文章信息
- Zhao Changling, Guo Weiming, Chen Junyu
- 赵昶灵, 郭维明, 陈俊愉
- Isolation and Structural Identification of the Anthocyanins from the Flower Color Pigment of Prunus mume 'Nanjing Hongxu' (Nanjing Red-Bearded)
- 梅花'南京红须'花色色素花色苷的分离与结构鉴定
- Scientia Silvae Sinicae, 2006, 42(1): 29-36.
- 林业科学, 2006, 42(1): 29-36.
-
文章历史
Received date: 2004-06-15
-
作者相关文章
2. 云南农业大学农学与生物技术学院 昆明 650201;
3. 南京农业大学园艺学院 南京 210095;
4. 北京林业大学园林学院 北京 100083
2. College of Agricultural Sciences and Biotechnology, Yunnan Agricultural University Kunming 650201;
3. College of Horticulture, Nanjing Agricultural University Nanjing 210095;
4. College of Landscape Architecture, Beijing Forestry University Beijing 100083
Mei (Prunus mume Sieb. et Zucc.) flower is regarded as one of the candidates of the national flower of P. R. China. So far in this world, Mei flower is the first horticultural plant which is accredited Chinese scientist, namely Chen Jun yu, as International Cultivar Registration Authority (Zhao et al., 2003). Mei is very floriferous, producing mauve, pink, pure white, greenish white, light yellow or double color blooms (Chen, 1989). However, the study on the flower color of Mei is almost a blank all the times. P. mume 'Nanjing Hongxu' (Nanjing red-bearded), belonging to Form Cinnabar, is the typical representative of the mauve. We have confirmed that its mauve is due to anthocyanins (Zhao et al., 2004b). Furthermore, we have systematically studied the effects of physical and chemical factors on the stability of the anthocyanins (Zhao et al., 2004a). But the molecular structures of the anthocyanins have not been reported up to the present.
This paper deals with the extraction, purification and molecular structure-elucidating of the main anthocyanins from the petals of P. mume 'Nanjing Hongxu', which could be a reference to the exploitation on the expression mechanism of the red flower color of Mei and the flower color-meliorating of Mei by gene engineering.
1 Material and methods 1.1 GeneralPartial acid hydrolysis was accomplished by the procedure reported by Harborne (1984).
UV-Vis spectra were measured at room temperature in a 1 cm pathlength quartz cell in the 200~700 nm range using a Shimadzu UV-1601 UV-Vis spectrophotometer.
High Performance Liquid Chromatography (HPLC) analysis of phenolic acids was carried out on Waters 600 system using a reversed-phase C18 column, SuperPac Pep-S (5 μm, 4×250 mm). The equipment consisted of a liquid chromatography system equipped with a Waters 2 467 dual λ absorbance detector. The gradient solvent system of Gao and Mazza (1994) was modified as follows: Solvent A was MeOH, solvent B was 0.2% acetic acid (in water), and the elution profile was modified as follows: 0~5 min, 100% B; 5~10 min, 100~50%B; 10~45 min, 50%B. Sample injections (5 μL) were made via a Waters 600 controller. Peaks were monitored by their absorbances at 273 nm. The flow rate was 0.9 mL·min-1. Peak assignments were made by comparing the retention times of the acid standards with those of anthocyanin hydrolysates.
Gas chromatography (GC) was carried out using Shimadzu GC-14B gas chromatography equipped with a flame ionization detector (FID). A SUPELCO SPBTM-5 fused silica capillary column (30 m×0.25 mm×0.25 μm film thickness) was used. The analysis program for sugar derivatives was as follows: column temperature starting at 120 ℃ for 2 min, increased to 185 ℃ at a linear rate of 20 ℃·min-1, followed by increase at 1 ℃·min-1 to 192 ℃, and followed again by increase at 5 ℃·min-1 to 200 ℃. Injector and detector temperature were 250 ℃. The analysis program for aliphatic acids was that reported by Gao and Mazza (1994).
Nuclear Magnetic Resonance (NMR) spectra were obtained at 500 MHz and 125 MHz for 1H and 13C respectively, on a Bruker AM-400 instrument at 25 ℃ using tetramethylsilane (TMS) as internal standard. The 13C and the residual 1H signals of the solvent CD 3OD-CF3COOD (95:5, V/V) were used as secondary references, namely δ49.0 and δ3.4 ppm from TMS respectively.
