文章信息
- Li Guoping, Huang Qunce, Yang Lusheng, Qin Guangyong
- 李国平, 黄群策, 杨鹭生, 秦广雍
- Microtubule Organization in Germinated Pollen of Pinus thunbergii
- 黑松花粉与花粉管中的微管分布
- Scientia Silvae Sinicae, 2006, 42(9): 13-16.
- 林业科学, 2006, 42(9): 13-16.
-
文章历史
Received date: 2005-11-23
-
作者相关文章
2. 莆田学院环境与生命科学系 莆田 351100
2. Department of Environment and Life Sciences, Putian University Putian 351100
Pollen developswithin the anther in seed plants.After pollination, pollen germinates and grows a tube that delivers sperm cells for fertilization.Pollen tubes are a tip-growing systemin plants.Angiosperm pollen tubes also act as a model forthe investigation of the cytoskeleton in plant cells.Like every other eukaryotic cell, the pollen tube contains an elaborate cytoskeletal apparatus, which mainly consists of microtubules and actin filaments (Tiezzi, 1991; Cai et al., 1997).In angiosperms, actin filament system is essential for the germination and tube growth of pollen (Pierson et al., 1992; Gibbon et al., 1999; Raudaskoski et al., 2001).
The role of microtubules in angiosperms pollen tube growth has been a puzzle, because anti-microtubules drugs has little effecton pollen germination or tube elongation (Pierson et al., 1992; Åström et al., 1995; Raudaskoski et al., 2001).On the other hand, the presence of microtubules in the pollen tube apex is still controversial (Pierson et al., 1992; Li et al., 1997).The presence of microtubules in the growth region (the apical domain) is still debated.Although standard immunocytochemistry afterchemical fixation has shown the occurrence of shortand twistedmicrotubules (del Casino et al., 1993), electron microscopy after freeze-fixation has not confirmed such finding (Lancelle et al., 1987).
Gymnosperms differs from angiosperms in the number of mitotic divisions between the microspore and the sperm, the growth rate of the pollen tube, the length of time between pollination and fertilization, and the absence of double fertilization (Singh, 1978).Previous studies have found that the cytoskeleton in pollen tubes of gymnosperms (Terasaka et al., 1994; Lazzaro, 1996; 1999;Liu et al., 2005) differs from that seen in angiosperm pollen tubes (Taylor et al., 1997; Heslop-Harrison et al., 1988; Raudaskoski et al., 2001; Pierson et al., 1992).Microtubules extend to the pollen tube apex.Anti-microtubules drugs partially block gymnosperm pollen germination and growth, induce tube branching and tip swelling (Anderhag et al., 2000).
However, to date, microtubule organization in the pollen and pollen tubes of gymnosperms has been examined only in Picea abies (Lazzaro, 1999), Pinus densiflora (Terasaka et al., 1994), Pinus sylvestris (de Win et al., 1996), and Ginkgo biloba (Liu et al., 2005).In order to reveal the importance of microtubules in gymnosperms pollen tube elongation, detailed studies on microtubule organization in othergymnosperms species are needed.In the present study, the distribution of microtubuleswas investigated in the pollen tubes of Pinus thunbergii (Japanese Black Pine) using a confocal laser scanning microscope after immunofluorescence labeling.
1 Materials and methods 1.1 Pollen collection and storageMature pollen grains were collected from trees of P.thunbergii (Pinaceae), growing in the Binhe Park, Zhengzhou, Henan Province, at the shedding time in April, 2005.The collected pollen grains were dried at room temperature and stored at -20℃ until use.
1.2 Pollen germination in vitroPollen grains were cultured on a liquid medium containing 0.25% sucrose, 0.01% boric acid and 0.01% calcium chloride in distilled water.Germination took place in dark on a shaker (100 r·min-1) at 28℃.
1.3 Colchicine treatmentFor disruption of microtubules, the colchicine was used.Colchicine was dissolved in the culture mediumand used at concentrations of 1 000μmol·L-1 colchicines.
1.4 Morphological measurementsAfter 60 h incubation, pollen tube morphology was analyzed under a Olympus CH20 microscope, measuring tube length and width, and frequency of germination and tip swelling.All experiments were performed in triplicate.
For visualization of pollen and pollen tubes, the germinated pollen grains were fixed with 4% glutaraldehyde and stained with 10μg·mL-1 DAPI (4′, 6-diamidino-2-phenylindole) in TAN buffer (Nemoto et al., 1988).The samples were examined with a Leica TCS-SP2 laser scanning confocal microscope (LSCM) equipped with a Leica DMIRE2 microscope.
