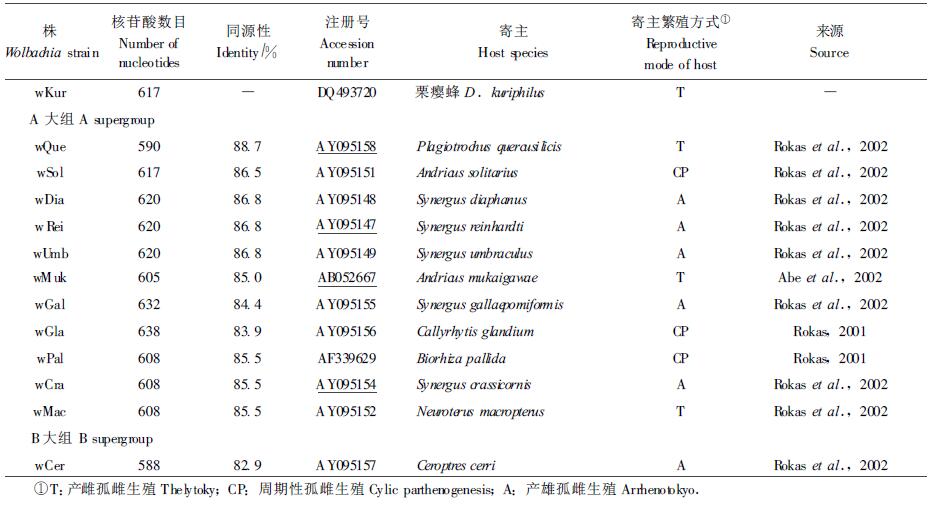
将栗瘿蜂共生Wolbachia的wsp基因序列利用NCBI网站提供的BLAST分析工具,与GenBank中注册的瘿蜂种类共生Wolbachia的wsp基因序列进行了同源性比较(表 1)。栗瘿蜂体内的Wolbachia的wsp基因序列与GenBank中A大组的其他瘿蜂Wolbachia株的同源性在88.7%~83.9%之间,与同为产雌孤雌生殖的Plagiotrochus quercusilicis体内的wQue株的同源性最高。与B大组Ceroptres cerri体内的wCer株的同源性为82.9%。
|
|
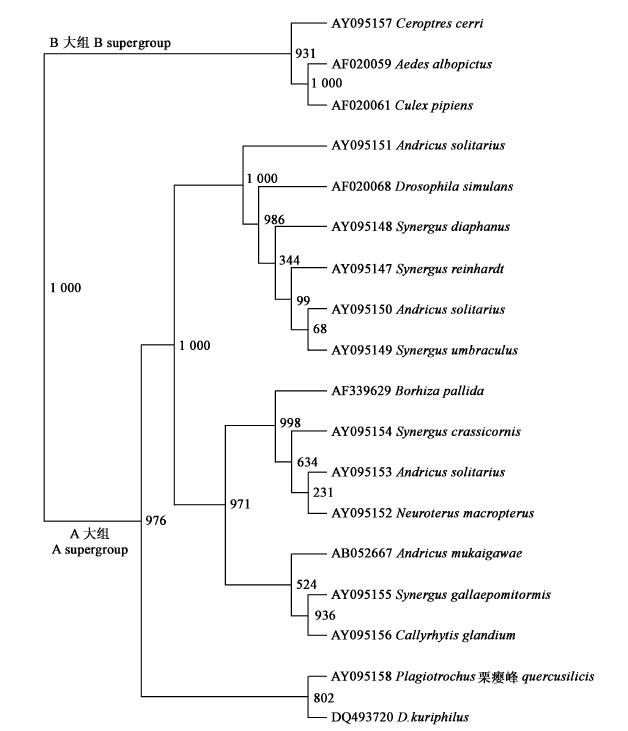
利用Clustal X软件对所测得序列、GenBank中已知的其他瘿蜂以及A大组代表性菌株wSim和B大组代表性菌株wPip、wAlb的wsp基因序列进行排列,用PHYLIP软件进行1 000次的自导复制,获得了NJ系统进化树(图 2)。从系统进化树中可以看出,栗瘿蜂体内共生的Wolbachia与其他多数瘿蜂种类的共生菌株属于A大组。
|
图 2 栗瘿蜂与其他昆虫种类Wolbachia的wsp基因序列的系统进化关系 Fig. 2 The phylogenetic relationship of wsp gene sequence of wolbachia in D. kuriphilus and other insects |
Wolbachia是节肢动物体内最为丰富的共生微生物之一(Werren, 1997)。本研究发现栗瘿蜂体内存在Wolbachia的共生,在NJ系统进化树中可以看出,栗瘿蜂体内的Wolba chia与大多数瘿蜂科种类的Wolbachia相同,属于A大组。
瘿蜂科昆虫的生殖方式比较复杂,有产雄孤雌生殖、产雌孤雌生殖以及周期性孤雌生殖;但是,其遗传机制尚不十分清楚(Hebert, 1987; Suomalainen et al., 1987)。Rokas等(2002)对欧洲的64种瘿蜂是否存在Wolbachia的共生进行了检测,仅仅7种检出了Wolbachia,检测种类的共生率较低,为10.9%。瘿蜂科Cynipini族的许多种类营周期性的孤雌生殖(Lijeblad et al., 1998; Ronquist, 1999),对于存在Wolbachia共生的Cynipini族营产雌孤雌生殖的种类,Rokas等(2002)推测其祖先繁殖方式是周期性的孤雌生殖,由于Wolbachia的共生,而导致产雄孤雌生殖世代的缺失。但是,已知感染Wolbachia的种类中,既有产雌孤雌生殖的种类,也有周期性孤雌生殖及产雄孤雌生殖的种类(表 1),可见Wolbachia对瘿蜂生殖的影响较为复杂。就栗瘿蜂而言,产雌孤雌生殖是否与共生的Wolbachia有关,调控机制如何,有待进一步探讨。
丁玉洲, 毕守东, 方国飞, 等. 2004. 栗瘿蜂虫瘿形成及发育与发生量关系研究. 应用生态学报, 15: 108-110. DOI:10.3321/j.issn:1001-9332.2004.01.024 |
靳杏蕊, 田士波, 赵淑娥, 等. 1995. 控制栗瘿蜂形成虫瘿的研究. 林业科学, 31: 77-80. |
吴晖, 陈顺立, 黄金聪, 等. 2004. 锥栗品种抗栗瘿蜂性状的评价. 福建林学院学报, 24: 344-348. DOI:10.3969/j.issn.1001-389X.2004.04.013 |
Abe Y. 1986. Taxonomic status of the Andricus mukaigawae complex and its speciation with geographical parthenogenesis (Hymenoptera: Cynipidae). Appl Entomol Zool, 21: 436-447. DOI:10.1303/aez.21.436 |
Abe Y, Miura K. 2002. Does Wolbachia induce unisexuality in oak gall wasps?(Hymenoptera: Cynipidae). Ann Entomol Soc Am, 95: 583-586. DOI:10.1603/0013-8746(2002)095[0583:DWIUIO]2.0.CO;2 |
Arakaki N, Oishi T, Noda H. 2001. Parthenogenesis induced by Wolbachia in Gronotoma micromorpha (Hymenoptera: Eucoilidae). Entomol Sci, 4: 9-15. |
Askew R R. 1984. The biology of gall wasps//Ananthakrishnan T N. The Biology of Galling Insects. New Delhi: IBH Publishing Co, 223-271
