文章信息
- 吴均章, 甘习华, 韩素芬.
- Wu Junzhang, Gan Xihua, Han Sufen.
- 根瘤菌诱发合欢根毛的增生和根表层传递细胞的形成
- Proliferation of Root Hairs and Formation of Transfer Cells of Root Out-Layers in Albizia julibrissin Induced by Rhizobia
- 林业科学, 2005, 41(6): 179-180.
- Scientia Silvae Sinicae, 2005, 41(6): 179-180.
-
文章历史
- 收稿日期:2003-10-13
-
作者相关文章
2. 南京林业大学森林资源与环境学院 南京 210037
2. College of Forest Resources and Environment, Nanjing Forestry University Nanjing 210037
传递细胞作为一种溶质短途运输的特殊结构最初是由Gunning等(1968)提出来的。现已在苔藓、蕨类、被子植物的生殖器官、茎、节、叶和根的维管系统中都发现了传递细胞的存在(Gunning et al., 1968;1969;Gunning, 1977;Folsom et al., 1986;Pate et al., 1969;朱徵等,1999);但是在植物根表面上少有传递细胞的报道。自韩素芬等(1998)首次报道了根瘤菌可诱发刺槐根表面产生传递细胞,笔者在对合欢的研究中又发现了类似的情况,同时还发现根瘤菌不仅能诱导合欢根表面传递细胞的形成,而且在水培的情况下还能诱导合欢侧根根毛的产生。
1 材料与方法 1.1 合欢苗的培养1) 合欢无菌对照苗的培养 将灭菌吸胀的种子在YEMA平板上催芽,待胚根长到2~3 cm,并检查确无根瘤菌生长后,将苗转移到灭菌无氮培养液中。2)合欢接菌苗的培养 经灭菌处理并吸胀的种子在灭菌滤纸培养皿中催芽,待胚根长至2~3 cm,置合欢根瘤菌液中浸根接菌3 h,再转移至灭菌无氮培养液的广口瓶中,将接菌与对照苗都置25 ℃左右光照培养室,在光照强度为2 000 lx,每天16 h光照/8 h黑暗培养,观察根部变化。
1.2 半薄切片的制备和光镜观察接菌处理的合欢幼苗培养2周后,取根毛密集、形变明显的侧根根段于FAA液中固定,同时取对照培养的相应部位的根段也于FAA液中固定,并分别用GMA包埋,制成塑料半薄切片,PAS反应染色(吴均章等,2003),在显微镜下观察拍照。
1.3 超薄切片制备和透射电镜观察上述接菌处理的合欢根段在FAA液中固定的同时,另取部分根段固定于4%戊二醛磷酸缓冲液中,经缓冲液冲洗,锇酸(OSO4)后固定,清洗,乙醇系列脱水,环氧丙烷过渡,环氧树脂Epon812渗透包埋,聚合后,在LKB-V型超薄切片机上切片(切片厚度约70 nm),切片经醋酸铀和柠檬酸铅双染色,H-600透射电镜观察拍照。
2 结果接菌处理的合欢幼苗培养2周后,主根无根毛,但侧根基部根毛较密且弯曲形变明显(图版Ⅰ-1,4),通过塑料半薄切片光镜观察侧根根段横切面,从外向内分别为表皮、皮层、维管柱。表皮细胞排列整齐、紧密、无细胞间隙,皮层细胞大、排列疏松,外皮层不明显,内皮层细胞小而排列整齐紧密,维管柱最外为中柱鞘,内为初生木质部和初生韧皮部以及它们之间的薄壁细胞。从初生木质部辐射角看,其根为三原型(图版Ⅰ-2)。油镜观察,在其表皮细胞和外皮层细胞的外切向壁上发育出大量的壁内突结构(图版Ⅰ-3),具有传递细胞结构的典型特征,但在皮层和维管柱的薄壁细胞中未观察到这种壁内突结构。不接菌对照的合欢无菌苗,培养2周后,主根和侧根都不形成根毛(图版Ⅰ-5),在相对应部位根段的塑料半薄切片中,显示其初生结构与接菌培养的合欢幼苗相同(图版Ⅰ-6),但在表皮细胞和内皮层细胞内均无壁内突结构(图版Ⅰ-7),不形成传递细胞。通过超薄切片透射电镜观察接菌合欢幼苗侧根的表皮和外皮层细胞,其壁内突更为清淅,刚开始形成时为一小突起(图版Ⅰ-8),逐渐发育成乳头状(图版Ⅰ-9)、棒状(图版Ⅰ-10,11)和分枝状(图版Ⅰ-12)。连续观察接菌处理的合欢苗,根瘤大多产生在侧根基部根毛密集生长并发生形变的部位。