1.5 Immunolabeling of pollen tubes for microtubulesImmunolabeling of pollen tubeswas carried out as described by Lazzaro (1999).Briefly, samples of pollen tubes were fixed in 4% paraformaldehyde in PEMbuffer, incubated in 1% cellulase (Sigma C1184) and 1% pectinase (Sigma P2401).After enzymatic digestion, samples were rinsed three times in blocking buffer (phosphate-buffered saline, 1% bovine serum albumin, 1% TX-100), incubated for 16 h at 20℃ in a solution of mouse anti-alpha-tubulin (Sigma; diluted 1:100 in blocking buffer), then incubated for 1 h at 37℃ in a second antibody solution of fluorescein isothiocyanate (FITC)-conjugated sheep anti-mouse IgG (Sigma; diluted 1:100 in blocking buffer).Controls omitting either the primary antibody or primary and secondary antibodies were also prepared.Samples were examined and photographed in a confocal microscope as described above.Optical serial sectionswere collected at a thickness of 500 or1 000 nm and confocal projections were used for the measurement of pollen tube tips.
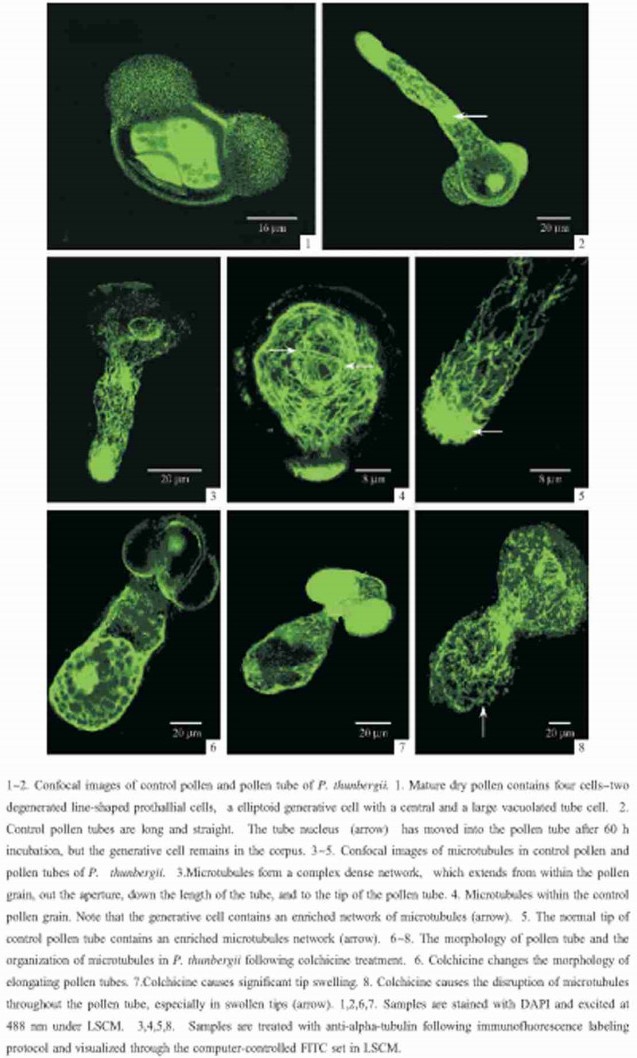
2 ResultsIn P.thunbergii, pollen is released in the spring in a four-cell stage containing two degenerated prothallial cells, the generative cell and the vegetative cell (PlateⅠ-1).Pollen grains grown on media without colchicine have a 77.70% germination rate.Pollen tubes elongate with a uniform diameter (19.04μm), normal length (154.00μm) and regular shape (PlateⅠ-2).During tube growth, the vegetative nucleus has moved into the elongating tube, while the reproductive cells remain within the pollen grain (PlateⅠ-2).These tubes have very low frequencies of tip swelling (0.58%).
|
图版Ⅰ 图版Ⅰ |
Microtubule organization in germinated pollen and tube was examined using immunofluorescence labeling coupled with laser scanning confocal microscopy.Microtubules form a dense network within pollen tubes of the gymnosperm P.thunbergii.Microtubules emanate from within the pollen grain and form long, longitudinal arrays passing through the aperture and down the length of the pollen tube to the tip (PlateⅠ-3).Within germinated pollen, microtubules are arranged obliquely or transversely.The generative cell, which remains within the grain in vitro, contains an enriched network of microtubules (PlateⅠ-4).Microtubules in pollen tube tip are enriched and forma dense radial network (PlateⅠ-5).