Fast Atom Bombardment Mass Spectrometry (FAB MS) was obtained in a positive ion mode on a VG AutoSpec 3000 mass spectrometer using glycerol as a matrix. The anthocyanins were dissolved in 1% TFA (in MeOH). The shoot voltage and the shoot electric current of cesium ion were 25 kV and 1 μA respectively. The scanning rate was 5 Sec·Dec-1.
All solvents used were of analytical grade. Silica gel G was used for Thin Layer Chromatography (TLC). Sephadex LH-20 (Pharmacia, Uppsala, Sweden) was used for Column Chromatography (CC). Paper Chromatography (PC) was carried out on Whatman No. 3 filter paper. Freezing-drying was performed on Savant PM-1330S freezing-dryer (USA).
The structure-drawings of the anthocyanins were performed with the ChemDraw of Chemoffice 2000 (CambridgeSoft Corp.).
1.2 Plant materialAll flowers were obtained at Mei Flower Hill of Sun Yat-sen Mausoleum Administrative Office of Nanjing. During the full florescence, the blooming flowers were collected randomly in the morning just after the dew evaporated completely, encased in ordinary kraft envelopes, and immediately frozen at -20~-22 ℃until extraction.
1.3 Isolation and purification of the anthocyaninsAway from androecia, the frozen petals were rapidly pulled out. 50.0 g petals were ground quickly and at 5~10 ℃ after mixing with MeOH-HOAc-H2O (10:1:9, V/V). Extracts were filtered and the residue was washed till it became full white. The filtrate was primarily purified by partition against n-hexane, and the final extract expressed purely mauve. TLC on the crude extract was carried out at room temperature using BAW [n-butanol-acetic acid -water (4:1:2, V/V)] as eluent. Only one dominant scarlet spot was observed and the Rf was 0.64, there was also a blurry light yellow spot before the scarlet one. The crude extract was concentrated to almost dryness under reduced pressure using a rotary evaporator below 35 ℃ (Zhao et al., 2004a), then frozen at -20~-22 ℃ in darkness.
The concentrated crude extract was elementar ily purified via PC using BAW [n-butanol-acetic acid-water (4:1:5, V/V, top layer)] as solvent (Harborne, 1984; Jackman et al., 1987). Only single scarlet spot was generated on the chromatogram. The PC was repeated 250 times, and the spots were cut out from the dried chromatograms, combined and extracted completely with above MeOH-HOAc-H2O. After filtration, the eluate was concentrated to almost half of the primitive volume under reduced pressure below 35 ℃, transferred into 250 mL white plastic beaker and frozen to dryness. About 4.3 g scarlet crystal powder was obtained, then stored in a desiccator with silica gel as desiccant in darkness at 5~10 ℃。
Further purification was done by CC (2.5 cm×30 cm) using above MeOH-HOAc-H2O as eluent at 7 mL·h-1 successively. Two fractions were observed, collected respectively and named AN2, AN1 according to the sequence being eluted. Every fraction was concentrated, frozen to dryness and stored in desiccator. AN1 is about 1.3 g and AN2 is about 2.5 g.
1.4 Elucidation of the molecular structures of the anthocyanins by chemical reactions, chromatography and spectroscopy 1.4.1 Specific color reactionsThe anthocyanins' colors in MeOH (pH 2.0) were observed in Visible and UV light before and after the addition of 5 drops of 25% ammonia solution. In the meantime, three color reactions were designed according to the literature of Robinson and Robinson (1931).
1.4.2 Paper chromatographic and spectroscopic identificationPC was performed using BuHCL and 1% HCL as solvents respectively (Harborne, 1984). Only single scarlet spot was observed in either of the solvents. Thus, the anthocyanins were assumed to be pure. It was also found that AN2 produced two spots on re-PC, indicating it must be acylated with acid (Harborne, 1984).
The UV-Vis spectra of the anthocyanins were recorded according to the method of Harborne (1958).
1.4.3 Preparation of the anthocyanin hydrolysates and the determination of corresponding aglycones, sugars, aliphatic acids and phenolic acidsPreparation of the anthocyanin hydrolysates were accomplished in 25 mL securely stoppered test tube according to the standard procedure developed by Gao and Mazza (1994). The mixture was flushed with nitrogen for 5 min to remove any oxygen before hydrolysis, then capped quickly.