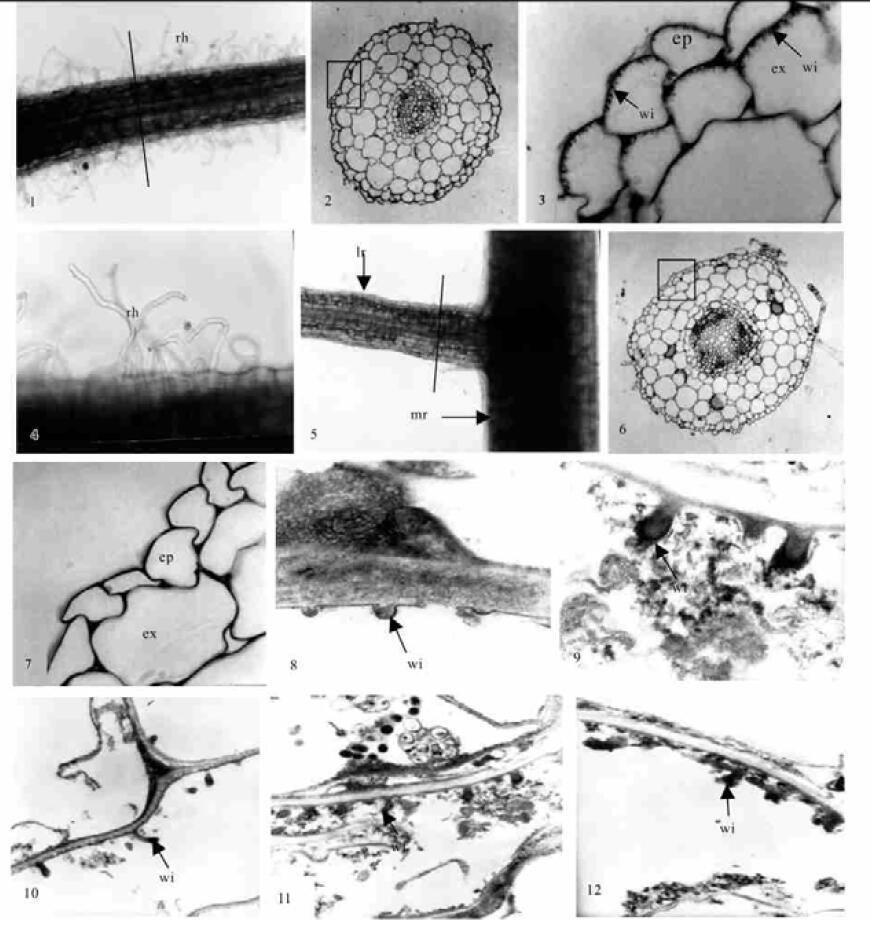
|
图版Ⅰ Plate Ⅰ
rh:根毛;ep:表皮;ex:外皮层;wi:壁内突;lr:侧根;mr:主根。 1.接菌合欢侧根形变根毛形态(20×);2.通过图 1直线处横切面, 示整体细胞结构(50×);3.图 2方框放大,箭头所指为表皮细胞和外皮层细胞外切向壁上的壁内突结构(500×);4.图 1根毛放大,示根毛弯曲形变(50×);5.对照合欢主根和侧根,示无根毛形成(20×);6.图 5直线部位横切,示对照合欢侧根横切面整体细胞结构图(×50);7.图 6方框放大,示表皮和外皮层细胞内无壁内突结构(300×);8~12.接菌合欢传递细胞的透射电镜照片。8.箭头所指为壁内突的形成起始(30 000×);9.箭头所指为乳头状壁内突结构(25 000×);10~11.箭头所指为棒状壁内突结构(8 000×);12.箭头所指为分枝状壁内突结构(15 000×)。 rh: root hairs; ep: epiderm; ex: exodermis; wi: wall ingrowth; lr: lateral root; mr: main root. 1. The lateral root hair's morpha of Albizia julibrissin inoculated by rhizobia (20×); 2. The lateral root's cross section of the treated Albizia julibrissin, showing whole primary structure of the root (50×); 3. Amplification of the section in the rectangle of the picture 2, and the arrows indicate the wall ingrowths on the tangential exowall of the epiderm and exodermis cells (500×); 4. Amplification of the root hairs in picture 1, showing the deformation of the root hairs (50×); 5. The main root and the lateral root of the contrastive Albizia julibrissin, showing no forming of the root hairs (20×); 6. The cross section along the line in the picture 5, showing the whole primary structure of the lateral root (50×); 7. Amplification of the section in the rectangle of the picture 6, showing no forming of the wall ingrowths in the cells of the epiderm and the exodermis (300×); 8~12. The transfer cell's transmission electron microscope photoes of the Albizia julibrissin; 8. The arrow indicates the beginning of the wall ingrowths (30 000×); 9. The arrow indicate the papillate wall ingrowths (25 000×); 10~11. The arrow indicates the claviform wall ingowths (8 000×); 12. The arrow indicates the ramose wall ingrowths (15 000×). |
