文章信息
- Dietrich Ewald, Hu Jianjun
- Dietrich Ewald, 胡建军
- Influence of Cytokinin and Ammonium Nitrate on Elongation of Adventitious Buds in Norway Spruce(Picea abies)
- 细胞分裂素和硝酸铵对欧洲云杉不定芽伸长的影响
- Scientia Silvae Sinicae, 2007, 43(1): 28-34.
- 林业科学, 2007, 43(1): 28-34.
-
文章历史
Received date: 2005-11-24
-
作者相关文章
2. 中国林业科学研究院林业研究所 国家林业局林木培育重点实验室 北京 100091
2. Key Laboratory of Tree Breeding and Cultivation, State Forestry Administration Research Institute of Forestry, CAF Beijing 100091
The induction of adventitious buds on Norway Spruce (Picea abies) zygotic embryos or seedlings as well as for other spruce species was described by several authors (Bornman, 1983; von Arnold et al., 1987; 1988; Drake et al., 1997). The induction step very often included BA(N6-benzyladenine) as the most effective cytokinin concerning the induction of adventitious buds (Ellis et al., 1991). However, these inductions as described, were carried out only once or a few times. The buds were thereafter elongated on media free of plant growth regulators and rooted in general by the use of an auxin treatment. Adventitious buds induced on vegetative buds from adult Norway Spruce trees in vitro however showed no shoot elongation (von Arnold et al., 1987). Earlier studies had shown (Kunze et al., 1993; Kunze, 1994; Ewald et al., 1993) that combinations of zeatin and kinetin can be used to propagate adventitious buds continuously, induce on juvenile Norway Spruce material. The experiments described here were carried out to study the influence of cytokinins as well as of ammonium nitrate on the induction and elongation behaviour of such buds.
1 Materials and methods 1.1 Plant materialNorway Spruce seeds (provenance Marienberg, Saxony) were disinfested and germinated on a plant growth regulator free nutrient medium for 4 weeks as described earlier (Ewald et al., 1993). Seedlings with fully expanded cotyledons were chosen for the experiments testing primary and secondary inductions. The root was removed and they were used as hypocotyl cuttings (appr. 30 mm long).
To study the effects of varying ammonium nitrate concentrations on the elongation behaviour of adventitious buds, one clone was used which was subcultured for 6 years as adventitious bud clusters (Ewald et al., 1993). This line originated from seeds of the Norway Spruce of provenance Marienberg, Germany. The continuous cyclic propagation led to adventitious bud clusters with buds smaller than 2 mm showing no elongation tendency. All cultures were kept in continuous red light and at 23 ℃ as already described elsewhere (Ewald et al., 1993).
1.2 Primary induction experimentsThe term 'primary induction' was used to describe the very first induction which was carried out only once and was aimed at an initial induction process for the following, repeated inductions later called 'secondary inductions' to distinguish them from the very first induction step. Fifty-four hypocotyl cuttings per variant in total were put in an upright position into the induction medium (modified MCM medium)(Ewald et al., 1993) and induced for three weeks. Plant growth regulators BA, kinetin, zeatin and 2iP 〔N6-(Δ2-isopentenyl) adenine〕were used in equal concentrations of 5, 2.5, 1.0, 0.5 and 0.25 μmol·L-1 for the induction treatment. Afterwards the explants were cultured for 12 weeks on a solidified nutrient medium free of growth regulators (BEMB) (Ewald et al., 1993). The number of surviving explants as well as the number of adventitious bud forming explants and the number of adventitious buds formed were counted.
1.3 Secondary induction experimentsThe experiments concerning secondary inductions were planned to study the influence of repeated inductions. Fifty hypocotyl cuttings were used in total per variant in these experiments. Three induction treatments were carried out:
1) The primary induction with 0.232 mmol·L-1 kinetin and 0.221 mmol·L-1 BA (50 mg·L-1 each) for 24 hours in liquid medium; after that the explants were transferred onto a growth regulator free medium (BEMB) for 4 weeks;
2) The 1st secondary induction on a solid medium with either BA, kinetin, zeatin or 2iP used at concentrations of 2.5, 1.0, 0.5 and 0.25 μmol·L-1 for 3 weeks, MCM13 (basal medium for induction but without growth regulators) was used as control;
3) The 2nd secondary induction was started after 12 weeks on plant growth regulator free medium (BEMB) with identical concentrations and duration as the 1st induction.