Aglycones were extracted by amyl alcohol. PC on the aglycone was performed using Forestal, Formic and BAW as solvents respectively (Harborne, 1984). The UV-Vis spectra of the aglycones were also measured in methanol containing 0.01% concentrated HCL(V/V).
Sugars were identified by PC and GC, using the aqueous phase produced by above standard procedure. Authentic samples of D-glucose, D-galactose, D-arabinose, D-xylose and L-rhamnose were used as standards (Harborne, 1984; Markham, 1982). As to PC, being heated by vapor, the aqueous phase was flushed to minimum volume with nitrogen and was analyzed by PC using n-butanol-pyridine-water (6:4:3, V/V) as solvent. The air-dried filter paper was sprayed with 2% diphenylamine (in acetone)-2% aniline (in acetone)-85% phosphoric acid (5:5:1, V/V). After air-dried again, the paper was heated at 80 ℃ for 15 min. AS to GC, after the aliquot was taken for PC analysis, the remaining aqueous solution was heated by vapor again, and evaporated to dryness with nitrogen, then stored in the desiccator at least overnight. 0.5 mg sugar was placed in a 50 mL securely stoppered conical flask, added 1 mg HONH3Cl, 0.5 mL pyridine, and heated in 90 ℃ water bath for 30 min while being surged now and then. The solution was cooled to room temperature rapidly under tap water, added 0.5 mL acetic anhydride, and heated again in 90 ℃ water bath for 30 min while being surged at times. The consequent solution was concentrated to minimum volume under reduced pressure at 85 ℃, and flushed to dryness with nitrogen and ivory-white unshaped solid emerged. Chloroform was used to dissolve the solid and injected into GC.
Aliphatic acids were also determined by GC after they prepared by the method introduced by Gao and Mazza (1994). Aliphatic acid standards include succinic, malonic, DL-malic, maleic and acetic acid (Markham, 1982).
Phenolic acids were elucidated by HPLC. Phenolic acid standards include p-hydroxybenzoic, ferulic, gallic and cinnamic acid (Markham, 1982).
2 Results and Discussion 2.1 ResultsPreliminary tests by TLC suggested that only one kind of major anthocyanin exists in the flower color pigment of P. mume 'Nanjing Hongxu'.
The two anthocyanins all expressed pure blue, from pure blue to colorless and pure blue respectively when respectively added "Na2CO3", "Na2CO3+NaOH" and "Na2CO3+CH3COCH3", showing they are cyanins (Robinson et al., 1931). Furthermore, they all expressed red in Visible light. But in UV light, they were dull red, and became blue after addition of ammonia solution. Thus, the anthocyanins are 3-glycoside derivatives (Harborne, 1984).
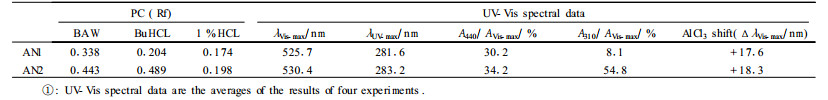
The UV-Vis spectra of the anthocyanins and the aglycone showed characteristic end absorption, a small absorption maxima in UV light region and a strong absorption maxima in Visible light region (Tab. 1, 2). The positive effect, namely a bathochromic shift, of aluminium ion on the λVis-max revealed the existence of o-dihydroxyl (Tab. 1). The hypsochromic shift of λVis-max caused by glycosylation of anthocyanin (Tab. 1, 2) and the pronounced shoulder in the 440~460 nm region (Tab. 1) indicated again the position of glycosidic substitution is at C3. The A440/AVis-max ratio indicated the 5-hydroxyl is free and the position of λVis-max also showed the 7-hydroxyl is free. In the UV-Vis spectra of AN2, the weak peak in the neighborhood of 310 nm implied AN2 is acylated by acids (Tab. 1) (Harborne, 1958; Andersen, 1985).
|
|
|
|
The aglycones, sugars and acids of the anthocyanins were produced by complete acid hydrolysis.
Aglycones, namely anthocyanidins, were all determined as cyanidin by the Rf on PC, the magenta color in Visible light, the λVis-max and the color shift with AlCl3(Tab. 2), which are very in accordance with the data found in literature of cyanidin (Harborne, 1984).