传递细胞普遍被认为是一种具细胞间短距离营养物质吸收及转运功能的细胞类型(Gunning et al., 1969;Gunning,1977),其发育产生的大量壁内突增加了沿着它表面分布的质膜的面积,同样也增加了细胞质膜表面积和细胞体积之比,从而有利于物质的吸收和转运。本研究发现,只有接菌处理的合欢侧根上具较多形变的根毛,表皮及外皮层细胞可发育形成传递细胞,而对照苗的侧根上不发育出根毛,且相应根段上,其表皮和内皮层细胞上均不形成壁内突结构。因而,笔者认为,根表层传递细胞和根毛的大量产生是由根瘤菌诱导所致,根毛与根表皮和外皮层细胞中外切向壁上壁内突的大量产生可增加对水分和某些营养物质的吸收和转运,这与根瘤形成过程中,由于细胞快速分裂、增长需要更多的水分和各种营养物质是相关的。这也说明,根瘤菌诱发合欢根毛增生和根表皮及外皮层细胞上传递细胞的形成,是合欢根瘤形成过程中表现出来的形态结构与功能的高度统一。
韩素芬, 甘习华, 黄金生. 1998. 接种根瘤菌后刺槐根表皮形态和超微结构的变化. 林业科学, 34(4): 109-110. DOI:10.3321/j.issn:1001-7488.1998.04.014 |
吴均章, 韩素芬. 2003. 塑料半薄切片观察豆科植物根表面传递细胞. 南京林业大学学报, 27(2): 65-68. DOI:10.3969/j.issn.1000-2006.2003.02.016 |
朱徵, 胡适宜. 1999. 植物生殖系统中传递细胞的研究进展. 植物学通报, 16(专辑): 35-41. |
Folsom M W. 1986. Change in transfer cell distribution in the ovule of soybean after fertilization. Can J Bot, 64: 965-972. DOI:10.1139/b86-130 |
Gunning B E S, Pate J S. 1969. "Transfer cells" plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—their occurrence, structure and development. Protoplasma, 68: 107-133. DOI:10.1007/BF01247900 |
Gunning B E S. 1977. Transfer cells and their roles in transport of solutes in plants. Sci Prog Oxf, 64: 539-568. |
Gunning B E S, Pate J S, Britarty L G. 1968. Specialised "Transfer cells" in minor veins of leaves and their possible significance in phloem translocation. J Cell Biol, 37: 7-12. DOI:10.1083/jcb.37.3.C7 |
Pate J S, Gunning B E S. 1969. Vascular transfer cells in angiosperm leaves a taxonomic and morphological survey. Protoplasma, 68: 135-156. DOI:10.1007/BF01247901 |
 2005, Vol. 41
2005, Vol. 41