After additional 6 weeks on a plant growth regulator free medium (BEMB) to allow the development of the induced buds they were counted and measured according to their size as buds smaller or larger than 4 mm. Elongation of buds referred to an elongated stem axis of the bud, which was clearly to identify on the bud cluster surface. The control was induced with the primary induction step exclusively and subcultured monthly on the plant growth regulator free medium (BEMB). A standard induction medium for adventitious bud propagation (Ewald et al., 1993) was also included 〔variant 13IB containing 2.27 μmol·L-1 zeatin (0.5 mg·L-1)+0.236 μmol·L-1 kinetin (0.05 mg·L-1)〕 with 150 hypocotyl cuttings. The experiments were carried out in two repetitions. The results were combined afterwards and the statistical differences among the variants (in pairs) were calculated with the SAS-package (PROC FREQ/CHI-square test).
1.4 Influence of enhanced cytokinin and ammonium nitrate concentrations on the elongation behaviourThe explants (small adventitious bud clusters, approximately 5 mm in diameter) were used at the end of two 4-week-subculture periods on a medium free of growth regulators (BEMB), containing 2.5 mmol·L-1 ammonium nitrate (200 mg·L-1). They were placed onto three variants of induction medium for 4 weeks. The following cytokinin variants were used: 6.84, 13.68, 20.52 μmol·L-1 zeatin (1.5, 3.0 and 4.5 mg·L-1) together with a constant concentration of 0.69 μmol·L-1 kinetin (0.15 mg·L-1). To stimulate the bud development a subculture on the above mentioned growth regulator free medium was carried out for 4 weeks. The bud clusters of each induction variant were subcultured then on the same growth regulator free medium (basal BEMB) but with different concentrations of ammonium nitrate (7.5, 12.5 and 17.5 mmol·L-1—600, 1 000 and 1 400 mg·L-1, respectively) for 4 weeks. Two final subculture steps (8 weeks) on a BEMB medium with 2.5 mmol·L-1 ammonium nitrate followed. The number of living adventitious buds was counted afterwards according to their size: non-elongated, elongated < 4 mm and elongated > 4 mm. The control variant was induced with the lowest cytokinin combination (6.84 μmol·L-1 zeatin+0.69 μmol·L-1 kinetin) and subcultured monthly on BEMB medium with 2.5 mmol·L-1 ammonium nitrate.
Because the number of elongated buds (larger and smaller than 4 mm) showed no normal distribution, a Poisson-distribution was assumed. A generalised linear model (Poisson/Log) taking this into account was used (SAS procedure GENMOD under SAS system V8.1) to distinguish between the variants and the traits observed.
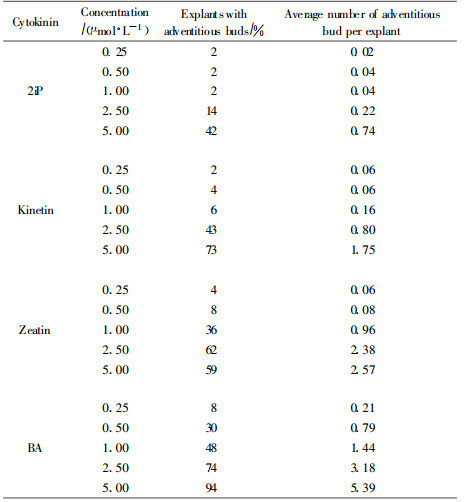
2 Results and discussion 2.1 Primary inductionThe survival rates of all hypocotyl cuttings ranged between 92% and 100%. The results showed that among the cytokinins which can be used for a first induction step to induce a larger number of adventitious bud forming explants only zeatin in the highest concentration used was in a comparable range with BA or kinetin (Tab. 1). Zeatin and 2iP in lower concentrations were not as effective as far as it concerned the portion of explants showing adventitious buds. It became obvious that BA at the highest concentrations induced the largest amount of explants forming adventitious buds and also revealed the highest number of buds per explant. BA and kinetin in this experiment were the most effective cytokinins concerning the amount of explants which were stimulated to form adventitious buds.