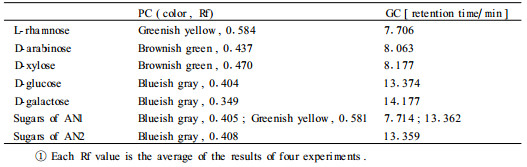
Sugars were synchronously identified by the Rf and color characteristics on PC and by the retention time on GC (Tab. 3). Therefore, AN1 contains glucose and rhamnose and AN2 contains only glucose respectively. Furthermore, GC analysis also revealed that the molar ratio of glucose to rhamnose of AN1 is about 1:1.02 and the glucose molar ratio of AN2 to AN1 is about 1.97:1.
|
|
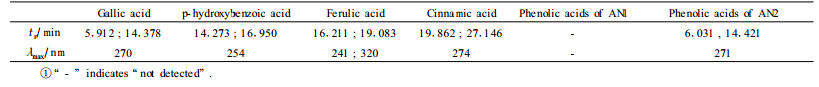
It was indicated by GC that the two anthocyanins do not contain any aliphatic acid moiety. HPLC analyses revealed that AN1 contains no any acid moiety and AN2 contains galloyl moiety (Tab. 4).
|
|
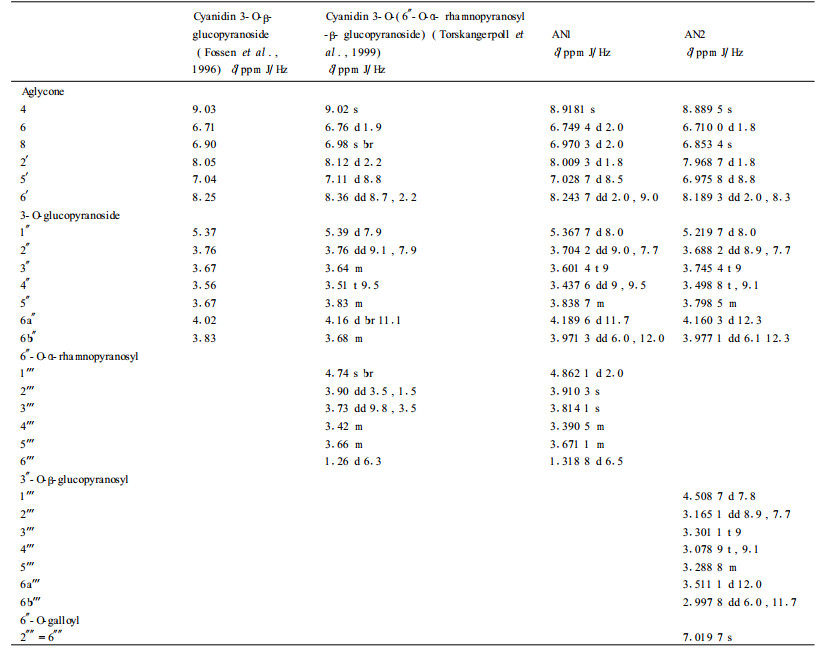
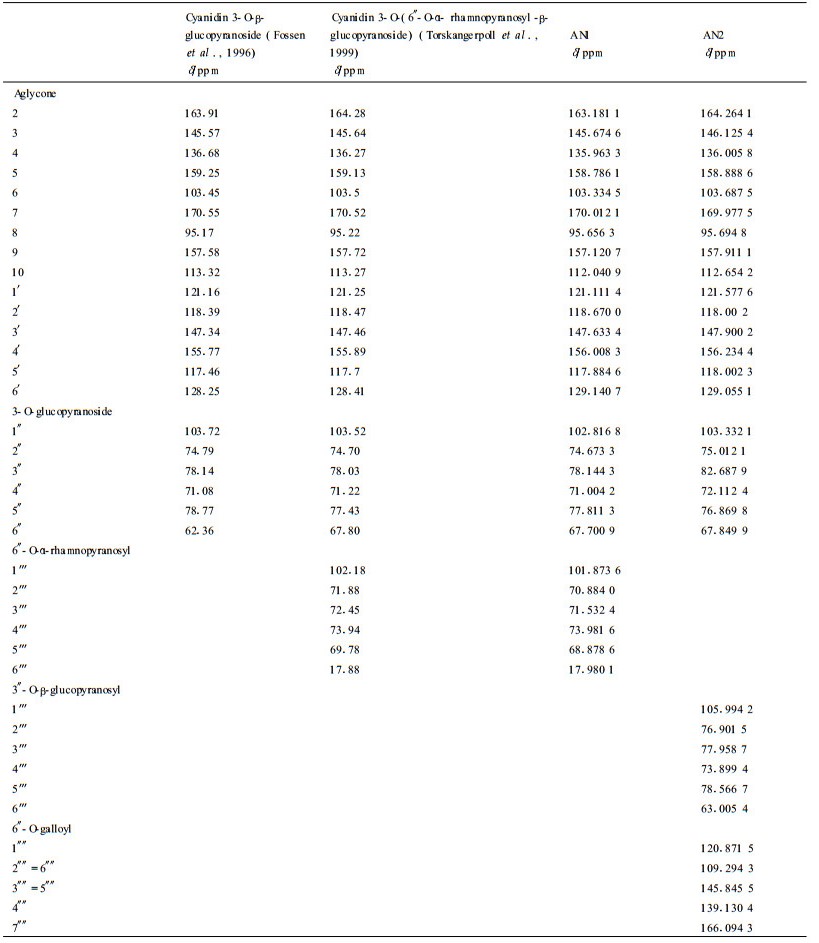
It was found that, in 1H NMR spectral data, the chemical shifts and the coupling constants (7.8~8.0) of the anomeric protons of the three glucose residues of the two anthocyanins showed the glucose residues have β-configuration and all the sugar ring protons are positioned di-axial to each other (Tab. 5) (Fossen et al., 2000). In 13C NMR spectral data, four hexose redsidues with pyranose forms were identified by the chemical shift values from about δ63 to 82 of the non-anomeric carbons (except that of the C6 of rhamnose residue) (Tab. 6) (Harborne et al., 1982).