Treatment with colchicine significantly affects pollen tube growth. When pollen grains germinate and tubes grow in the presence of colchicine, they are significantly shorter (83.05μm) and wider (31.11μm) than normal tubes (154.00μm) (PlateⅠ-6).In addition, a significant percentage of pollen tubes (17.88%) have swollen tips (PlateⅠ-7).However, there is no significant change in germination frequency following colchicine treatment.On the other hand, treatmentwith colchicines results in a disordered arrangement of microtubules throughout the pollen tube.The induction of tip swelling by colchicine is coincident with the disruption of microtubules in swollen tips, where microtubules form random short, fragmented bundles (PlateⅠ-8).
3 DiscussionConfocalmicroscopy indicates that microtubules in pollen tubes of P.thunbergii form a contiguous network that extends fromwithin the pollen grain to the tube tip.Microtubules in pollen tube tip are enriched.This is similar to the overall organization of microtubules in P.abies (Lazzaro, 1999), P.densiflora (Terasaka et al., 1994), and Ginkgo biloba (Liu et al., 2005) pollen tubes.Picea abies pollen tubes are used as a model system for the investigation of cytoskeleton's role in polarized growth at the Lazzaro Lab of Department of Biology, College of Charleston, Charleston, US. In Picea abies pollen tubes, microtubules are enriched at the elongating tip, where they form a radial array beneath the plasma membrane that is perpendicular to the direction of tube growth and a longitudinal array within the tip center (Lazzaro, 1999).Previous studies and our results have shown that the organization of microtubules in pollen tubes of gymnosperms does differ from that seen in angiosperm pollen tubes.
Pollen tubes of gymnosperms exhibit some evolutionarily primitive characteristics, i.e.slow germination and growth, branching and longevity (Singh, 1978).Elongation in gymnosperms pollen tubes is fundamentally distinct from angiosperms (de Win et al., 1996; Dawkins et al., 1993).This makes sense that cytoskeletal function also differs.In present study, the results of colchicine treatment show that colchicine significantly induces tip swelling and disrupts microtubule organization especially in swollen tips.The results indicate that disruption of microtubules induced by colchicine treatmentis responsible forthe formationof tip swelling.We believe that microtubules have a unique functional role in maintaining the tip integrality of pollen tube in gymnosperms.Anderhag et al. (2000) found that microtubules and microfilaments are both required for the elongation of pollen tubes in P.abies and the specific disruption of these microtubules causes tip swelling and induces tube branching.Justus et al. (2004) reported that microtubules and microfilaments coordinate to direct a fountain streaming pattern in elongating conifer pollen tube tips and the specific disruption of microtubules stops streaming.
Pollen tubes grow by the deposition of cell wall components at the tip (Taylor et al., 1997; Derksen et al., 1995).In conifer, the microtubules at the tip may orient the deposition of cellulose microfibrils at the tip region.According to Lazzaro (1999), the pollen tube wall may develop in the terminal 20μm but may be mature in older parts of the tube.Lazzaro et al. (2003) found that the specific inhibition of cellulose microfibril deposition leads to the disorganization of these microtubules and also causes tip swelling, indicating that the microtubules in the tip also direct the deposition of cellulose and do maintain tip integrity.
In conclusion, Pinus thunbergii pollen tube contains a contiguous microtubules network which is enriched in the elongating tip.Microtubules have a unique functional role in tip extension of gymnosperms pollen tubes.It is suggested that the gymnosperm pollen tubeselongate in a mechanism thatis differentfrom angiosperm pollen tubes.However, there has notyetbeen enough information to reveal the underlying mechanisms.To explore the mechanisms, further studies are expected. The present study may provide a clue for further research on cytoskeletal organization in gymnosperms pollen and pollen tube.