|
|
This confirmed the results of other authors who mentioned BA as the most effective cytokinin for a single adventitious bud induction step (Bornman, 1983; von Arnold et al., 1988; Kolevska-Pletikapic et al., 1995; Selby et al., 2005). A support of the development of buds induced by kinetin was also reported by other authors (von Arnold, 1982; Kolevska-Pletikapic et al., 1995; Lpez-Escamilla et al., 2000). In the following experiments BA together with kinetin was used for the primary induction step. However, to avoid long lasting negative effects on the later bud development during the following two repeated induction steps with different cytokinins, the primary induction was carried out as a short-time treatment (pulse) in liquid medium (von Arnold et al., 1987).
2.2 Secondary inductionsWhen comparing the adventitious buds induced by repeated induction steps we could find not only differences in the number and elongation behaviour but also in the morphological appearance of adventitious buds. Buds induced with BA often had a globular form and developed brown bud scales, especially after repeated inductions. Their growth often started with a needle elongation. This is in accordance with earlier results of other authors (Bornman, 1983; Drake et al., 1997). A difference in the developmental behaviour of in vivo and in vitro (BA-induced) spruce buds was reported by Cesar et al. (1997). They found that the mitotic index of Norway Spruce vegetative buds in vivo during spring time increased dramatically (0.1×10-3 to 40.7×10-3) while those after a BA pulse induction stayed at a lower level (5.8×10-3) which demonstrated an obvious difference in the development of in vivo and in vitro regenerated vegetative buds. As earlier mentioned (Ewald et al., 1993) the adventitious buds which could be propagated repeatedly showed an appearance of structures described by Bornman (1987) as 'pseudobuds'. Their development started with an axis elongation. The needle elongation followed after the axis elongation has already begun. That is why elongating buds could be easily detected and counted.
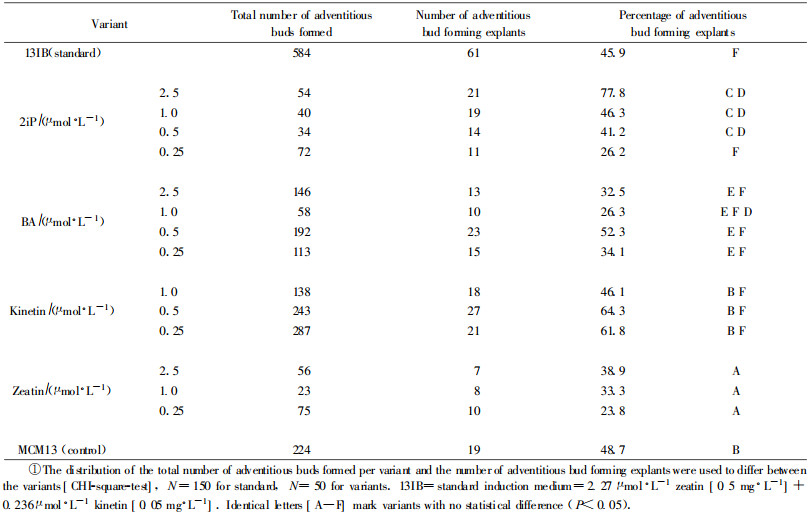
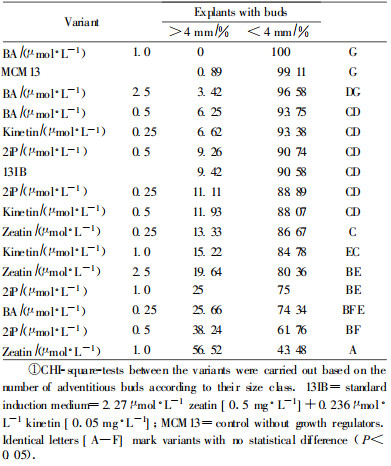
The result concerning the quantity of adventitious buds formed is shown Tab. 2. Two variants (2.5 μmol·L-1 kinetin and 0.5 μmol·L-1 zeatin) were influenced by contaminations and thus not measured. After 2 secondary induction cycles and a period free of growth regulators the observed number of adventitious bud forming explants showed that BA and kinetin at a concentration of 0.5 μmol·L-1 (variant d) were able to induce a maximum of explants. Zeatin was less effective compared with the other cytokinins. 2iP on the other hand showed a decrease in effectiveness with the decreasing concentration. Thus the highest concentration tested (2.5 μmol·L-1) was the most effective one. The variants used for comparison [standard variant—13IB, which was tested with a higher explant number (N=150)] and the control variant induced almost equal amounts of hypocotyl cuttings to form adventitious buds (45.9%, 48.7% respectively). The result observed for the control demonstrated the long-lasting effect of the primary induction step. Deviations from the control variant, either positive or negative, thus showed the influence of the secondary inductions. To decide about the quality of adventitious buds concerning their elongation behaviour the development of the bud axis was measured as a marker. Previous observations of spruce buds from different groups (> 2 mm