|
|
|
|
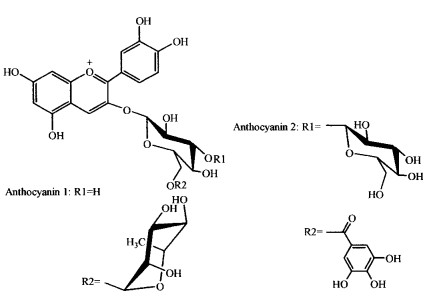
The 1H and 13C NMR spectral data of AN1 were found to be in close agreement with those of cyanidin 3-O-(6″-O-α- rhamnopyranosyl -β- glucopyranoside) reported previously (Tab. 5, 6) (Fossen et al., 1996). The proton signals of cyanidin were assigned according to information regarding coupling constant and chemical shifts. The doublet at δ 4.862 1 and the doublet of a methyl group at δ 1.318 8 showed a α-L-rhamnose residue (Torskangerpoll et al., 1999; Li et al., 2000). The down field shift of the H-6a″ (from 4.02 to 4.1896), H-6b″ (from 3.83 to 3.971 3) and the down field shift of C-6″ (from 62.36 to 67.700 9) indicate the rhamnosyl moiety is attached to the glucosyl 6-position (Torskangerpoll et al., 1999) (Tab. 5, 6). The FAB MS spectrum of AN1 gave the [M+] at m/z 595.2, with fragment ions of m/z 449.2 [M-146]+ (loss of rhamnose residue), 287.0 [M-146-162]+ (loss of rhmanose and glucose residues). Based on the above comparison and analyses, AN1 was identified as cyanidin 3-O- (6″-O-α-rhamnopyranosyl-β-glucopyranoside), namely cyanidin 3-rutinoside (Fig. 1).