|
Atkinson R J, McVean G A T, Stone G N. 2002. Use of population genetic data to infer oviposition behaviour: species-specific patterns in four oak gallwasps (Hymenoptera: Cynipidae). Proceedings of the Royal Society of London: Series B, 269: 383-390. DOI:10.1098/rspb.2001.1820 |
Breeuwer J A J, Werren J H. 1993. Cytoplasmic incompatibility and bacterial density in Nasonia vitripennis. Genetics, 135: 565-574. |
Chen B H, Kfir R, Chen C N. 1992. The thelytokous Trichogramma chilonis in Taiwan. Entomol Exp Appl, 65: 187-194. DOI:10.1111/j.1570-7458.1992.tb01642.x |
Hebert P D N. 1987. Genotypic characteristics of cyclic parthenogenesis and their obligately asexual derivatives//Stearns S C. The Evolution of Sex and its Consequences. Basel: Birkhauser-Verlag, 403
|
Huigens M E, De Almeida R P, Boons P A H, et al. 2004. Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proceedings of Royal Society of London: Series B, 271: 1538-1545. |
Jeyaprakash A, Hoy M A. 2000. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of 63 arthropod species. Insect Mol Bio, 9: 393-405. DOI:10.1046/j.1365-2583.2000.00203.x |
Liljeblad J, Ronquist F. 1998. A phylogenetic analysis of higher-level gall wasp relationships (Hymenoptera: Cynipidae). Sys Entomol, 23: 229-252. DOI:10.1046/j.1365-3113.1998.00053.x |
Perrot-Minnot M J, Guo L R, Werren J H. 1996. Single and double infections with Wolbachia in the parasitic wasp Nasonia vitripennis: effects on compatibility. Genetics, 143: 961-972. |
Plantard O, Rasplus J Y, Mondor G, et al. 1998. Wolbachia-induced thelytoky in the rose gallwasp Diplolepis spinosissimae (Giraud) (Hymenoptera: Cynipidae), and its consequences on the genetic structure of its host. Proceedings of the Royal Society of London, Series B, 265: 1075-1080. DOI:10.1098/rspb.1998.0401 |
Plantard O, Rasplus J Y, Mondor G, et al. 1999. Distribution and phylogeny of Wolbachia inducing thelytoky in Rhoditini and 'Aylacini' (Hymenoptera: Cynipidae). Insect Mol Bio, 8: 185-191. DOI:10.1046/j.1365-2583.1999.820185.x |