and > 4 mm) had shown that buds larger than 4 mm more often continued their elongation, whereas a possible elongation of buds in the size range between 2 and 4 mm was difficult to predict. However the effectiveness of zeatin and 2iP was confirmed in our experiments in the size range of buds larger than 4 mm (Tab. 3). This demonstrated a continuous support of these cytokinin variants concerning the bud elongation behaviour after repeated induction cycles. Thus our results were in accordance with the results of Ellis et al. (1991) who found a zeatin treatment (50 μmol·L-1) together with TDZ as the best cytokinin for bud survival and development. The following conclusions about the actions of different cytokinins on the induction and elongation of adventitious buds could be drawn:
|
|
|
|
1) The choice of the cytokinin used did not only influence the number, but also the later development of the adventitious buds.
2) BA stimulated most of the explants (hypocotyl cuttings) to form a large number of buds. The elongation of these buds was inhibited beyond a concentration of 0.5 μmol·L-1 (0.112 6 mg·L-1). That is why BA can be used preferably for the first induction or a single induction step.
3) Kinetin acted similar compared with BA concerning the effectiveness of bud induction. The maximal number of adventitious buds per explant was reached at the lowest concentration tested (0.25 μmol·L-1—0.053 8 mg·L-1) under our conditions, whereas the highest percentage of buds larger than 4 mm was reached at 1.0 μmol·L-1 (0.215 2 mg·L-1).
4) Although zeatin and 2iP formed a lower number of adventitious buds in total, these buds kept their capability to elongate even after repeated inductions. Zeatin had its maximum at 1 μmol·L-1 (0.219 3 mg·L-1) and 2iP at 0.5 μmol·L-1 (0.101 6 mg·L-1).
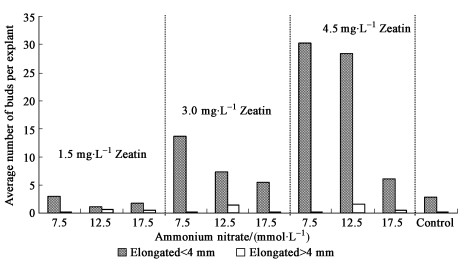
2.3 Influence of enhanced cytokinin and ammonium nitrate concentrations on the elongation behaviourIn the first years after establishing of adventitious bud lines from 4-week-old hypocotyl cuttings it was possible to elongate the shoots formed and to produce plants by rooting them regularly. Approximately one thousand plants of several clones were produced via this way in total. With the prolonged subcultivation it was still possible to propagate the bud cluster lines, but the elongation behaviour decreased. That is why an increase in zeatin concentration as well as the introduction of one subculture step with an enhanced ammonium nitrate concentration was tested on the later elongation behaviour. As Fig. 1 showed there was an increase in the number of elongating buds depending on the zeatin concentration used for induction. An increase in the ammonium nitrate concentration used during one subculture showed an inhibiting effect of concentrations beyond 7.5 mmol·L-1. The significances between the variants concerning the elongated buds smaller than 4 mm were depicted in Fig. 1. The distribution of buds larger than 4 mm was statistically significant for the induction with 4.5 mg·L-1 zeatin between all ammonium nitrate variants (7.5, 12.5 and 17.5 mmol·L-1). There was a statistical significant difference also for the induction with 3.0 mg·L-1 zeatin. Concerning the lowest zeatin concentration used a statistical significant difference was shown between 7.5 mmol·L-1 and 17.5 mmol·L-1. The presence of interactions between the ammonium nitrate concentration and the zeatin concentration was also statistical significant (P=0.000 1). The situation was similar as far as it concerned the