|
Fig.1 The molecular structures of AN1 and AN2 isolated from the flower color pigment of P. mume 'Nanjing Hongxu' |
In 1H NMR spectra, the chemical shifts of the two glucosidic anomeric protons of AN2 appeared at δ 5.219 7 (d, J=8.0 Hz, H-1″) and δ4.508 7 (d, J=7.8 Hz, H-1'''). The proton and carbon signals of galloyl were assigned according to the literature (Fossen et al., 1999, Lee et al., 2000). The chemical shifts of the protons of the second glucose unit, the down field shift of the H-3″ (from 3.67 to 3.874 4), the H-6a″ (from 4.02 to 4.120 3), H-6b″ (from 3.83 to 3.977 1), together with the down field shift of C-3″ (from 78.14 to 82.687 9) and C-6″ (from 62.36 to 67.849 9) indicated both the glucopyranosyl and the galloyl moiety are attached to the glucosyl 3″ or 6″-position (Tab. 5, 6), which was confirmed by the fact that the FAB MS spectrum of AN2 establiehed [M+] at m/z 763.4, with fragments corresponding to cyanidin 3-glucoside (m/z 449.1), cyanidin 3-glucosylglucoside (m/z 611.3) and cyanidin 3-galloylglucoside (m/z 601.2). Moreover, it was quite possible that the galloyl moiety was positioned at the 6″ because the comparatively big down field shift (0.1403 of H-6a″, 0.1471 of H-6b″) of H-6″ implied the spacial proximity of an aromatic ring (Fossen et al., 2000). Partial acid hydrolysis of AN2 produced cyanidin 3-O- (6″-O-galloyl-β-glucopyranoside), confirming that the galloyl moiety is attached to the glucosyl 6″-position of the inner glucose residue and the second glucopyranosyl moiety is attached to the 3″-position. As a result, AN2 was identified as cyanidin 3-O- (6″-O-galloyl-3″-O-β-glucopyranosyl-β-glucopyranoside) (Fig. 1).
2.2 Discussion 2.2.1 Biological implication of the anthocyaninsBeing the core of the flower color pigment of P. mume 'Nanjing Hongxu', cyanidin 3-O- (6″-O-α-rhamnopyranosyl -β- glucopyranoside) and cyanidin 3-O- (6″- O- galloyl- 3″- O- β- glucopyranosyl- β- glucopyranoside), may possess of some remarkable biological functions. It radically contributes to the blazing mauve of the flower, which lures the bird pollinators to optimize the diffuseness of pollen on the basis of wind-pollination in winter and early spring (Harborne, 1973). Furthermore, the cyanins may play a role in protecting Mei flower against cold (Leng et al., 2003), which perhaps means the cultivars with red flowers are more adaptive to colder circumstance.
In cyanidin 3-O- (6″-O-galloyl-3″-O-β-glucopyranosyl-β-glucopyranoside), the fact that the galloyl moiety is attached to the 6″-position of the inner glucose residue enables the free rotation of the galloyl group and results in the folding of the planar ring of the galloyl over the pyrilium ring, leading the weak intramolecular copigmenttation to stabilize the cyanin in dilute solution (Rodr guez-Saona et al., 1999). However, the simplex structure-elucidating of the anthocyanin in this paper can not completely clarify the exact coloration behavior of the anthocyanin in vivo. This is because, when anthocyanin occurs in high concentration in the petals, it appears in crystalline forms due to metal chelation, copigmentation and being absorbed onto soluble polysaccharides (Harborne, 1973).
2.2.2 One question about the structure elucidation of the anthocyaninsIt has been confirmed that many anthocyanins do not have sharp melting points (m. p.) and do not give meaningful results on elementary analysis (Harborne, 1958; Harborne, 1973). As a result, the anthocyanin in this paper is not characterized by m. p. or elementary analysis.
AcknowledgementsWe are grateful for the cultivar-verifying and flower-collecting assistance by Mr. Zhao Xingfa and Ms. Liu Xuelan of the Research Centre of Mei flower of Sun Yat-sen Mausoleum Administrative Office of Nanjing. NMR was carried on by the State Key Laboratory of Coordination Chemistry of Nanjing University. FAB MS was performed by the phytochemistry laboratory of Kunming Institute of Botany, Chinese Academy of Sciences.