Rokas A, Atkinson R J, Brown G S, et al. 2001. Understanding pattern of genetic diversity in the oak gallwasp Biorhiza pallida: demographic history or a Wolbachia selective sweep?. Heredity, 87: 294-304. DOI:10.1046/j.1365-2540.2001.00872.x |
Rokas A, Atkinson R J, Nieves-Aldrey, et al. 2002. The incidence and diversity of Wolbachia in gallwasps (Hymenoptera; Cynipidae) on oak. Mol Ecol, 11: 1815-1829. DOI:10.1046/j.1365-294X.2002.01556.x |
Ronquist F. 1999. Phylogeny, classification and evolution of the Cynipidae. Zool Scipta, 28: 139-164. DOI:10.1046/j.1463-6409.1999.00022.x |
Stone G N, Schonrogge K, Atkinson R J, et al. 2002. The population biology of oak gallwasps (Hymenoptera: Cynipidae). Ann Rev Entomol, 47: 633-668. DOI:10.1146/annurev.ento.47.091201.145247 |
Stouthamer R, Breeuwer J A J, Hurst G D D. 1999. Wolbachia pipientis: microbial manipulator of arthropod reproduction. Ann Rev Micro, 53: 71-102. DOI:10.1146/annurev.micro.53.1.71 |
Stouthamer R, Luck R F. 1993. Influence of microbe-associated parthenogenesis on the fecundity of Trichogramma deion and T. pretiosum. Entomol Exp Appl, 67: 183-192. DOI:10.1111/j.1570-7458.1993.tb01667.x |
Suomalainen E, Saura A, Lokki J. 1987. Cytology and evolution in parthenogenesis. Boca Raton: CRC Press.
|
Vavre F, Fleury F, Varaldi J, et al. 2000. Evidence for female mortality in Wolbachia-mediated cytoplasmic incompatibility in haplodiploid insects: epidemiologic and evolutionary consequences. Evolution, 54: 191-200. |
Werren J H. 1997. Biology of Wolbachia. Ann Rev Entomol, 42: 587-609. DOI:10.1146/annurev.ento.42.1.587 |
Werren J H, Wan Z, Lirong G. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proceedings of the Royal Society of London: Series B, 261: 55-63. DOI:10.1098/rspb.1995.0117 |
Werren J H, Windsor D M. 2000. Wolbachia infection frequencies in insects: evidence of a global equilibrium?. Proceedings of Royal Society of London: Series B, 267: 1277-1286. DOI:10.1098/rspb.2000.1139 |
West S A, Cook J M, Werren J H, et al. 1998. Wolbachia in two insect host-parasitoid communities. Mo Ecol, 7: 1457-1465. DOI:10.1046/j.1365-294x.1998.00467.x |
 2007, Vol. 43
2007, Vol. 43