average number of non-elongated buds per explant. The lowest ammonium nitrate treatment (7.5 mmol·L-1) showed a more than twofold increase in the number of non-elongated buds from 1.5 to 3 mg·L-1 zeatin, this was 17.1 adventitious buds per explant and 39.1 respectively. From 3.0 to 4.5 mg·L-1 there was no further increase in the formation of non-elongated buds. At the 12.5 mmol·L-1 ammonium nitrate level the number of adventitious buds per explant of all zeatin variants differed significantly with the increasing zeatin concentration (22.2; 35.4; 40.3). The highest ammonium nitrate concentration used (17.5 mmol·L-1) did not result in significant differences at increasing zeatin levels (29.9; 28.9; 30.7). The increasing ammonium nitrate concentration increased the number of dead buds counted at each explant when they were induced with 1.5 mg·L-1 and 3.0 mg·L-1 zeatin. The number of dead buds per explant after the highest zeatin induction (4.5 mg·L-1) was lower (43 buds/explant) compared with the two lower concentrations (63 and 77 buds/explant respectively). A possible explanation for this result is the stimulating effect of increasing cytokinin concentrations on the survival rate of adventitious buds induced. The conclusions from these results were:
|
Fig.1 Influence of the zeatin concentration (mg·L-1) used for induction and one subculture step with an enhanced ammonium nitrate concentration on the average number of elongated buds formed in a Norway Spruce bud cluster line 30 explants were used per variant, control: continuous subcultures on BEMB nutrient medium with 2.5 mmol·L-1 ammonium nitrate. |
1) The maximal number of elongating buds was reached after a treatment with the highest zeatin concentration tested (4.5 mg·L-1) and a 4-week-pulse with 7.5 mmol·L-1 ammonium nitrate.
2) An increase in ammonium nitrate did not result in a further increase of elongating buds, but enhanced the number of dead buds.
Comparing the results from the first experiments and the experiments carried out with the long-term bud cluster line showed that the cytokinin concentrations were higher to induce buds with the ability to elongate. For the long-term maintaining of bud clusters a lower cytokinin concentration was sufficient (PlateⅠ-1, 2, 3) and kept the capability to elongate shoots in juvenile Norway Spruce and their transfer after spontaneous rooting to soil (PlateⅠ-4, 5). Juvenile material of Norway Spruce and larch (Ewald et al., 1997) is able to elongate a shoot axis spontaneously but this capability is depending on a proper cytokinin induction and can decrease with the number of subcultures, mainly in larch. Although there were no experiments carried out to explain this phenomenon it is justified to assume that there is an ageing effect in tissue cultures as well. Adult bud clusters of larch, although showing the capability to be propagated, refused to form an elongating shoot axis regularly (Ewald, 1998). Thus the triggering of the shoot axis elongation in winter buds or adventitious buds of different conifer species (e.g. Norway Spruce, larch) is still the main problem of an in vitro propagation over longer time periods or for adult material especially. Nevertheless these recent results showed the influence of two main factors (cytokinin, nitrogen availability) which will be tested to a larger extent in conifers to understand the regulation of shoot elongation and to improve the organ development for ongoing research tasks which need applicable regeneration systems (rejuvenation, gene transfer).