Andersen ϕ M. 1985. Chromatographic separation of anth ocyanins in cowberry (Lingonberry) Vaccinium vites-idaea L. J Food Sci, 60: 1 230-1 232. |
Chen Junyu(陈俊愉). 1989.Chinese Mei Flower Cultivars (中国梅花品种图志). Chinese Forestry Press, Beijing, China, 43 -101 (In Chinese)
|
Fossen T, Andersen ϕ M, ϕvstedal D O, et al. 1996. Characteristic anth ocyanin pattern from onions and other Allium spp. J Food Sci,, 61: 703-706. DOI:10.1111/j.1365-2621.1996.tb12185.x |
Fossen T, Andersen M. 1999. Cyanidin 3-(2″, 3″-digalloylglucoside) from red leaves of Acer platanoides. Phytochem, 52: 1697-1700. DOI:10.1016/S0031-9422(99)00188-0 |
Fossen T, Slimestad R, ϕvstedal D O, et al. 2000. Covalent anthocyanin-fla vonol complexes from flowers of chive, Allium schoeno prasum. Phytochem, 54: 317-323. DOI:10.1016/S0031-9422(00)00102-3 |
Gao L, Mazza G. 1994. Rapid method for complete chemic al characterization of simple and acylated anthocyanins by High-Performance Liqu id Chromatography and Capillary Gas-Liquid Chromatography. J Agric Food Chem, 42: 118-125. DOI:10.1021/jf00037a020 |
Harborne J B, Mabry R. 1982.The flavonoids: Advances in Research. Lon don: Chapman and Hall, 19-19
|
Harborne J B. 1973. Flavonoids//Lawrence P Miller. Phytochemistry organic metabolites (Volume Ⅱ). Toronto London Melbourne: Va n Nostrand Reinhold Company, 345-380
|
Harborne J B. 1984.Phytochemical Methods. 2nd ed. London, New York: Chapman an d Hall, 55-68
|
Harborne J B. 1958. Spectral methods of mcharacterizin g anthocyanins. Biochemical J, 70: 22-28. DOI:10.1042/bj0700022 |
Jackman R L, Yada R Y, Tung M A. 1987. A review: separ ation and chemical properties of anthocyanins used for their qualitative and qua ntitative analysis. J Food Biochem, 11: 279-308. DOI:10.1111/j.1745-4514.1987.tb00128.x |
Lee D, Kang S J, Lee S H, et al. 2000. Phenolic compounds from the leaves of Cornus controversa. Phytochem, 53: 405-407. DOI:10.1016/S0031-9422(99)00502-6 |
Leng P, Qi J X. 2003. Effect of anthocyanin on David Peach (Prunus davidiana Franch) under low temperature. Scientia Horticulturae, 97: 27-39. DOI:10.1016/S0304-4238(01)00374-0 |
Li Y M, Jiang S H, Gao W Y, et al. 2000. Phenylpropanoid glycoside s from Scrophularia ningpoensis. Phytochem, 54: 923-925. DOI:10.1016/S0031-9422(00)00171-0 |
Markham K R. 1982. Techniques of flavonoid identification. New York: Academic Press.
|
Robinson G M, Robinson R. 1931. A survey o f anthocyaninsⅠ. Biochemical J, 25: 1687-1705. DOI:10.1042/bj0251687 |
Rodr guez-Saona L E, Giusti M M, Wrolstad R E. 1999. Color and pigmen t stability of red raddish and red-fleshed potato anthocyanins in juice model sy stems. J Food Sci, 64: 451-456. DOI:10.1111/j.1365-2621.1999.tb15061.x |
Torskangerpoll K, Fossen T, Andersen ϕ M. 1999. Anthoc yanin pigments of tulips. Phytochem, 52: 1687-1692. DOI:10.1016/S0031-9422(99)00328-3 |
Zhao Changling (赵昶灵), Guo Weiming (郭维明). 2003. Mei flower and Tree peony-the first choice of the national flower of P. R. China. J Beijing Forestry University (北京林业大学学报), 25 (Special issue): 107-110 (In Chine se)
|
Zhao Changling (赵昶灵), Chen Junyu (陈俊愉), Liu Xuelan(刘雪兰), et al. 2004a. Effects of physical and chemical factors on the color expression of the flower color pigment of Prunus mume Sieb. et Zucc. 'Nanjing Hongxu'(Nanjing red- bearded). J Nanjing Forestry University (南京林业大学学报), 28(2): 27-32 (In Chinese)
|
Zhao Changling (赵昶灵), Guo Weiming (郭维明), Chen Junyu (陈俊愉). 2004b. Preliminary study on the categories and contents of the flower color pi gments of Prunus mume Sieb. et Zucc. J Beijing Forestry Univer sity (北京林业大学学报), 26(2): 68-73 (In Chinese)
|
 2006, Vol. 42
2006, Vol. 42