Anderhag P, Hepler P K, Lazzaro MD. 2000. Microtubules and microfilaments are both responsible for pollen tube elongation in the conifer Picea abies (Norway Spruce). Protoplasma, 214: 141-157. DOI:10.1007/BF01279059 |
Åström H, Sorri O, Raudaskoski M. 1995. Role of microtubules in the movement of the vegetative nucleus and generative cell in tobacco pollen tubes. Sexual Plant Reproduction, 8: 61-69. |
Cai G, Moscatelli A, Cresti M. 1997. Cytoskeletal organization and pollen tube growth. Trends Plant Sci, 2(3): 86-91. DOI:10.1016/S1360-1385(96)10057-1 |
Dawkins MD, Owens J N. 1993. In vitro andin vivo pollen hydration, germination, and pollen tube growth in white spruce, Picea glauca (Moench) Voss. IntJ Plant Sci, 154: 506-521. DOI:10.1086/297134 |
del Casino C, Li Y, Moscatelli A, et al. 1993. Distribution of microtubules duringthe growth of tobacco pollen tubes. Biol Cell, 79: 125-132. DOI:10.1111/j.1768-322X.1993.tb00902.x |
Derksen J, Rutten T, van Amstel T, et al. 1995. Regulation of pollen tube growth. Acta Botanica Neerlandica, 44: 93-119. DOI:10.1111/j.1438-8677.1995.tb00773.x |
de Win A H N, Knuiman B, Pierson E S, et al. 1996. Development and cellular organization of Pinus sylvestris pollen tubes. Sex Plant Reprod, 9: 93-101. DOI:10.1007/BF02153056 |
Gibbon B C, Kovar D R, Staiger C J. 1999. Latrunculin B has different effects on pollen germination andtube growth. Plant Cell, 11: 2349-2363. DOI:10.1105/tpc.11.12.2349 |
Heslop-Harrison J, Heslop-Harrison Y, Cresti M, et al. 1988. Cytoskeletal elements, cell shapingand movement in the angiosperm pollen tube. J Cell Sci, 91: 49-60. |
Justus C D, Anderhag P, Goins J L, et al. 2004. Microtubules and microfilaments coordinate to direct a fountain streaming patternin elongating conifer pollen tube tips. Planta, 219: 103-109. DOI:10.1007/s00425-003-1193-2 |
Lancelle S A, Cresti M, Hepler P K. 1987. Ultrastructure of cytoskeleton in freeze-substituted pollen tubes of Nicotiana tabacum. Protoplasma, 140: 141-150. DOI:10.1007/BF01273723 |
Lazzaro M D. 1999. Microtubule organizationin germinated pollen of the conifer Picea abies (Norway Spruce, Pinaceae). AmJ Bot, 86: 759-66. DOI:10.2307/2656696 |
Lazzaro M D. 1996. The actin microfilament network within elongating pollen tubes of the gymnosperm Picea abies (Norway Spruce). Protoplasma, 194: 186-194. DOI:10.1007/BF01882026 |
Lazzaro M D, Donohue J M, Soodavar F M. 2003. Disruption of cellulose synthesis by isoxaben causes tip swelling and disorganizes cortical microtubules inelongating conifer pollen tubes. Protoplasma, 220: 201-207. DOI:10.1007/s00709-002-0042-7 |
Li Y, Moscatelli A, Cai G, et al. 1997. Functional interactions among cytoskeleton, membranes, and cell wall in the pollen tube of flowering plants. International Reviewof Cytology, 176: 133-199. DOI:10.1016/S0074-7696(08)61610-1 |
Liu J M, Zhang H, Li Y. 2005. Cytoskeleton in pollen and pollen tubes of Ginkgo biloba L. Journal of Integrative Plant Biology, 47(8): 952-958. DOI:10.1111/j.1744-7909.2005.00123.x |
Nemoto Y, Kawano S, Nakamura S, et al. 1988. Studies on plastid-nuclei (nucleoids) in Nicotiana tabacum L.Ⅰ:Isolation of proplastid-nuclei from cultured cells and identification of proplastid-nuclear proteins. Plant Cell Physiol, 29: 167-177. |
Pierson E S, Cresti M. 1992. Cytoskeleton and cytoplasmic organization of pollen and pollen tubes. International Reviewof Cytology, 140: 73-125. DOI:10.1016/S0074-7696(08)61094-3 |
Raudaskoski M, Ström H, Laitiainen E. 2001. Pollentube cytoskeleton:structure andfunction. J Plant Growth Regul, 20(2): 113-130. DOI:10.1007/s003440010015 |
Singh H.1978.Embryology of gymnosperms∥Zimmermann W, Carlquist S, Ozenda P, et al. Handbuch der Pflanzenanatomie: vol.10, part 2.Gebrüder Borntraeger, Berlin
|
Taylor L P, Hepler P K. 1997. Pollen germination andtube growth. Annu Rev Plant Physiol Plant Mol Bio, 48: 461-491. DOI:10.1146/annurev.arplant.48.1.461 |
Terasaka O, Niitsu T. 1994. Differential roles of microtubule and actin-myosin cytoskeleton in the growth of Pinus pollen tubes. Sex Plant Reprod, 7: 264-272. |
Tiezzi A. 1991. The pollen tube cytoskeleton. Electron Microsc Rev, 4: 205-219. DOI:10.1016/0892-0354(91)90003-U |
 2006, Vol. 42
2006, Vol. 42