|
PlateⅠ PlateⅠ 1,2,3. Elongation of induced adventitious buds after a combined treatment of enhanced zeatin (4.5 mg·L-1) and a 4-week ammonium nitrate (600 mg·L-1) pulse:1. Bud cluster after long-term subcultures before the experiment;2. Beginning of the bud axis elongation;3. Development of shoots 16-weeks after induction.4,5. Elongated shoots from adventitious bud cluster cultures of Norway Spruce after 9 years of continuous subculture:4. Induction of line in 1995;5. Micropropagated Norway Spruce plant after 8 years in the field. |
Bornman C H. 1983. Possibilities and constraints in the regeneration of trees from cotyledonary needles of Picea abies in vitro. Physiologia Plantarum, 57(1): 5-16. |
Bornman C H. 1987. Picea abies//Bonga J M, Durzan D J. Cell and tissue culture in forestry: Vol. 3. Case histories: gymnosperms, angiosperms and palms. Kluwer Academic Publishers, Dordrecht, 2-29
|
Cesar V, Papeš D, Bornman C H. 1997. Nuclei distribution pattern in the tissue of vegetative buds of spruce species (Picea abies, Picea pungens and Picea omorika) grown in vivo and in vitro. Periodicum Biologorum, 99(1): 107-116. |
Drake P M W, John A, Power J B, et al. 1997. Cytokinin pulse-mediated shoot organogenesis from cotyledons of Sitka spruce [Picea sitchensis (Bong.) Carr.] and high frequency in vitro rooting of shoots. Plant Cell, Tissue and Organ Culture, 50: 147-151. DOI:10.1023/A:1005998812774 |
Ellis D D, Barcynska H, McCown B H, et al. 1991. A comparison of BA, zeatin and thidiazuron for adventitious bud formation from Picea glauca embryos and epicotyl explants. Plant Cell, Tissue and Organ Culture, 27: 281-287. DOI:10.1007/BF00157592 |
Ewald D, Kretzschmar U, Chen Y. 1997. Continuous micropropagation of juvenile larch from different species via adventitious bud formation. Biologia Plantarum, 39(3): 321-329. DOI:10.1023/A:1000959621891 |
Ewald D, Suess R. 1993. A system for repeatable formation of elongating adventitious buds in Norway Spruce tissue culture. Silvae Genet, 42(4/5): 169-175. |
Ewald D. 1998. Advances in tissue culture of adult larch. In vitro Cell Dev Biol Plant, 34: 325-330. DOI:10.1007/BF02822742 |
Kolevska-Pletikapic B, Bututrovic-Deric Z. 1995. Regeneration of Picea omorika plants via organogenesis. Plant Cell, Tissue and Organ Culture, 41: 189-192. DOI:10.1007/BF00051590 |
Kunze I, Grafe R, Schiemann J. 1993. Continuous in vitro multiplication of shoot buds of Norway Spruce (Picea abies L.) by intermittent application of growth regulators. Biologia Plantarum, 35(1): 11-15. DOI:10.1007/BF02921111 |
Kunze I. 1994. Influence of the genotype on growth of Norway Spruce (Picea abies L.) in vitro meristem culture. Silvae Genetica, 43(1): 36-41. |
Lpez-Escamilla A L, Olguin-Santos L P, Márquez J, et al. 2000. Adventitious bud formation from mature embryos of Picea chihuahuana Martnez, an endangered Mexican spruce tree. Annals of Botany, 86: 921-927. DOI:10.1006/anbo.2000.1257 |
Selby C, Waston S, Harvey B M R. 2005. Morphogenesis in Sitka Spruce (Picea sitchensis [Bong.] Carr.) bud cultures-tree maturation and explants from epicormic shoots. Plant Cell, Tissue and Organ Culture, 83: 279-285. DOI:10.1007/s11240-005-7016-3 |
von Arnold S, Hakman I. 1988. Plantlet regeneration in vitro via adventitious buds and somatic embryos in Norway Spruce (Picea abies)//Hanover J W, Keathley D E. Genetic manipulation of woody plants. New York: Plenum Press: 199-215. |
von Arnold S, Tillberg E. 1987. The influence of cytokinine pulse treatments on adventitious bud formation on vegetative buds of Picea abies. Plant Cell, Tissue and Organ Culture, 9: 253-261. DOI:10.1007/BF00040811 |
von Arnold S. 1982. Factors influencing formation, development and rooting of adventitious shoots from embryos of Picea abies (L.) Karst. Plant Science Letters, 27: 275-287. DOI:10.1016/0304-4211(82)90130-4 |
 2007, Vol. 43
2007, Vol. 43